Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Cost-Benefit Analysis: Hemopatch® Vs Standard of Care in The Incidence of Postoperative Pancreatic Fistula in a Observational Study
*Corresponding author: Ramirez Manuel G, Global Advanced Surgery Clinical/HEOR, Advanced Surgery, Baxter Healthcare Corporation, 1 Baxter Way, Deerfield, IL 60015, USA. Email: manuel_ramirez@baxter.com
Received: December 22, 2018; Published: January 08, 2019
DOI: 10.34297/AJBSR.2019.01.000504
Abstract
Objective: To determine the efficacy and the impact on postoperative morbidity, mortality, and direct hospitalization costs of using the hemostatic-sealant Hemopatch® in patients undergoing pancreaticoduodenectomy.
Method: A retrospective observational data review (26 consecutive pancreaticoduodenectomies) from July 2015 to October 2016 using the same surgical technique, 13 procedures reinforcing the duct-to-mucosa pancreaticojejunostomy with Hemopatch® and 13 procedures without Hemopatch® at Miguel Servet University Hospital, Zaragoza, Spain. Both groups were statistically homogenous. Demographic data and rates of postoperative complications were collected. To extrapolate the average cost for pancreaticoduodenectomy treatment in a larger population with a normal distribution, a Monte Carlo simulation was run with a 1,000-procedure scenario.
Results: Results of the Monte Carlo simulation determined: the incidence of postoperative pancreatic fistula in the Hemopatch® group reported was 7.7% vs 30.9% (p value: 0.05), biliary fistula 7.7% vs 15.4% (p value: 0.05), hemorrhage 7.7% vs 15.5% (p value: 0.05), mean stay 20.8 vs 26.3 days (p value: 0.01), ICU stay 7.1 vs 9.1 days (p value: 0.01). Therefore, improved outcomes of Hemopatch® vs SoC, respectively resulted in a cost offset of: ICU length of stay $16,254.18 vs $21,000.41, total hospital length of stay $13,659.92 vs $17,233.67 (excluding ICU stay), postoperative pancreatic fistula $427.90 vs $2,234.05 biliary fistula $1,119.21 vs $2,238.43 and hemorrhage $427.90 vs $855.80.
Conclusions: Sealing with Hemopatch® as an adjuvant after a pancreaticoduodenectomy might offer a new possibility to decrease postoperative pancreatic fistula, with fewer severe fistulas, shorter hospital stays and reduced healthcare costs. The use of Hemopatch® resulted in a substantial savings of $11,109.00 (-24%)per patient. Hemopatch® might be an effective and cost-beneficial alternative against the standard of care.
Clinical relevance: The use of Hemopatch® as a sealant may result in better clinical (less morbidity) and possibly economic outcomes in pancreaticoduodenectomy.
Keywords: Postoperative pancreatic fistula; Pancreaticoduodenectomy; Duct-to-mucosa pancreaticojejunostomy; Hemopatch®
Introduction
The Whipple procedure (also known as pancreaticoduodenectomy [PD]) is the most common operation for pancreatic cancer. It’s also used to treat patients with tumors and cancers of the duodenum, ampulla of Vater and the bile duct, and chronic pancreatitis. Whipple surgery is a complex surgery that requires great expertise on the part of the surgeon. Recent papers suggest that institutions should perform a minimum of 11 Whipple procedures annually to maintain the team’s level of expertise and skills required by this complex surgery. At the University of Virginia, surgeons perform more than 50 Whipple procedures per year -five times that benchmark [1]. The IMS Health data related to hepato-pancreato-biliary procedures, volumes and Health and Safety penetration in premier on pancreas Whipple procedures reported around 11,000 procedures completed in 2015 [2]. The Spanish national registry of Whipple procedure in 2015 reported a total of 1,016 procedures (70% of Whipple surgeries in Spain in one year) [3]. At Miguel Servet University Hospital (Zaragoza, Spain) 50-60 Whipple surgeries are performed per year. Postoperative pancreatic fistula (POPF) is the most common major complication after pancreatectomy and it is a potentially serious and lifethreatening complication that may lead to prolonged hospital stay and increased costs [4].
Besides POPF, other serious postoperative complications are delayed gastric emptying (DGE), postoperative bleeding, and infectious complications [5,6]. POPF derives from an anastomotic leakage after a pancreatectomy. It is such leakage that determines the other major complications, such as peripancreatic collections, intraabdominal abscesses, DGE, and postoperative hemorrhage [7- 12]. The most important risk factors identified are a soft pancreatic texture and a main pancreatic duct diameter of 3 mm or less. POPF not only prolongs hospital stay and increases healthcare costs, but also plays a central role in the development of life-threatening events such as intra-abdominal abscess and postoperative hemorrhage [7]. The International Study Group of Pancreatic Fistula (ISGPF) provided a unique and universally accepted definition, that it has been adopted worldwide and applied to over 320,000 patients [13]. Despite its overall acceptance and success, following studies point out some major limitations [13-16] and, in 2016, the International Study Group for Pancreatic Surgery (ISGPS) revised the POPF definition and introduced new criteria to better characterize the different severity grades. Grade A POPF has been replaced with a new category characterized by an asymptomatic pancreatic leak called a “biochemical leak” (BL) [17].
Postoperative Fistula, Complications and Costs
POPF is still the most common and dangerous severe complication after PD, the incidence of which ranges widely in reported series from 10% to more than 34% in high volume centers [18,19]. POPF also represent a significant economic burden. Healthcare expenditure is currently rising exponentially, including in surgical fields. One of the reasons for this is the use of new surgical techniques and devices. However, postoperative complications are the main reason for increased costs in surgery [20]. Patients who develop complications consume a disproportionately greater share of the available resources. Moreover, postoperative morbidity may not only increase the costs of care, but may also lead to prolonged sick leave or even to permanent incapacity [21]. Since healthcare resources are limited, the funders of healthcare should know how complications increase the cost of the treatment.
Gani et al. reported on the total hospital costs of 971 patients who underwent pancreatic resection at a high volume academic center [22]. The median total costs nearly doubled in those who developed a postoperative complication following pancreatic resection compared with those who did not. Enestvedt et al. [23] reported similar results in a review of 144 patients undergoing PD in a network of community-based hospitals composed of both low- and high-volume centers [23]. The median cost was also nearly doubled for those with major complications, from $29,038 to $56,224 [22]. The development of POPF was an independent predictor of increased total cost, and fistula alone accounted for 1.3 times increase in the total cost, similar to the doubling in cost we report in our study in patients with POPF. Enestvedt [23] also found that postoperative complications tended to occur in clusters, and pancreatic fistula was generally associated with at least four other complications, further contributing to the accumulation of incurred costs.
Complications after pancreatic resection represent an important area for quality improvement in order to maximize the value of care. Postoperative complications, in particular POPF, are associated with increased length of stay (LOS) and higher hospital costs. Any measure to reduce the incidence and severity of complications after PD will save hospital costs [24]. Several studies have shown that POPF, as the most ominous complication after pancreatic resection, increases the cost of treatment and has severe clinical consequences as well [25-27]. Hence the attention of healthcare funders (government and insurance companies) must focus on the precise economic impact of the quality of healthcare delivery. Cost analysis of major surgical procedures was published by Vonlanthen et al. [28]. The study revealed that pancreatic surgery is different from other surgical procedures. The costs of pancreatic surgery were significantly higher compared with all other procedures, and the difference was even greater in patients with complications.
While surgical technique, comorbidities, surgical history, anticoagulation therapies, type of surgery, and other individual risks may all influence hemostasis during surgery, surgical technique is the usual cause of both bleeding and reoperation, signifying the need for effective hemostasis strategies [29,30]. Collagen hemostatic patches have been used for surgical hemostasis and healing for decades and been enhanced since their development in the early 1980s [31]. Hemopatch® is a recently developed hemostatic patch consisting of a specifically formulated collagen matrix, a protein-reactive monomer, and a biocompatible dye. The soft, thin, pliable, high liquid-absorption capacity, self-adherence, and sealing properties of Hemopatch® make it easy to use in open and minimally invasive surgery. The active side of the patch is coated with the rapid protein-reactive monomer, N-hydroxylsuccinimide functionalized pentaerythritol polyethylene glycol ether tetrasuccinimidyl glutarate (NHS-PEG). Hemopatch® has a two-fold mechanism of action. The thin layer of NHS-PEG provides rapid tissue attachment and sealing of the bleeding surface. Additionally, the collagen activates both the coagulation cascade and platelets, and releases coagulation factors, thereby enabling fibrin formation. The clot is further strengthened by the structural collagen fibers [32].
Methods
A retrospective observational analysis of 26 consecutive PD performed by the same surgeon from July 2015 to October 2016 using the same surgical technique at Miguel Servet University Hospital, Zaragoza, Spain, assessed clinical and healthcare resource utilization outcomes between the use of Hemopatch® reinforcing the duct-to-mucosa pancreaticojejunostomy or the control group without Hemopatch® (Standard of Care – SoC). The incidence of POPF in matched groups treated described above was compared. Hemopatch® cost as compared to the associated costs related to the post-operative management of POPF was examined. Both groups were statistically homogenous. Demographic data was collected (age, gender, diagnosis, comorbidities), and rates of postoperative complications (pancreatic fistula, biliary fistula, DGE, hemorrhage, readmission, exitus, and hospital length of stay –LOSand ICU LOS). To extrapolate the average cost of PD treatment and follow a normal distribution, a Monte Carlo simulation was used to populate a 1,000-procedure scenario.
Data source
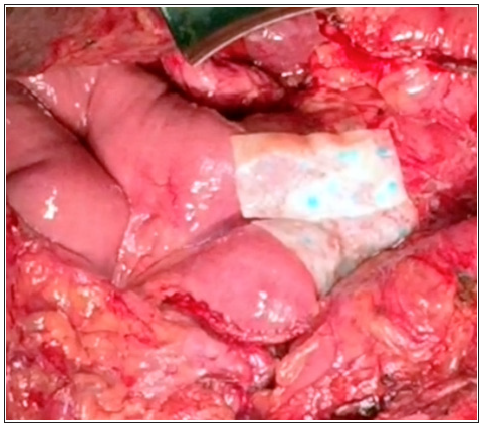
Data for this retrospective database analysis was obtained from Miguel Servet University Hospital, Zaragoza, Spain, analyzing a repository of clinical, economic and resource-use data developed for quality and utilization benchmarking purposes. The database includes nationally representative hospital data based on bed size, geographic region, and teaching hospital status. The database comprises data from 26 consecutive PD performed by the same surgeon from July 2015 to October 2016 using the same surgical technique reconstruction with duct-to-mucosa pancreaticojejunostomy. In the Hemopatch® group, two patches of Hemopatch® 9 x 4.5cm were placed, one on the underside and another on the anterior once the anastomosis was complete (Figure 1). To protect confidentiality, patient-specific data was de-identified according to US Health Insurance Portability and Accountability Act (HIPAA) regulations. Therefore, this analysis using the registry database does not constitute human subject research and is not subject to International Review Board approval.
Database case selection criteria
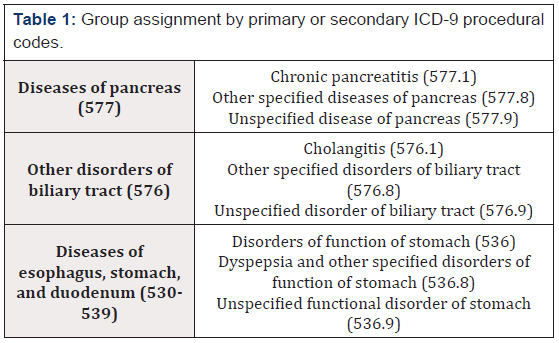
The records of PD cases included in this retrospective database analysis were of patients that underwent elective surgery with hospitalizations and discharges that occurred between July 2015 to October 2016; the patients were randomized in an alternative way preoperatively (Hemopatch® group vs SoC), independently of the characteristics of the patient and the main; and were at least 18 years old on the day of hospital admission. Table 1 lists the ICD-9 codes used in the identification of the cases and in characterizing their primary or secondary surgical type and severity (i.e., minor, major/severe). In cases of multiple hospitalizations within the same hospital during the study period, only the first hospitalization for one of the target procedures was identified and considered. Database records indicating those patients with or without Hemopatch® and those that lacked complete demographic/ baseline values and/or had unevaluable outcome measures were excluded from the analysis.
Data extracted for analyses
Data regarding patient characteristics (e.g., age at the time of admission, gender, diagnosis, Charlson Comorbidity Index -CCI-) were extracted. Outcome variables extracted from the records included complication outcomes, and healthcare resource utilization outcomes.
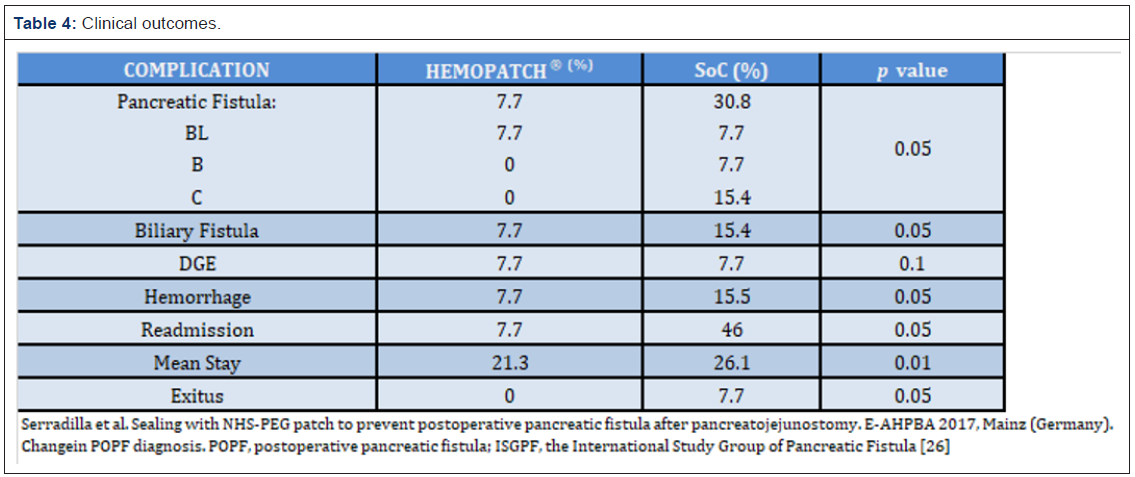
Healthcare resource utilization outcomes extracted were: a) average Hemopatch® cost per procedure vs associated costs (system costs) of POPF; b) hospital LOS (in days); c) ICU LOS (in days), incorporating the clinical differences in using Hemopatch® vs SoC; d) average base cost of a PD without complications, revision, hemorrhage, biliary fistula or DGE; e) average costs of the PD with major or minor complication, including the incidence of POPF but with revision, hemorrhage, biliary fistula or DGE (Table 4).
Statistical Analyses
All patient and hospital characteristics were summarized descriptively by hemostat charge cohort (i.e., Hemopatch® or SoC). Continuous variables were summarized using descriptive statistics (number, mean, standard deviation, median, minimum, and maximum) and categorical variables were presented as counts and percentages. To compare the equivalency of the cohorts, t-tests and Chi-square tests were performed on continuous and categorical variables, respectively. Baseline and demographic variables were used in the propensity score model to obtain a propensity score for every subject in the Hemopatch® and SoC cohorts. The propensity score for each subject was calculated from a logistic regression model that included all baseline and demographic data as covariates and the dependent variable of study treatment (i.e., Hemopatch® or SoC).
Methodology
Cost analyses: Cost analysis was performed by examining the cost (system cost) of using
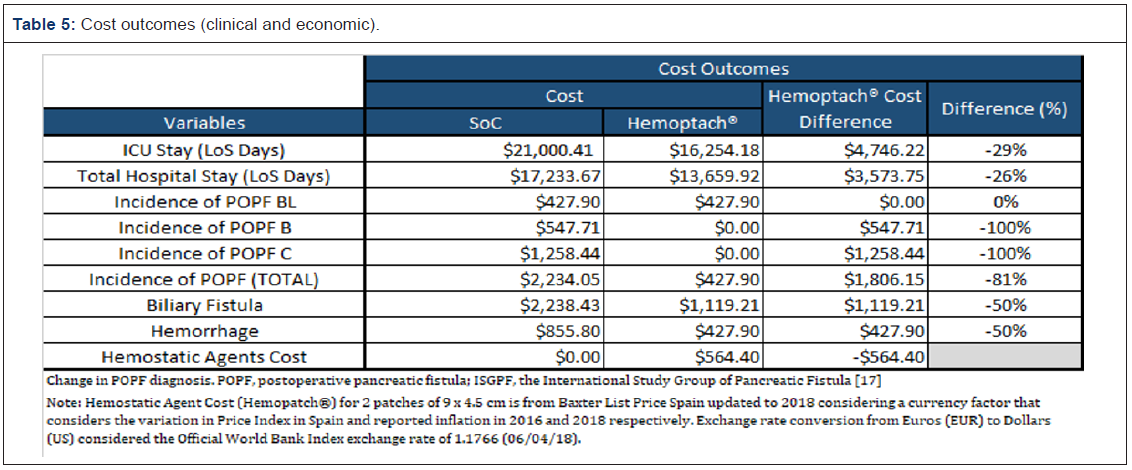
Hemopatch®, compared with the costs (system costs) of postoperative management of POPF at Miguel Servet University Hospital, Zaragoza, Spain (Table 5).
Surgery and complication costs: Surgery-related costs include: a) average Hemopatch® cost
per procedure vs associated costs (system costs) of POPF at Miguel Servet University Hospital, Zaragoza, Spain; b) hospital LOS (in days); c) ICU LOS (in days), incorporating the clinical differences in using Hemopatch® vs SoC; d) average base cost of a PD without complications, revision, hemorrhage, biliary fistula or DGE; e) average costs of the PD with major or minor complication, including the incidence of POPF but with revision, hemorrhage, biliary fistula or DGE (Table 5).
All base costs were updated to 2018 considering a currency factor that considers the variation in Price Index in Spain and reported inflation in 2016 and 2018 respectively. Exchange rate conversion from Euros (EUR) to Dollars (US) considered the Official World Bank Index exchange rate of 1.1766 (06/04/18).
The updated costs were used to feed a 1,000 procedures scenario using a Monte Carlo simulation following a normal distribution to extrapolate the median average cost in the treatment of PD (surgery, POPF treatment, biliary fistula, hemorrhage, ICU and overall stay).
Model analyses: This model estimated the results at Miguel Servet University Hospital, Zaragoza, Spain, performing 26 patients who underwent PD. For this economic model, Hemopatch® was used in 13 of PD and the other 13 PD were SoC procedure. The economic outputs of this model were calculated in terms of the annualized comparative clinical outcomes (ICU stay, overall stay-including readmissions-), incidence of biliary fistula and the corresponding cost savings of using Hemopatch® vs SoC. By doing so, the net cost impact of using Hemopatch® versus SoC was estimated.
Monte carlo simulation: Monte Carlo simulation is, in essence, the process that generates a sample with the characteristics of a desirable and finite population in order to calculate a certain value that follows a certain distribution (with independent and identically distributed values). The inherent randomness of the MCM is not only essential for the simulation of real-life random systems, it is also of great benefit for deterministic numerical practical problems [33].
For that, MCM was applied to iterate parameters such as the Average costs of the Whipple Surgery procedures without complication, including the incidence of POPF leaks but with revision, hemorrhage, biliary fistula or DGE, Product Cost in the use of Hemopatch®, Hospital stay (Including Readmission) and ICU Stay.
The initial Justification of the Monte Carlo method comes from two of the main theorems in Statistics and Probability: The Weak Law of Large Numbers and The Central Limit Theorem, which concludes that with an adequately high number of samples is possible to estimate one desirable parameter by incurring in a minimal error that allows to asymptotically quantify the relation between these two (Number of samples-Error) [34].
To evaluate significance and independence among alternatives of treatment, Fisher exact test was performed on variables such as POPF leaks, Biliary Fistula and Hemorrhage, due to the treatment of data as a contingency table, besides that in this retrospective analysis the Chi square assumption was violated because dataset contains <20 observations and at least one of the outcomes to test contains <5 observations. For Overall Stay (Including Readmission) and ICU Stay reported as continuous variables T Test was performed to determine Independence and goodness of fit among alternatives.
Results
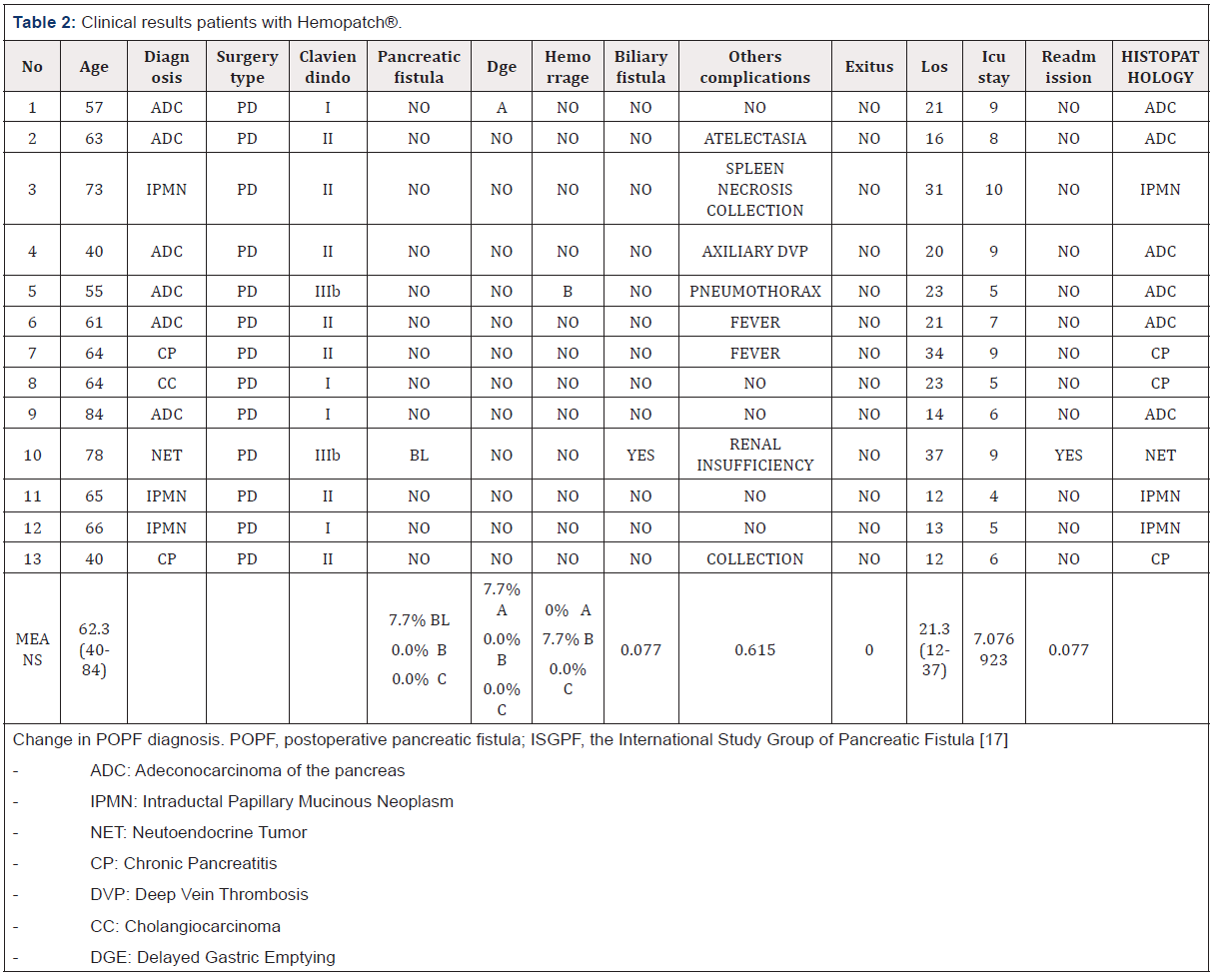
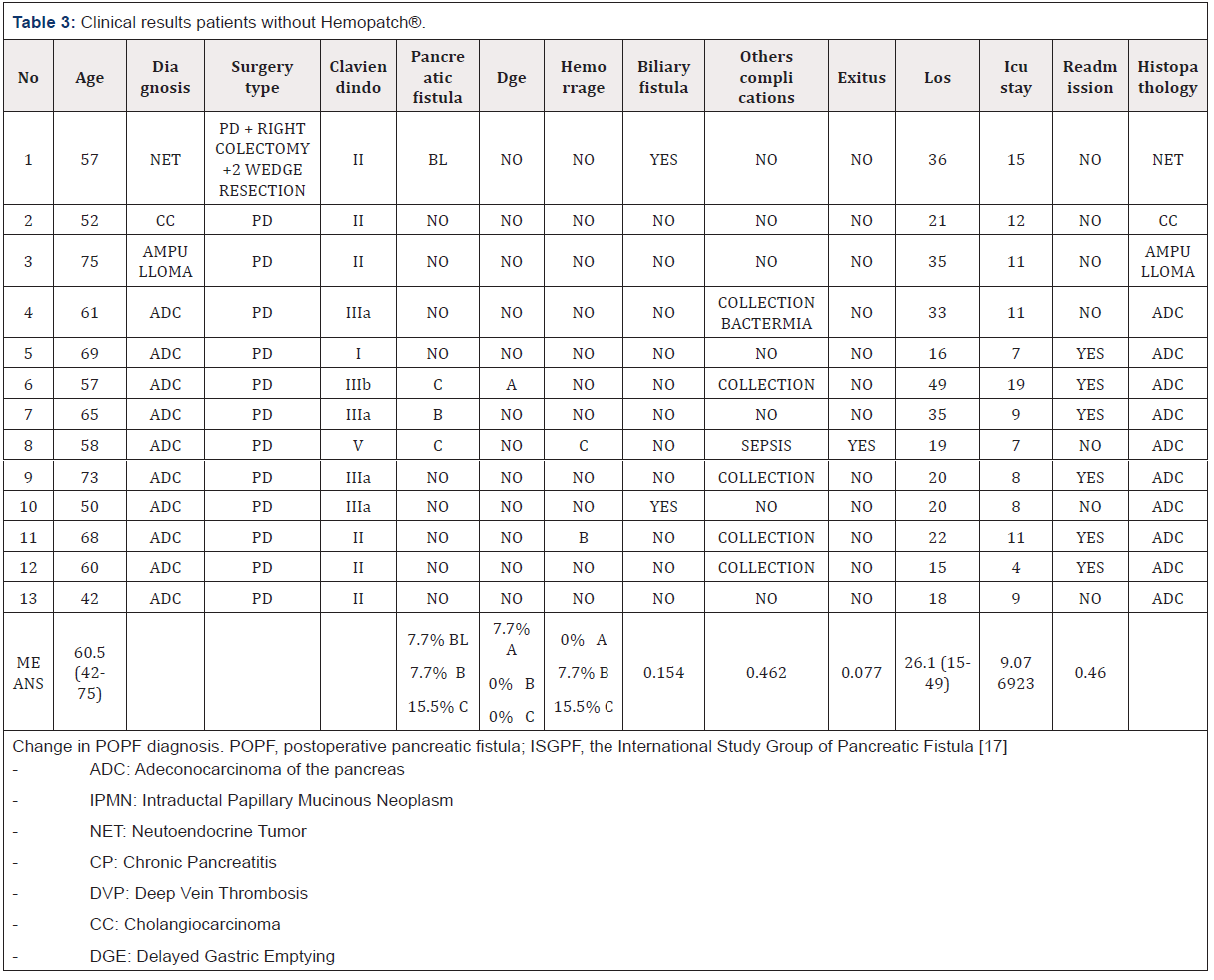
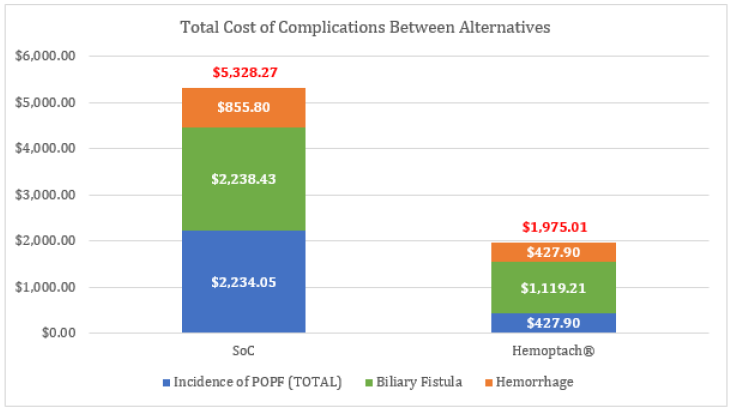
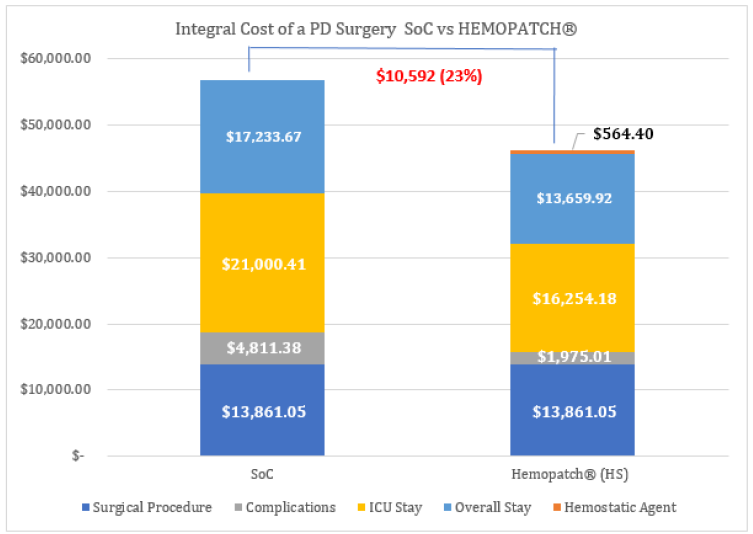
After the 1,000 procedures scenario, a total of 26 consecutive PD were identified with the following outcomes: the incidence of POPF in the Hemopatch® group reported was 7.7% vs SoC 30.9% (p value: 0.05), biliary fistula 7.7% vs 15.4% (p value: 0.05), hemorrhage 7.7% vs 15.5% (p value: 0.05), mean stay 20.8 vs 26.3 days and ICU stay 7.1 vs 9.1 days (p value: 0.01) (Tables 2-4). Therefore, improved outcomes of Hemopatch® vs SoC, respectively resulted in a cost offset of: ICU length of stay $16,254.18 vs $21,000.41, total hospital length of stay $13,659.92 vs $17,233.67 (excluding ICU stay), postoperative pancreatic fistula $427.90 vs $2,234.05 biliary fistula $1,119.21 vs $2,238.43 and hemorrhage $427.90 vs $855.80 (Table 5 & Figure 2). The use of Hemopatch® resulted in substantial savings of $10,592.00 (-23%) per patient (Figure 3). Because of the clinical and economic outcomes described above Hemopatch® is an effective and cost-benefit alternative against the SoC group.
Discussion
Physiopathology of POPF
POPF is generally defined as an abnormal communication between the pancreatic ductal epithelium and another epithelial surface containing pancreas-derived, enzyme-rich fluid [10]. POPF corresponds to a failure of healing of a pancreatic-enteric anastomosis. Nonetheless, it may derive from a parenchymal leak, that is not directly related to an anastomosis; for example, one that originates from the raw pancreatic surface (in patients with central or left pancreatectomy or enucleation). The pancreatic juice, which is rich in protease, is the most important factor involved in the inception and evolution of the POPF. When the proteases that it contains get activated, they determine the digestion and the destruction of the tissue, leading to partial or complete anastomotic dehiscence [4]. Furthermore, through the fistulation of the pancreatic-enteric anastomosis, the pancreatic juice induces inflammation and auto destruction of the peripancreatic and retroperitoneal tissues and of the surrounding vessels and viscera. As a result of these phenomena, hemorrhage, intraabdominal abscess, peripancreatic and retroperitoneal collections, and delayed gastric emptying may occur. Additional and more severe complications that could be associated with those previously mentioned are sepsis, shock, organ failure, and death [4].
Limitations
All economic models are a simplification of a complex healthcare situation and, hence, bear the limitations associated with a simple representation of the reality. Limitations of the analysis include its observational evaluation of a registry hospital (Miguel Servet University Hospital, Zaragoza, Spain) database design, which is less robust than the conduct of prospective randomized trials and inherently associated with selection biases. As mentioned, the utilization of propensity score matching was utilized to reduce the differences between the two cohorts. Specifically, after propensity score matching methodology was applied the two cohorts were closely matched with the exceptions of race, admission type, and several hospital-related characteristics. Further, well-controlled clinical trials and cost-consequence studies are needed to confirm and further elucidate these findings and to quantify the costsavings that may be realized with the utilization of a single flowable hemostatic matrix versus combination management of bleeding with flowable and non-flowable hemostatic agents in patients undergoing spine surgery. In addition, the sample size is small, so the results may be due to chance. To avoid this, a randomized clinical trial with a larger number of patients should be carried out.
Conclusion
Sealing the ducto-to-mucosa pancreaticojejunostomy with Hemopatch® after a PD might offer a new possibility to decrease POPF (-23.2%), with less fistula rate B and C, hemorrhage (-7.7%) and biliary fistula (-7.7%), less hospital stays (-4.8 days) and less healthcare costs. The use of Hemopatch® resulting in substantial savings of $10,592.00 (-23%) per patient. Hemopatch® might be an effective and cost-beneficial alternative against the SoC. Randomized controlled trials with larger number of patients should be performed to support this theory.
References
- University of Virginia. Health System.
- Truven Marketscan. Trend between years 2015 and 2016 may be impacted by change from ICD-9 to ICD-10. HPBA Procedure Volumes and H&S Penetration in Premier. Source: Premier.
- Martin Perez E, Poves I, Moya Herraiz A, Members of the Spanish Association of Surgeons (AEC). Section of Hepatic-Biliary-Pancreatic Surgery. Spanish National Registry of patients undergoing pancreaticoduodenectomy for periampullary tumors: Effects of hospital volume on outcomes E-AHPBA 2017, Mainz, Germany.
- Butturini G, Daskalaki D, Molinari E, Copelliti F, Casarotto A, et al. (2008) Pancreatic fistula: definition and current problems. J Hepatobiliary Pancreat Surg 15(3): 247–251.
- Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, et al. (2011) A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg 254(5): 702–707.
- DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, et al. (2006) Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 244(6): 931–937.
- Gómez T, Palomares A, Serradilla M, Tejedor L (2014) Reconstruction after pancreatoduodenectomy: Pancreatojejunostomy vs pancreatogastrostomy. World J Gastrointest Oncol 6(9): 369-376.
- Aranha GV, Aaron JM, Shoup M, Pickleman J (2006) Current management of pancreatic fistula after pancreaticoduodenectomy. Surgery 140(4): 561–568.
- Butturini G, Marcucci S, Molinari E, Mascetta G, Landoni L, et al. (2006) Complications after pancreaticoduodenectomy: the problem of current definitions. J Hepatobiliary Pancreat Surg 13(3): 207–211.
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, et al. (2005) International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138(1): 08–13.
- Hashimoto M, Koga M, Ishiyama K, Watarai J, Shibata S, et al. (2007) CT features of pancreatic fistula after pancreaticoduodenectomy. Am J Roentgenol 188(4): 323–327.
- Payne RF, Pain JA. (2006) Duct-to-mucosa pancreaticogastrostomy is a safe anastomosis following pancreaticoduodenectomy. Br J Surg 93(1): 73–77.
- Eshmuminov D, Schneider MA, Tschuor C, Kambakamba P, Muller X, et al. (2018) Systematic review and meta-analysis of postoperative pancreatic fistula rates using the updated 2016 International Study Group Pancreatic Fistula definition in patients undergoing pancreatic resection with soft and hard pancreatic texture. HPB 20(11): 992-1003.
- Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, et al. (2007) Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg 245(3): 443- 451.
- Hackert T, Hinz U, Pausch T, Fesenbeck I, Strobel O, et al. (2016) Postoperative pancreatic fistula: We need to redefine grades B and C. Surgery 159(3): 872-877.
- Hassenpflug M, Hinz U, Strobel O, Volpert J, Knebel P, et al. (2016) Teres Ligament Patch Reduces Relevant Morbidity After Distal Pancreatectomy (the DISCOVER Randomized Controlled Trial). Ann Surg 264(5): 723- 730.
- Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, et al. (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 161(3): 584-591.
- Pulvirenti A, Marchegiani G, Pea A, Allegrini V, Esposito A, et al. (2018) Pancreatic Surgery Definition and Grading of Postoperative Pancreatic Fistula on 775 Consecutive Pancreatic Resections. Ann Surg 268(6): 1069-1075.
- Sato N, Yamaguchi K, Chijiiwa K, Tanaka M (1998) Risk analysis of pancreatic fistula after pancreatic head resection. Arch Surg 133(10): 1094–1098.
- Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, et al. (2011) The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 254(6): 907–913.
- Lang M, Niskanen M, Miettinen P, Alhava E, Takala J (2001) Outcome and resource utilization in gastroenterological surgery. Br J Surg 88(7): 1006–1014.
- Gani F, Hundt J, Makary MA, Haider AH, Zogg AH, et al. (2016) Financial Impact of Postoperative Complication Following Hepato-Pancreatico- Biliary Surgery for Cancer. Ann Surg Oncol 23(4): 1064–1070.
- Enestvedt CK, Diggs BS, Cassera MA, Hammil C, Hansen PD, et al. (2012) Complications nearly double the cost of care after pancreaticoduodenectomy. Am J Surg 204(3): 332-338.
- Topal B, Peeters G, Vandeweyi H, Aerts R, Penninckx F (2007) Hospital Cost-categories of Pancreaticoduodenectomy. Acta Chirurgica Belgica 107(4): 373-377.
- Cecka F, Jon B, Subrt Z, Ferko A (2013) Clinical and economic consequences of pancreatic fistula after elective pancreatic resection. Hepatobiliary Pancreat Dis Int 12(5): 533–9.
- Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, et al. (2000) Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 232(6): 786-795.
- Daskalaki D, Butturini G, Molinari E, Crippa S, Pederzoli P, et al. (2011) A grading system can predict clinical and economic outcomes of pancreatic fistula after pancreaticoduodenectomy: results in 755 consecutive patients. Langenbecks Arch Surg 396(1): 91–98.
- Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, et al. (2011) The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 254(4): 907-913.
- Natour E, Suedkamp M, Dapunt OE (2012) Assessment of the effect on blood loss and transfusion requirements when adding a polyethylene glycol sealant to the anastomotic closure of aortic procedures: a casecontrol analysis of 102 patients undergoing Bentall procedures. J Cardiothorac Surg 7: 105.
- Vivacqua A, Koch CG, Yousuf AM, Nowicki ER, Houghtaling PL, et al. (2011) Morbidity of bleeding after cardiac surgery: is it blood transfusion, reoperation for bleeding, or both? Ann Thorac Surg 91: 1780-1790.
- Schelling G, Block T, Blanke E, Hammer C, Brendel W, et al. (1987) The effectiveness of a fibrinogen-thrombin-collagen-based hemostatic agent in an experimental arterial bleeding model. Ann Surg 205(4): 432-435.
- Lewis KM, Kuntze CE, Gulle H (2016) Control of bleeding in surgical procedures: critical appraisal of HEMOPATCH (Sealing Hemostat). Med Devices (Auckl) 9: 1-10.
- Kroese DP, Brereton T, Taimre T, Botev ZI (2014) Why the Monte Carlo method is so important today. WIREs Comput Stat 6(6): 386-392.
- Del Moral, Pierre (2013) Mean field simulation for Monte Carlo integration. Monographs on Statistics & Applied Probability”. Chapman & Hall/CRC Press. pp. 626.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.