Perspective 
 Creative Commons, CC-BY
Creative Commons, CC-BY
A Stepwise Infection and Immunity Strategies to Prevent and Treat an Emerging Infection
*Corresponding author: Kuender D Yang, MacKay Children’s Hospital, Institute of Clinical Medicine, National Yang Ming University, Department of Microbiology and Immunology, National Defense Medical Center, Taipei, Taiwan.
Received: October 24, 2020; Published: December 04, 2020
DOI: 10.34297/AJBSR.2020.11.001602
Abstract
Global warming, transportation and urbanization expose humans to novel pathogens for emerging infections arising from microbial mutation, vector-borne and/or zoonotic transmission. We have experienced and studied immunopathogenesis of 4 emerging infections including enterovirus 71 encephalitis, dengue hemorrhagic fever, severe acute respiratory syndrome (SARS), and novel influenza A(H1N1) in the past 2 decades. Based on our studies and references from literature, here, we have summarized a stepwise infection and immunity regimens to prevent and treat an emerging infection. A consensus to monitor virus-host-environment interactions in the global village is the most important thing, particularly the potential mutants of emerging RNA viruses and herd immunity of risk populations, poultries, vectors and wild animals. In this article, we provide a stepwise infection and immunity strategies to make an emerging infection preventable and treatable by monitoring virus-host-environment interactions, developing vaccines, anti-virus agents and/or immunotherapies.
Environmental Evolution of Emerging Infections
Changes of global ecology, e.g. global warming, transportation and urbanization, expose humans to novel pathogens for emerging infections arising from microbial mutation, vector-borne and/or zoonotic transmission. Most of the common emerging infections are mediated by RNA viruses which pose a higher rate of genetic mutation, sequence deletion, recombination and reassortment of RNA virus codes [1-3]. Severe acute respiratory syndrome (SARS), avian flu and seasonal flu are known to emerge from sequence mutation, deletion, recombination and/or reassortment of RNA segments. Vector-borne diseases such as yellow fever, dengue hemorrhagic fever and West Nile virus encephalitis are transmitted by mosquitos and affected by weather and global warming [4,5]. Zoonotic diseases: Ebola, Lassa and Hantavirus infections are affected by urbanization, migration of animals and social culture [6,7].
Host Immunity and Herd Immunity for Emerging Infections
Host individual immunity and herd immunity determine the transmission and reproduction number (Ro) of an emerging infection. Individual immunity is largely influenced by age, genetic inheritance and comorbidities. For instances, it is known that elders with comorbidities have a higher fatality in the outbreaks of seasonal flu and SARS (SARS-CoV-1, SARS-CoV-2 and MERS-CoV) [8-10]. Genetic variants in IL6R, TLR3, and DC-SIGN genes were associated with susceptibility and/or severity of dengue fever (DF) [11]. We have found that CD209 genotypes are significantly associated with the susceptibility of DHF [12]. TLR7 genetic variants cause predisposition to severe COVID-19 infections [13]. Interferon-inducible transmembrane protein 3 (IFITM3) gene are associated with susceptibility to and protection of severe influenza [14,15]. Herd immunity is another key factor that determines the transmission on endemic or epidemic spread of an emerging infection. Each year, human seasonal flu emerges with certain serotype of a mutant with antigen drift resulting in endemic or epidemic of influenza depending on herd immunity and coverage of population immunization. The seasonal flu endemic or epidemic is usually occurring in autumn and winter while humans live in an atmosphere with a shorter social distance, lower temperature and humidity [16]. Based on the equation (1-1/Ro x 100%) to control an infection calculated by a reproduction number, Ro, to cease an epidemic of seasonal flu requires a herd immunity or coverage of population vaccination over 20% population (1-1/Ro = 1-1/1.25=20%) while the seasonal flu has a Ro value between 1.2 and 1.3. The Ro value for SARS-CoV-2 is estimated at 2.3 [17], whose control of the pandemic requires the population herd immunity or mass vaccination over 57% (1-1/2.3 x100%= 57%).
A Total Solution of Infection and Immunity Regimens for Preventing an Emerging Infection
We have experienced and studied immunopathogenesis of 4 emerging infections including enterovirus 71 encephalitis [18- 20], dengue hemorrhagic fever [21-23], severe acute respiratory syndrome (SARS) [24-26], and novel influenza A(H1N1) [27-29] in the past 2 decades. Based on our studies and references from literature, here, we have summarized a stepwise infection and immunity regimens to prevent and treat an emerging infection by 10 sequential steps for infection and immunity control below:
Monitor of mutant viruses, herd immunity and vectors. Infection immunity of an emerging infectious disease is determined by virushost- environment interactions. Emerging infections are frequently derived from RNA viruses which usually lack a 3-exonuclease that is present in DNA-dependent polymerases providing proofreading ability for the genome stability during replication [30]. Global warming, extreme climate and global transportation have promoted the widespread of the vector-born transmitted diseases to different regions of the world [31]. Wet markets could also transmit zoonotic diseases [32]. The best way to prevent emerging infections is to monitor mutant viruses, herd immunity and vectors for early containment of a potential emerging pathogen.
Development of useful vaccines. As progress of the vaccinology, many useful platforms have been made in genuine vaccine designs that induce effectively protective immunity but less side effects by a recombination with an avirulent vector that encodes a vaccine antigen gene for producing a viral antigen (glycoprotein) responsible for active immunization. Once an emerging infectious pandemic occurs, the vaccine platforms can be applied to make a useful vaccine as shown successful in the development of Ebolavirus vaccines [33,34].
Blockade of virus entry by neutralizing Abs. Both polyclonal and monoclonal antibodies have been shown to rescue fatal emerging infections. Early administration of convalescent plasma containing specific polyclonal antibodies has been shown to significantly reduce the mortality of hospitalized Covid-19 patients [35]. Similarly, convalescent plasma or neutralizing monoclonal antibodies (MoAbs) have also been demonstrated to rescue patients with Ebola, SARS and MERS [36-38] infections. Thus, hyperimmune or recombinant MoAbs of Covid-19 with neutralizing Abs titers administrated as early as possible should be able to decrease virus load and raise better immune response toward balanced Th17/ Treg reaction resulting in less severity and also less autoimmunity.
Inhibition of viral replication. Several potential anti-RNA virus agents have been shown to block SARS-CoV-2 entry, replication and/or shedding [39]. The decrease of viral replication and shedding could be made by inhibition of virus-cell fusion, virus and host proteases, lysosome acidification, RNA synthetase and virus budding [39,40]. A proper regimen to combine more than one antivirus agent may effectively reduce the virus transmission between infected and non-infected cells and raise a better immune response and less mortality [40,41]. A combination of neutralizing MoAbs and anti-virus agent may induce a synergistic effect.
Inhibition of viral shedding. RNA viruses although code 10 more or less structure and nonstructure proteins for replication and evasion of human defense, these viral antigens (glycoproteins) may hijack immunity and/or mediate an enhancement of filopodial protrusion for viral shedding [39]. A recent study in SARS-CoV-2 cell model has shown that inhibition of casein kinase II (CK2) can block viral shedding and suppress inflammatory response [41].
Screening of genetic susceptibility. While a RNA virus invades mucosal epithelium or blood cells, human RNA sensing receptors such as TLR3, RIG-1 (DDX58), TLR7, and/or TLR8, detect the virus and induce interferon production via MyD88, TRIF (TICAM1), IRF3 and/or IRF7 pathways, for suppression of infection by innate immunity [42,43]. While the innate immunity does not eradicate the virus, the viral antigen(s) is(are) presented to T cell-mediated adaptive immunity via recognition of HLA molecules. Different HLA subtypes would cause different disease susceptibility and severity. For instance, the severity of Covid-19 infection has been proposed to be associated with HLA-B*46:01 in a computational simulation by simulating the binding of HLA molecules with Covid-19 whole genome peptides [44]. Deletion or mutation of TLR7 has been also attribute to severity of Covid-19 in young adults [45]. While encountering certain patients who revealed a unique severity different from general patients or a treatment resistance, we need to clarify individual genetic variants and susceptibility.
Regimens to target cytokine storm. Certain RNA viruses do not cause systemic dissemination, but cause systemic immunopathology, resulting in hemorrhage, coaulopathy and/or vascular leakage [46,47]. The emerging infections with immunopathology such as cytokine storm and complement activation of vascular leakage require administration of cytokine antagonist, inhibition of complement cascade or anticoagulant treatment [48-50].
Correcting Th17/Treg imbalance. In response to an RNA virus infection, the host’s antigen presenting cells would present the viral antigen to T cells for polarization of naive T helper cells (Tho) toward a proper Th1 cell immunity and/or Th2 humoral (B cell) response of neutralizing antibody production for viral clearance. Abnormal immune responses with Th17-/Treg imbalance have been shown in some infectious diseases [51,52]. Induction and/or stabilization of Treg cell development is capable to reverse the altered relationship between Th17 and Treg [53]. Microbiota and vitamins have been shown to upregulate Treg functions [54-56]. Treg cells and Vitamin D levels were lower in many Covid-19 patients and associated with an increase in inflammatory cytokines and a risk to severity of pneumonia [56,57]. Moreover, microbiota has been recently shown to coordinate adipocyte-derived mesenchymal stem cells to combat autoimmunity of type 1 diabetes in mice [58], and mesenchymal stem cells (MSC) or their exosomes have been proposed to eliminate hyperinflammation of Covid-19 [59,60]. Appropriate applications of vitamin D, microbiota, MSC and their exosomes may rescue hyperinflammation of an emerging infection with Th17/ Treg imbalance (Figure 1).
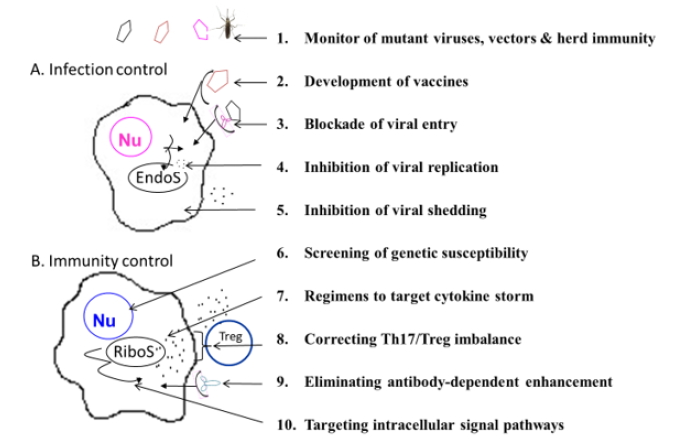
Figure 1: A stepwise infection & immunity controls of an emerging infection Abbreviations: Nu: Nucleus, EndoS: Endosome, RiboS: Ribosome, Th17: T helper cell type 17, Treg: Regulatory T cells
Eliminating ADE. Antibody-dependent enhancement (ADE) in emerging infections has been concerned in dengue fever and different coronaviruses [61,62]. ADE usually occurs to a sub neutralizing antibody titer or presence of heterotypic antibodies [21,23]. A good way to avoid ADE is to prevent infections or to provide effective immune response. Effective vaccines or neutralizing antibodies are required, but it is not guaranteed whether an effective vaccine could induce another ADE in a subsequent heterotypic infection or the neutralizing antibody titers could decay with time to a sub neutralizing antibody titer for ADE. Another approach to prevent ADE is recently proposed by the elimination of the glycosylation site at N297 of the IgG Fc portion or by a mutation in the Fc region resulting in an effective immune response antibody neutralization but not ADE [61].
Targeting intracellular signal pathways. We and others have shown that certain emerging infections induce hyperactivation of MAPK (e.g. ERK and p38) pathways. Inhibition of p38 activation has been shown to decrease viral replication and cytokine induction in an in vitro cell model. Inhibitors of the phosphokinases which are activated in an in vitro Covid-19 infection, including CK2, CDK, AXL, and PIKFYVE kinases, possess antiviral efficacy. A combination of different inhibitors of the kinases may offer a synergistic effect on anti-virus and anti-inflammatory effects. A recent study showing a combination of viral protease inhibitor, GC376, with the RNAdependent RNA synthetase inhibitor, remdesivir, causes a sterilizing additive effect. For some infections which could induce infectionassociated hemophagocytosis syndrome also called macrophage activation syndrome showing anemia, thrombocytopenia, hyperferritinemia and hypertriglyceridemia may require a combination of IVIG with cyclosporin-A, and/or anti-TNFα [63].
In summary, although each individual emerging infection requires individual strategies to prevent and/or treat the disease morbidity and mortality, the applicable pattern and principle for prevention of an emerging infection may be made in advance for mitigating the pandemic and reducing fatality. A consensus to monitor virus-host-environment interactions in the global village is the most important thing, particularly the potential mutants of emerging RNA viruses and herd immunity of human populations, poultries, vectors and wild animals. A couple of platforms for developing vaccines with safety and efficacy have been made possible by a recombination of viral antigen gene to an avirulent vector, and a number of models for making monoclonal antibodies have been made capable to neutralize an emerging infection and avoid viral evasion. A combined therapy with different antivirus agents to inhibit both virus replication and shedding is also feasible. A sequential treatment begins with an anti-viral agent followed by an immunoregulation with neutralizing antibodies or anti-inflammatory regimens may be required for those with potential dissemination or autoimmunity of an emerging infection. In case of certain portion of patients who revealed a treatment resistance, strategies to clarify individual genetic susceptibility and/or pathogenic signal transductions in virus-host-environment interactions are needed for prevention and immunotherapy of a life-threatening emerging infection.
Acknowledgement
None.
Conflict of Interest
No Conflict of interest.
References
- Domingo E, Holland JJ (1997) RNA virus mutations and fitness for survival. Annu Rev Microbiol 51:151-178.
- Sanjuán R, Domingo Calap P (2016) Mechanisms of viral mutation. Cell Mol Life Sci 73(23): 4433-4448.
- Bedford T, Riley S, Barr IG, Broor S, Chadha M, et al. (2015) Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature 523(7559): 217-220.
- Melissa Lee Phillips (2008) Dengue reborn: Widespread resurgence of a resilient vector. Environ Health Perspect 116(9): A382-A388.
- Johnson B, Chambers T, Crabtree M, Filippis A, Vilarinhos P, et al. (2002) Vector competence of Brazilian Aedes aegypti and Ae. albopictus for a Brazilian yellow fever virus isolate. Trans Royal Soc Trop Med Hyg 96(6): 611-613.
- Zeier M, Handermann M, Bahr U, Rensch B, Müller S, et al. (2005) New ecological aspects of hantavirus infection: a change of a paradigm and a challenge of prevention--a review. Virus Genes 30(2): 157-180.
- Leffel EK, Reed DS (2004) Marburg and Ebola viruses as aerosol threats. Biosecur Bioterror 2(3):186-191.
- (2020) CDC. Flu & People 65 Years and Older.
- Reilev M, Kristensen KB, Pottegaard A (2020) Characteristics and predictors of hospitalization and death in the first 11122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. dyaa140.
- Williamson E, Walker AJ, Bhaskaran KJ, Seb Bacon, Chris Bates, et al. (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821): 430-436.
- Avendaño Tamayo E, Rúa A, Parra Marín MV, Rojas W, Campo O, et al. (2019) Evaluation of variants in IL6R, TLR3, and DC-SIGN genes associated with dengue in sampled Colombian population. Biomedica 39(1): 88-101.
- Wang L, Chen RF, Liu JW, Lee IK, Lee CP, et al. (2011) DC-SIGN (CD209) Promoter-336 A/G polymorphism is associated with dengue hemorrhagic fever and correlated to DC-SIGN expression and immune augmentation. PLoS Negl Trop Dis 5(1): e934.
- van der Made CI, Simons A, Schuurs Hoeijmakers J, Guus van den Heuvel, Tuomo Mantere, et al. (2020) Presence of genetic variants among young men with severe COVID-19. JAMA 324(7): 1-11.
- Kenney AD, McMichael TM, Imas A, Chesarino NM, Zhang L, et al. (2019) IFITM3 protects the heart during influenza virus infection. Proc Natl Acad Sci 116(37): 18607-18612.
- Prabhu SS, Chakraborty TT, Kumar N, Banerjee I (2018) Association between IFITM3 rs12252 polymorphism and influenza susceptibility and severity: A meta-analysis. Gene 674: 70-79.
- Brooke CB (2017) Population diversity and collective interactions during influenza virus infection. J Virol 91(22): e01164-17.
- Zhang S, Diao M, Yu W, Pei L, Lin Z, et al. (2020) Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: A data-driven analysis. Int J Infect Dis 93: 201-204.
- Yang KD, Yang MY, Li CC, Lin SF, Chong MC, et al. (2001) Altered cellular but not humoral reactions in children with complicated enterovirus 71 infections in Taiwan. J Infect Dis 183(6): 850-856.
- Li CC, Yang MY, Chen RF, Lin TY, Tsao KC, et al. (2002) Clinical manifestations and laboratory assessment in an enterovirus 71 outbreak in southern Taiwan. Scand J Infect Dis 34(2): 104-109.
- Yang B, Chuang H, Yang KD (2009) Sialylated glycans as receptor and inhibitor of enterovirus 71 infection to DLD-1 intestinal cells. Virol J 6: 141.
- Yang KD, Yeh WT, Yang MY, Chen RF, Shaio MF (2001) Antibody-dependent enhancement of heterotypic dengue infections involved in suppression of IFNgamma production. J Med Virol 63(2): 150-157.
- Liu JW, Lee IK, Wang L, Chen RF, Yang KD (2013) The usefulness of clinical-practice-based laboratory data in facilitating the diagnosis of dengue illness. Biomed Res Int 2013: 198797.
- Lee IK, Liu JW, Yang KD (2012) Fatal dengue hemorrhagic fever in adults: emphasizing the evolutionary pre-fatal clinical and laboratory manifestations. PLoS Negl Trop Dis 6(2): e1532.
- Lee CH, Chen RF, Liu JW, Yeh WT, Chang JC, et al. (2004) Altered p38 mitogen-activated protein kinase expression in different leukocytes with increment of immunosuppressive mediators in patients with severe acute respiratory syndrome. J Immunol 172(12): 7841-7847.
- Lee YS, Chen CH, Chao A, Chen ES, Wei ML, et al. (2005) Molecular signature of clinical severity in recovering patients with severe acute respiratory syndrome coronavirus (SARS-CoV). BMC Genomics 6: 132.
- Ko SF, Lee TY, Huang CC, Cheng YF, Ng SH, et al. (2004) Severe acute respiratory syndrome: prognostic implications of chest radiographic findings in 52 patients. Radiology 233(1): 173-181.
- Wang L, Chang LS, Lee IK, Tang KS, Li CC, et al. (2014) Clinical diagnosis of pandemic A(H1N1) 2009 influenza in children with negative rapid influenza diagnostic test by lymphopenia and lower C-reactive protein levels. Influenza Other Respir Viruses 8(1): 91-98.
- Lee IK, Liu JW, Wang L, Yang KD, Li CC, et al. (2012) 2009 pandemic influenza A (H1N1): clinical and laboratory characteristics in pediatric and adult patients and in patients with pulmonary involvement. Influenza Other Respir Viruses 6(6): e152-161.
- Li CC, Wang L, Eng HL, You HL, Chang LS, et al. (2010) Correlation of pandemic (H1N1) 2009 viral load with disease severity and prolonged viral shedding in children. Emerg Infect Dis 16(8): 1265-1272.
- Hui EK (2006) Reasons for the increase in emerging and re-emerging viral infectious diseases. Microbes Infect 8(3): 905-916.
- Servadio JL, Rosenthal SR, Carlson L, Bauer C (2018) Climate patterns and mosquito-borne disease outbreaks in South and Southeast Asia. J Infect Public Health 11(4): 566-571.
- Webster RG (2004) Wet markets--a continuing source of severe acute respiratory syndrome and influenza? Lancet 363(9404): 234-236.
- Ollmann Saphire E (2020) A vaccine against Ebola virus. Cell 181(1): 6.
- Marzi A, Mire CE (2019) Current Ebola virus vaccine progress. BioDrugs. 33(1): 9-14.
- Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, et al. (2020) Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol 190(11): 2290-2303.
- Mulangu S, Dodd LE, Davey Jr RT, Mbaya OT, Proschan M, et al. (2019) A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 381(24): 2293-2303.
- Cheng Y, Wong R, Soo YOY, Wong WS, Lee CK, et al. (2005) Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 24(1): 44-46.
- Arabi YM, Hajeer AH, Luke T, Raviprakash K, Balkhy H, et al. (2016) Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis 22(9): 1554-1561.
- Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, et al. (2020) Immunology of Covid-19: Current state of the science. Immunity 52(6): 910-941.
- Fu L, Ye F, Feng Y, Yu F, Wang Q, et al. (2020) Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat Commun 11(1): 4417.
- Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, et al. (2020) The global phosphorylation landscape of SARS-CoV-2 infection. Cell 182(3): 685-712.e19.
- Jensen S, Thomsen AR (2012) Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol 86(6): 2900-2910.
- Schulz KS, Mossman KL (2016) Viral evasion strategies in type I IFN signaling-A summary of recent developments. Front Immunol 7: 498.
- Nguyen A, David JK, Maden SK, Wood MA, Weeder BR, et al. (2020) Human leukocyte antigen susceptibility map for severe acute respiratory syndrome coronavirus 2. J Virol 94(13): e00510-20.
- van der Made CI, Simons A, Schuurs Hoeijmakers J, van den Heuvel G, Mantere T, et al. (2020) Presence of genetic variants among young men with severe Covid-19. JAMA 324(7): 1-11.
- Khaiboullina SF, St Jeor SC (2002) Hantavirus immunology. Viral Immunol 15(4): 609-625.
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, et al. (2006) Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 12(10): 1203-1207.
- Nasonov E, Samsonov M (2020) The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomed Pharmacother 131: 110698.
- Carvelli J, Demaria O, Vély F, Batista L, Benmansour NC, et al. (2020) Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature.
- Carfora V, Spiniello G, Ricciolino R, Di Mauro M, Migliaccio MG, et al. (2020) Anticoagulant treatment in COVID-19: a narrative review. J Thromb Thrombolysis page: 1-7.
- Kanwar B, Favre D, McCune JM (2010) Th17 and regulatory T cells: implications for AIDS pathogenesis. Curr Opin HIV AIDS 5(2): 151-157.
- Muyayalo KP, Huang DH, Zhao SJ, Xie T, Mor G, et al. (2020) COVID‐19 and Treg/Th17 imbalance: Potential relationship to pregnancy outcomes. Am J Reprod Immunol: e13304.
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, et al. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441(7090): 235-238.
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, et al. (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317(5835): 256-260.
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, et al. (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504(7480): 451-455.
- Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA. Perspective: Vitamin D deficiency and Covid-19 severity - plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2, and thrombosis. J Intern Med.
- Ilie PC, Stefanescu S, Smith L. (2020) The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res 32(7): 1195-1198.
- Bassi ÊJ, Moraes Vieira PM, Moreira Sá CS, Almeida DC, Vieira LM, et al. (2012) Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes 61(10): 2534-2545.
- Tsuchiya A, Takeuchi S, Iwasawa T, Kumagai M, Sato T, et al. (2020) Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (Covid-19) cases. Inflamm Regen. 40: 14.
- Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, et al. (2020) Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe Covid-19. Stem Cells Dev 29(12): 747-754.
- Eroshenko N, Gill T, Keaveney MK, Church GM, Trevejo JM, et al. (2020) Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat Biotechnol 38(7): 789-791.
- Wang SF, Tseng SP, Yen CH, Yang JY, Tsao CH, et al. (2014) Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun 451(2): 208-214.
- Morimoto A, Nakazawa Y, Ishii E. (2016) Hemophagocytic lymphohistiocytosis: Pathogenesis, diagnosis, and management. Pediatr Int 58(9): 817-825.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.