Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Leaching of Phthalate from Medical Devices use in Pakistani Hospitals
*Corresponding author: Talpur FN, National Center of Excellence in Analytical Chemistry, University of Sindh, Jamshoro, 76080, Pakistan.
Received: November 14, 2021; Published: November 30, 2021
DOI: 10.34297/AJBSR.2021.14.002056
Abstract
Phthalates (PAEs) are widely used as plasticizers in medical devices to make polyvinyl chloride flexible and soft. However, PAEs can be leached out from plasticizers undesirably and can migrate within the material over the time and can end up with direct exposure to humans, because PAEs are not chemically bound, Particularly, (2-ethylhexyl) phthalate (DEHP) is present within various industrial and domestic goods along with medical devices. Leaching of PAEs is the main concern for human health due to toxic health effects, such as endocrine disruptor (EDCs) carcinogenicity, breast cancer, asthma, allergies and so on. PAEs are produced all over the world around 6.0 million metric ton per PAEs expose specifically from plastic containers, like cooler, plastic bottles, soft drinks, mineral water bottles and baby feeders and medical devices.
Therefore, in present an attempt is made to study the leaching of PAEs from medical devices abundantly use in local hospitals. After market survey in first step, infusion (IF) bottles of various brands available for infusion of dextrose, normal saline and mannitol packed in plastic bags/ packing along with this catheters and syringes were collected and coded to fulfill ethical requirements. Total 30 samples (103) of IF, 9 (3x3) samples of catheters along with 3 samples of syringes were collected and analyzed. The IF samples were classified on the basis of their composition, such as sugar (glucose/mannitol) and salt. Leached PAEs were extracted with dichloromethane following (EPA) standard method (606). Moreover DSC analysis showed clear Tg and Tm values for the infusion bottles, which lies in the category of amorphous nature. In addition IF bottles catheters and syringes were also analyzed by FTIR characterization. Results indicated that all IF solutions were acidic with pH ranges from 2.34 to 5.88. Gas chromatographic analysis showed that extent of leaching was different among brands; normal saline showed more PAEs leaching (78.60 μg/L) followed by dextrose (34.29 μg/L), mannitol (20.93 μg/L). In addition leaching studies of catheters was carried out with synthetic saline solution; results showed the total PAEs leached were in the range of 3.96 to 51.484 μg/L. In syringes PAEs leaching was not detected by Gas chromatography. Finally the GC MS of IF solution showed the presence of various PAEs like DEHP, DEP, DIBP, DBP BBzP by their mass fragmentation and from catheters additional 2 PAES i.e DiBP and DiProP. The Overall study indicated that due to medical devices such as infusion solutions, human are at direct exposure risk of phthalate accumulation.
Keywords: PAEs leaching, Exposure, Health implication Medical devices, PAEs alternatives
Abbreviations: PAEs: Phthalates; PVC: Polyvinyl chloride; DMP: Dimethyl Phthalate; DEP: Diethyl Phthalate; DPrP: Dipropyl Phthalate; DBP: Dibutyl Phthalate; DEHP: Di-2-ethylhexyl Phthalate; BBP: Benzylbutyl Phthalate; DiNP: Di-isononyl Phthalate; DOP: Di-n-Octyl Phthalate; DCHP: Dicylohexyl Phthalate; EDCs: Endocrine Disruptor Chemicals; BBP: Butyl Benzyl Phthalate; EDCs: Endocrine Disruptor; TOTM/TEHTM: Trioctyltrimellitate/ tri-(2-ethylhexyl) Trimellitate); DNA: Deoxyribos Nucleic Acid; IF: Infusion Bottles; DIDP: Di-Isodecy lPhthalate; DEHCH: Di(2‐ethylhexyl)‐1,2‐ Cyclohexane Dicarboxylate; DEHT: is(2-ethylhexyl) Terephthalate; MDs: Medical Devices.
Introduction
Phthalates are the diesteres of ortho-phthalic acid and are known as organic lipophilic compounds utilized to enhance the flexibility and softness in plastics [1]. Polyvinyl chloride (PVC) plasticizers compounds are extensively used in large range of substances which includes medical devices (MD), toys, shower, cables, curtains food packing etc. Plasticizers are used to soften and develop their elasticity. Specifically, they mixed into the composition of medical devices (MDs) synthesized by PVC like catheters and medical gloves [2]. PAEs are abundantly used in many goods like paints, fragrances, nail polish, skin care products, food packing, hair products cosmetics and printing inks [3]. The general figure of PAE is depicted in 1.1.
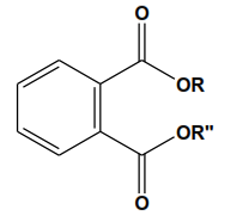
Figure 1-1: General structure of PAEs. (R and R’’ are the similar or different alkyl or aryl functional groups).
In MDs Phthalates (PAEs) like di-(2-ethylhexyl) (DEHP) is used as a major source which is about 30 to 40% of its weight [4]. In Europe about one million tons of PAEs are produced per year and the dominant PAEs are diisononyl phthalate (DiNP), diisodecyl phthalate (DiDP) and di-(2-ethylhexyl) phthalate (DEHP) are used as plasticizers [5]. PAEs are not chemically bound to plastic therefore leached into plastic material and spread into environment frequently [6]. PAEs are released out from MDs quickly by high temperature, lower pH, duration of use, infusion rate, and surface contact [7]. Human beings are highly affected by the PAEs over the larger scale [8] [DEHP, benzyl-butylphthalate (BBP) and dibutyl- phthalate (DBP)] have mutagenic and carcinogenic impact over the breastfeeding or pregnant women and children [9]. The DEHP, benzyl butyl phthalate (BBP) and dibutyl phthalate (DBP) are known as carcinogenic endocrine disruptors to humans and some of their studied showed their effects of mutagenic activity, peroxisome proliferation, infertility and carcinogenicity [10]. There are numerous ways through which human beings are exposed to PAEs such as foodstuff, dermal resorption or by inhaling air which contains PAEs [11].
The leaching and toxicity studies of PAEs were carried out by Duty et al [12], Schug et al; [13]; they reported the great impact of PAEs over the health of human beings through their studies. Previously the leaching study of PAEs from the PVC bags was reported by Bernard et al; [14], infusion sets Inoue et al [15], PVC tubing Takehisa et al; [16], blood bag shaz et al [17]. The Toxic and neurological effect over the animals and human beings [18] even can affect their liver [19]. The children are highly affected by the PAEs which results the great damage in their brain function. Moreover, the sperms of human beings along with their DNA are damaged due to the diethyl hexyl phthalate (DEHP) and DEP. The PAEs results in the destruction of lungs reported by Schutze et al; [13]. Along with this the exposure to PAEs from PVC material causes the enlargement of allergies, asthma [20]. The reproduction effects showed that the DEHP reduces the testicular weight as well as production of sperms and contributes to the atrophy of seminiferous tubules which results to infertility and damage kidney liver, spleen cancer and thyroid in male rodents [21] and PAEs lead to the disorders in the development of reproduction and in testicular dysgenesis in humans [22], keeping in view about the health effects of PAEs many of the countries like France in 2015 banned tubes used in neonatal possessing DEHP [23], US in 2012 [24], Canada in 2010 [25], USA in 2008 [26] and European union in 2005 [27] has banned PAEs to be used in PVC as plasticizers. Currently many of the countries like United States, Germany, Switzerland and Austria use the alternative of PAEs like Diisononyl cyclohexane dicarboxylate (DINCH) has substituted the DEHP [13]. The common alternates of PAEs are excessively used as free plasticizers in medical devices which have no health effect such as Diisononyl adipate (DINA), Bis(2- ethylhexyl) terephthalate (DEHT) [28,29].
In Pakistan generally the temperature reaches up to 50oC and it is reported in literature with the increasing effect of temperature the leaching from the PVC bottles takes place at higher rate [30]. Since MDs have direct impact on human health in our country due to having high temperature and MDs are stored at room temperature. The pH has direct impact over the leaching of PAEs from the MDs [31] and all the IF bottles are found to be in lower pH. Keeping in view of such risk factors leaching of PAEs from MDs like infusion bottles, catheters and syringes have not being conducted, therefore this study is evaluated first time in Pakistan to analyze the leaching of PAEs from MDs label in our country and identified by gas chromatography mass (GC - MS) and GC - FID technique. In addition, the PVC bottles were also characterized by DSC and FT - ATR to prove that MDs are the source of PAEs leaching.
Chemicals and Reagents
Chemicals and Reagents
Standards of all analytical grades of PAEs namely DMP, DEP, DBP, DiBP, BBP, DEHP, and DiProP of 99% purity were purchased from Deajung (Siheung, Korea) and Dichloromethane (DCM) (99.5% purity) were obtained from Scharlau (Barcelona, Spain).
Sampling Collection
All the PVC samples like IF bottles, catheters and syringes were purchased from local medical stores of Hyderabad Sindh Pakistan and were collected in triplicate and coded for each sample due to ethical issues.
Extraction of Leached Phthalates
Leaching of PAEs from the MDs was followed by liquid - liquid extraction as per reported method presented by Suhrhoff [32]. The 0.9% solution of normal saline was prepared and passed through catheters and syringes. 10 ml of each infusion solution was extracted with 20 ml DCM and extraction was repeated thrice for maximum recovery of leached PAEs. The anhydrous sodium sulphate (Na2SO4) was used to dry the combined organic phases. The gas chromatography (GC) combined along with the flame ionization detector (FID) was used to analyze samples.
Mds Characterization by Ftir - Atr
FTIR - ATR was used to analyze all MDs samples for the determination its functionality, samples were cut into 2 cm as reported in literature [3] and recorded by using Fourier transform infrared (FTIR) spectrometer Nicolet iS10 (Thermo Nicolet Analytical Instruments) Madison, WI, USA with diamond single bounce ATR accessory (spectra - Tech, Shelton, CT) with KBr beam splitter and deuterated triglycine sulphate (DTGS) detector. The acquisition of data and instrumental control the OMNIC software (version 7.3) was used and for the recording of spectra 24 accumulated scans having 4 cm-1 resolution in the range of 4000 - 650 cm-1 were set, before acquiring each spectrum of sample the background spectrum was collected. Soft tissue and dichloromethane were used for the cleaning of diamond crystal after every sample.
Gc - Fid Conditions for the Determination of Paes.
The leached PAEs were monitored by gas chromatograph The DB-1 (30m x 0.25 mm ID) m long polar capillary column, with the gas chromatograph (GC-8700 PERKIN ELMER) was used to analyze the substrate and as a carrier gas nitrogen was utilized, temperature of oven was raised from 120 to 220oC with the ramp rate of 4oC /min and final temperature 220oC was set for 30 minutes, 2 μL volume was used as injection. The temperature of detector and injector was set at 240°C and 260°.
Identification of Leached Paes By Gc - Ms
The leached PAEs from MDs were identified by using Agilent 6890 N gas chromatograph, equipped with a MS-5975, Injector auto sampler 7683-B, inert XL mass selective mass spectrometer (GCMS, Agilent Technologies, Santa Clara, USA). The Capillary column HP-5MS (30m x 0.25mm x 0.25 mm) used for gas chromatography and the maintained temperature was of 190°C till 60 seconds and raised from 15°C min-1 300°C for 15 minutes later on it was rapidly cooled to 60°C, by using helium of high ultra purity as carrier and its flow rate was 0.8 mL min-1. The detection and injection temperature were set as 310°C and 350°C. The temperature source was 250°C and 70eV as electron ionization energy with 50-550 m/z as mass range and to obtain the MS spectra in the positive electron ionization (EI) mode the 1mL of extracted sample was injected in split mode of (10:1). The mass of authentic compounds were identified by using program NIST Mass Spectral search (National Institutes of Standard and Technology, Gaithersburg, MD) in comparison to retention time.
Mds Characterization by Differential Scanning Calorimetry (Dsc)
The thermal behavior of PVC was analyzed by using differential scanning calorimetry (DSC). The rate of heating was 7°C/min in the range 60 to 150°C was set by using the stream of N2 gas (10 mL/min). In sample pan approximately the 5 mg of PVC tubes were sealed. The midpoint of a small endothermic rise of the pre- and post-transition baselines was used to determine the glass transition temperature (Tg) of PVC films, method described by Takehisa [16].
Results and Discussion
Ftir - Atr Characterization of Plastic Bottles
All the plastic infusion bottles syringes and catheters were studied by FTIR - ATR analysis in the region of 400 - 4000 cm-1. Results indicated that all IF bottles syringes and catheters contain variable amounts of PAEs. The peak in all spectra at the 717.06 to 731.17 cm-1 showed the existence of aromatic C - H wagging vibrations as reported by Kumar et al; PET bottles and found the characteristic peaks at 1713cm-1 which was attributed due to the presence of carbonyl attached with the aromatic ring [33]. Moreover by tracing the spectra peaks were detected at 2848, 2917 and 1462 cm-1 which were attributed due to the presence of PVC [34]. The result has confirmed the existence of PAEs from their characteristics peaks. The C=O ester peak present at 1710 cm-1 and the peak present at 717.06 to 731.17 cm-1 is the general peak of all PAEs present in all MDs. The similar observations were done by Chen et al; [34] the analysis of PAEs in plasticized polymer films at the molecular levels refff and Bagel et al; [35] from infusion sets and Jager et al; [35] from blood bags.
Gc - Fid Quantification of Leached Phthalates
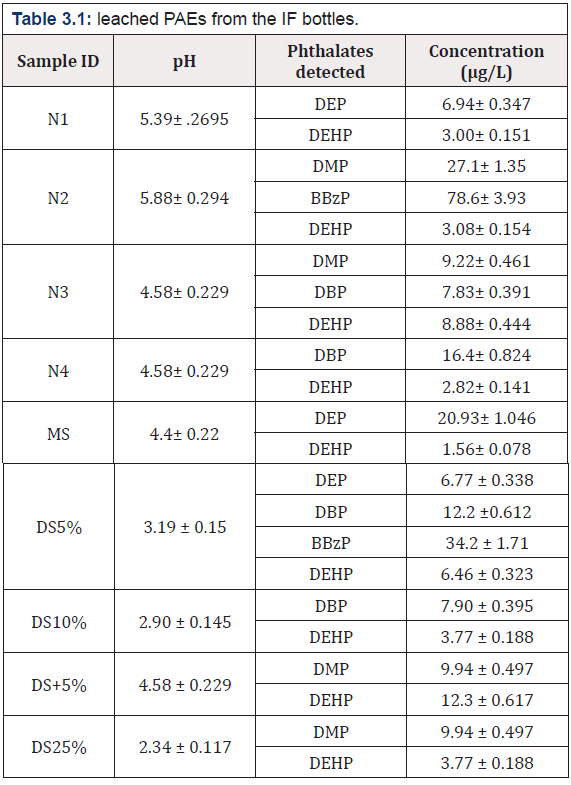
The current result indicats that all the brands of IF bottles like normal saline, dextrose sugar, catheters and syringes leached different amount of PAEs. All the IF solution are acidic in nature thus had great impact on the leaching of PAEs. The GC - FID study reveals that the leaching of PAEs found higher in infusion solution containing salts (N) (78.60up to μg/L) followed by dextrose (DS) (34.29 μg/L), and mannitol (MS) (20.93 μg/L). Among all IF solutions (DS) has leached five PAEs such as DMP, DEP, DBP, BBzP and DEHP. The GC - FID chromatogram of a (DS), b (MS) and c (N) is depicted in (Figure 3.1) while the quantification of all the leached PAEs from the IF bottles are shown in (Table 3.1).
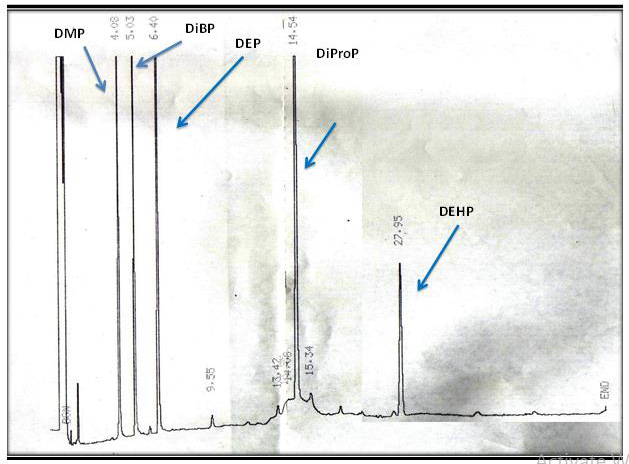
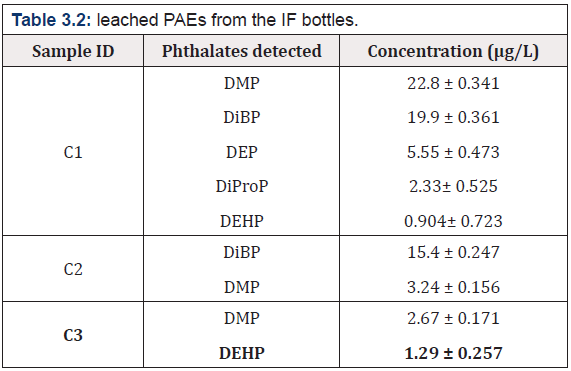
The addditional two PAEs known as DiProP and DiBP were leached out from the catheter (c) and the total concentartion of PAEs was (74. 08μg/L) shown in (Figure 3.2) while there was no any PAEs leached from the syringe. The total no: of PAEs leached from the catheters are depicted in (Table 3.2).
Gc - Ms. Quantification of Leached Phthalates
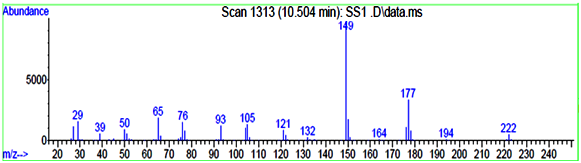
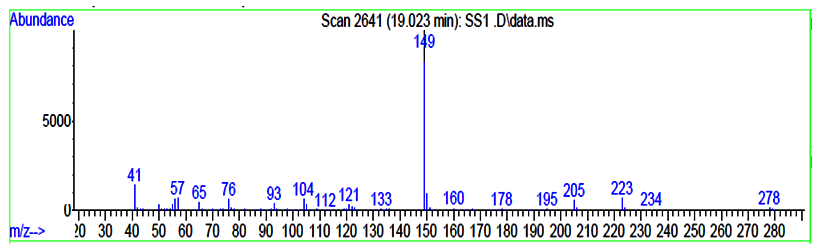
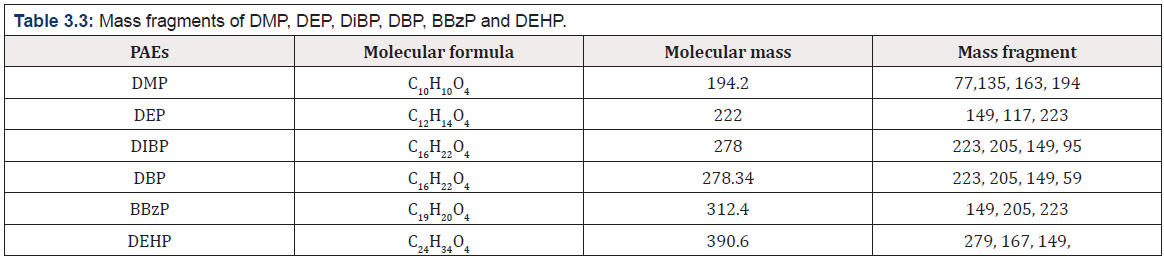
The identification of PAEs was also carried out by the GC - MS by measuring the mass fragments and molecular ion peak. The general characteristic peak for all the PAEs was 149 m/z was detected as a base peak in all the samples and same was reported by Xu during during the biodegradation studies of n - butyl benzyl phthalate by a bacterium species isolated from mangrove sediment and Murry 2015 [36] for detection of phthalates in bottled water by gas chromatography-mass spectrometry [37]. All the mass fragments of PAEs which were leached are present in (Table 3.3) and the mass spectra of leached PAEs are depicted in (Figure 3.3a and 3.3 b).
Mds Characterization by Differential Scanning Calorimetry (Dsc)
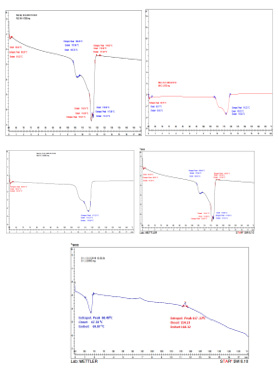
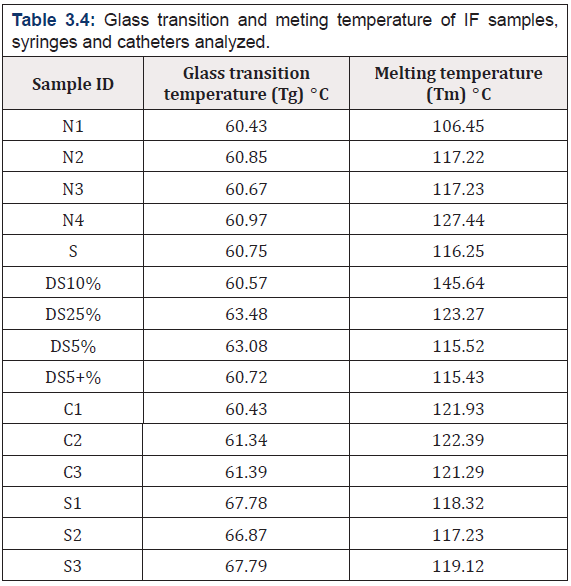
DSC analysis measures the heat absorbed when the temperature of the sample is raised at the linear rate and the process can be either exothermic or endothermic along with its morphology [38]. The experiment provides the (Tg) glass transition temperature and the percent crystallinities to assess the morphological state i.e (amorphous, crystalline and oriented). At the (Tg) the polymer is in its glassy state at which cold draw and plastic deformations are possible as the temperature is raised the secondary interaction between the adjacent polymers become weaker and the polymer lost its glassy state and is completely amorphous state [39]. all the samples of IF were found in the range of 106.45°C to 145.64°C, it has been found that low density poly ethylene (LDPE) has a melting temperature below 120oC, in our case sample DS5%, DS5+%, DS25% have melting point of 115.52°C, 115.43°C, 123.27°C respectively while two samples contain higher temperature of 127.44oC and 145.64oC which has been reported for high density poly ethylene (HDPE) materials [40]. Along with this all the PVC samples of catheters and syringes which start from 60.43°C to 67.79°C, whereas their Tm ranges from 117.23°C to 122.39°C .It has reported by Takehisa [16] which proved the leaching can take place at this range and this is due to the amorphous nature of samples.
The DSC thermogram of representative samples i.e. normal saline, mannitol, sugar, syringe and catheter (Figure 3.4 a,b c,d and e) respectively are given with details with transition and melting temperatures in (Table 3.4).
Conclusion
The present study has revealed that phthalates leaching from infusion bottles and catheters syringes were found at different ranges, while no PAEs leaching was found from different syringe samples. The GC - FID study reveals that the leaching of PAEs found higher in infusion solution containing salts (78.60up to μg/L) followed by dextrose (34.29 μg/L), and mannitol (20.93 μg/L). Among catheters C1 had higher PAEs leaching i.e. 51.484 μg/L than C2 (18.64 μg/L) and C3 (3.96 μg/L). Overall result indicated that mannitol has less phthalate leaching among IF bottles and C3 in catheters. Furthermore GC- MS analysis confirmed the presence of DMP, DEP, DIBP, DBP, BBzP and DEHP in IF bottles, whereas additional two PAEs i.e DiBP and DiProP were found in cathers. Moreover the pH of all infusion solutions was found acidic in the range of 2.34-5.88 which could be a contributing factor for PAEs leaching as acidic pH accelerate the leaching of PAEs on account of weak interaction forces/bond between polymers and PAEs. Furthermore plastic bottles and catheters were also analyzed by FTIR characterization and DSC thermal profiling.
DSC analysis showed clear Tg and Tm values for the infusion bottles and for catheter, which lies in the category of amorphous nature. Results confirmed the presence of phthalates from their characteristics peaks such as peak at 717.06 to 731.17 cm-1. The peak at 1710 which showed the carbonyl group in combination with aromatic ring, while other peak at 721.8 cm-1 showed the presence of aromatic C-H wagging vibrations in MDs. Moreover the peak present at 717 cm-1 is the general peak of all PAEs.
Our current study findings of FTIR-ATR for PAES leaching from infusion bottles associate well with the findings of PAES leaching in infusion solutions /catheter as determined by GC-FID and GC - MS. Hence it can be concluded the loosely bond phthalates from plastic bottles/catheters are major contributing factors of PAEs leaching in infusions solution along with storage conditions.
Competing Interests
All the authors declare that they have competing interest.
Acknowledgement
Shoaib Ahmed Hab is thankful to National Centre of Excellence in Analytical Chemistry, University of Sindh, Jamshoro, Sindh Pakistan for providing monthly stipend to carry out his research work.
References
- Fierens T, Servaes K, Van Holderbeke M, Geerts L, De Henauw S, et al. (2012) "Analysis of phthalates in food products and packaging materials sold on the Belgian market," Food and Chemical Toxicology 50(7): 2575-2583.
- Bernard L, Cueff R, Chagnon M, Abdoulouhab F, Decaudin B, et al. (2015) "Migration of plasticizers from PVC medical devices: Development of an infusion model," International journal of pharmaceutics 494(1): 136-145.
- Pradeep S and Benjamin S (2012) "Mycelial fungi completely remediate di (2-ethylhexyl) phthalate, the hazardous plasticizer in PVC blood storage bag," Journal of hazardous materials 235: 69-77.
- Marie C, Hamlaoui S, Bernard L, Bourdeaux D, and Sautou V, et al. (2017) "Exposure of hospitalised pregnant women to plasticizers contained in medical devices," BMC women's health 17: 45.
- Schettler T, (2006) "Human exposure to phthalates via consumer products," International journal of andrology 29(1): 134-139.
- Leitz J, Kuballa T, Rehm J, and Lachenmeier DW, (2009) "Chemical analysis and risk assessment of diethyl phthalate in alcoholic beverages with special regard to unrecorded alcohol," PloS one 4(12): e8127.
- Marie C, Hamlaoui S, Bernard L, Bourdeaux D, Sautou V, et al. (2017) "Exposure of hospitalised pregnant women to plasticizers contained in medical devices," BMC women's health 17:1-12.
- Kwapniewski R, Kozaczka S, Hauser R, Silva MJ, Calafat AM, et al. (2008) "Occupational exposure to dibutyl phthalate among manicurists," Journal of occupational and environmental medicine 50(6): 705-711.
- Regulation E (2008) "No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006," EC, and amending Regulation (E. Luxembourg: Official Journal of the European Union 13(20): 3-1357.
- Koch HM, Gonzalez Reche LM, and Angerer J (2003) "On-line clean-up by multidimensional liquid chromatography–electrospray ionization tandem mass spectrometry for high throughput quantification of primary and secondary phthalate metabolites in human urine," Journal of Chromatography B 784(1): 169-182.
- Bernard L, Cueff R, Breysse C, Decaudin B, Sautou V, et al. (2015) "Migrability of PVC plasticizers from medical devices into a simulant of infused solutions," International journal of pharmaceutics 485(1-2): 341-347.
- Hauser R, Meeker J, Singh N, Silva M, Ryan L, et al. (2006) "DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites," Human reproduction 22 (3): 688-695.
- Schütze A, Gries W, Kolossa Gehring M, Apel P, Schröter Kermani C, et al. (2015) "Bis-(2-propylheptyl) phthalate (DPHP) metabolites emerging in 24 h urine samples from the German Environmental Specimen Bank (1999–2012)," International journal of hygiene and environmental health 218(6): 559-563.
- Bernard L, Cueff R, Bourdeaux D, Breysse C, Sautou V, et al. (2015) "Analysis of plasticizers in poly (vinyl chloride) medical devices for infusion and artificial nutrition: comparison and optimization of the extraction procedures, a pre-migration test step," Analytical and bioanalytical chemistry 407(6): 1651-1659.
- Inoue K, Kawaguchi M, Yamanaka R, Higuchi T, Ito R, et al. (2005) "Evaluation and analysis of exposure levels of di (2-ethylhexyl) phthalate from blood bags," Clinica Chimica Acta 358(1-2): 159-166.
- Takehisa H, Naoko E, Masahiko S, Katsuhide T, Moriyuki O, et al. (2005) "Release behavior of diethylhexyl phthalate from the polyvinyl-chloride tubing used for intravenous administration and the plasticized PVC membrane," International journal of pharmaceutics 297(1-2): 30-37.
- Shaz BH, Grima K, and Hillyer CD (2011) "2‐(Diethylhexyl) phthalate in blood bags: is this a public health issue?," Transfusion 51(11): 2510-2517.
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, et al. (2009) "Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort," Neurotoxicology 30(4): 522-528.
- Rettberg HV, Hannman T, Subotic U, Brade J, Schaible T, et al. (2009) "Use of di (2-ethylhexyl) phthalate–containing infusion systems increases the risk for cholestasis," Pediatrics 124(2): 710-716.
- Jaakkola JJ and Knight TL (2008) "The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis," Environmental health perspectives 116(7): 845-853.
- Ventrice P, Ventrice D, Russo E, and De Sarro G (2013) "Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity," Environmental toxicology and pharmacology 36(1): 88-96.
- Eveillard A, Mselli Lakhal L, Mogha A, Lasserre F, and Polizzi, A et al. (2009) "Di-(2-ethylhexyl)-phthalate (DEHP) activates the constitutive androstane receptor (CAR): a novel signalling pathway sensitive to phthalates," Biochemical pharmacology 77(11): 1735-1746.
- Mallow E and Fox M, (2014) "Phthalates and critically ill neonates: device-related exposures and non-endocrine toxic risks," Journal of Perinatology 34(12): 892-897.
- Lioy PJ, Gennings C, Hauser R, Koch HM, and Kortenkamp A (2014) "Changing trends in phthalate exposures," Environmental health perspectives 122(10): A264-A264.
- Erythropel HC, Maric M, Nicell JA, Leask RL, and Yargeau V (2014) "Leaching of the plasticizer di (2-ethylhexyl) phthalate (DEHP) from plastic containers and the question of human exposure," Applied microbiology and biotechnology 98(24): 9967-9981.
- Flaherty E (2008) "Safety first: the consumer product safety improvement act of 2008," Loy. Consumer L Rev 21(3): 372-391.
- Fontelles J and Clarke C (2005) "Directive 2005/84/EC of the European Parliament and of the Council of 14 December 2005 amending for the 22nd time Council Directive 76/69/EEC on the approximation of the laws, regulations and administrative provisions of the Men Der States relating to restrictions on the marketing and use of certain dangerous substances and preparations (phthalates in toys and childcare articles)," Official Journal of the European Union 48(90): 40-43.
- Bernard L, Cueff R, Breysse C, Décaudin B, Sautou V, et al. (2015) "Migrability of PVC plasticizers from medical devices into a simulant of infused solutions," International journal of pharmaceutics 485(1-2): 341-347.
- Van Vliet E, Reitano E, Chhabra J, Bergen G, and Whyatt R (2011) "A review of alternatives to di (2-ethylhexyl) phthalate-containing medical devices in the neonatal intensive care unit," Journal of Perinatology 31(8): 551-560.
- Su PH, Chang YZ, Chang HP, Wang SL, Haung HI, et al (2012) "Exposure to di (2-ethylhexyl) phthalate in premature neonates in a neonatal intensive care unit in Taiwan," Pediatric Critical Care Medicine 13(6): 671-677.
- Bošnir J, Puntarić D, Galić A, Škes I, Dijanić T, et al. (2007) "Migration of phthalates from plastic containers into soft drinks and mineral water," Food Technology and Biotechnology 45(1): 91-95.
- Suhrhoff TJ and Scholz Böttcher BM (2016) "Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics—A lab experiment," Marine pollution bulletin 102(1): 84-94.
- Vijayakumar S and Rajakumar P (2012) "Infrared spectral analysis of waste pet samples," International Letters of Chemistry, Physics and Astronomy 4: 58-65.
- Zhang X and Chen Z, "Observing phthalate leaching from plasticized polymer films at the molecular level," Langmuir 30: 4933-4944.
- Bagel S, Dessaigne B, Bourdeaux D, Boyer A, and Bouteloup C et al. (2011) "Influence of lipid type on bis (2‐ethylhexyl) phthalate (DEHP) leaching from infusion line sets in parenteral nutrition," Journal of Parenteral and Enteral Nutrition 35(6): 770-775.
- Otero P, Saha SK, Moane S, Barron J, and Clancy G et al. (2015) "Improved method for rapid detection of phthalates in bottled water by gas chromatography–mass spectrometry," Journal of Chromatography B 997: 229-235.
- Xu XR, Li HB, and Gu JD (2006) "Elucidation of n-butyl benzyl phthalate biodegradation using high-performance liquid chromatography and gas chromatography–mass spectrometry," Analytical and bioanalytical chemistry 386(20: 370-375.
- Hodgson SC, Bigger SW, and Billingham NC (2001) "Studying Synthetic Polymers in the Undergraduate Chemistry Curriculum. A Review of the Educational Literature," Journal of Chemical Education 78(4): 555.
- Gunasekaran S, Natarajan R, and Kala (2007) "FTIR spectra and mechanical strength analysis of some selected rubber derivatives," Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 68(2): 323-330.
- Doh GH, Lee SY, Kang IA, and Kong YT (2005) "Thermal behavior of liquefied wood polymer composites (LWPC)," Composite structures 68(1): 103-108.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.