Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Effect of A Novel Formulated Food, Night CalmTM, On Sleep Quality and Mood in Healthy Adults with Self- Reported Poor Sleep: A Randomized, Double-Blind, Placebo-Controlled Study
*Corresponding author: Dan Cheng, Ph.D., R&D Center, Shanghai Lithy One Health Group, Shanghai, China.
Received: January 20, 2021; Published: January 29, 2021
DOI: 10.34297/AJBSR.2021.11.001674
Abstract
Poor sleep has not only led to low quality of life and stress but also jeopardized host physiology severely in the long run with huge socioeconomic losses. Given the market for sleep-aid supplement is tremendous, formula with evidence-based functionality and originality would be welcomed for individuals with poor sleep. This randomized, double-blinded, placebo-controlled study was carried out to investigate the effect of this novel formulated Night CalmTM on the sleep quality recovery and mood improvement for adults with self-reported poor sleep. The questionaries of PSQI and DASS-21 have shown greater amelioration in the treatment (Night CalmTM) group than that in the placebo group. Also, there has been no side effects of this supplement based on the satisfaction ratings or report during this study. It can be concluded administering Night CalmTM could be a more effective way in dealing with poor sleep, compared with the commonly used neuroinhibitory transmitter, γ-aminobutyric acid (GABA).
Introduction
Good sleep is essential for a good quality of life. However, people in the recent decades have suffered from poor sleep due to life pressure, irregular pattern of sleep and so on. Poor sleep do not only affect the working performance and mood of the next day, but also are associated with long-term impact on health and diseases such as immune system maturation [1], cardiovascular risks [2], skin aging [3], type II diabetes [4], energy metabolism [5]. For instance, a systematic review and meta-analysis of 10 studies showed the short duration of sleep (≤5–6 h/night) might increase the relative risk of development of type 2 disease by 28% [4]. Globally, poor sleep is prevalent among groups with different ages, genders and ethnicities, but usually underrecognized and underestimated [6].
Healthy subjects that are not medically diagnosed as insomnia or treated with prescribed medicines usually take sleep-aid food/supplements. One of the popular but inappropriate ones is melatonin. It is only suitable for the elders to use melatonin as they have deteriorated function of the pineal gland for secreting melatonin. Besides, the sides effects of melatonin administration including nausea, vomit, headache, drowsiness have been reported in various scientific research [7]. Moreover, supplementing external melatonin to adolescents may impair the normal function of pineal gland although more investigations should be warranted. Food/supplements from natural processing such as fermentation or extraction from plants have been demonstrated for their efficacies in treating sleep disorders especially insufficient sleep. γ-aminobutyric acid (GABA), which is produced from fermentation of sodium glutamate by Lactobacillus hilgardii, can restore the normal levels of sleep time and quality in the arousal animal model [8]. In Japan, GABA has gained great popularization as a functional food that can claim aid in sleep.
Tart cherry (Prunus cerasus), a fruit widely planted in Europe and North America and rich in numerous phytochemicals, has been widely applied as a key ingredient in various food/supplements due to its prestigious function in sleep-aid [9], uric acid reduction [10] and so on. Saffron (Crocus sativus L.), traditionally used in Ayurvedic health system as a sedative, has been in recent years obtaining more and more popularity for its great efficiency in relieve anxiety and recover sleep quality [11]. In the Chinese market, GABA has been used as a sleep-aid supplement for many years while new ingredients and formulas would be appreciated in terms of advanced functionality and originality. Although the benefits of GABA, tart cherry and saffron in restoration of sleep quality have been clinically investigated, it is still unknown if the combined three elements can lead to the synergistic effect. It is hypothesized the combination of three components could have better effect in aiding adults with sleep disorders. Therefore, a randomized, double blind, placebo-controlled study was designed and conducted to investigate this hypothesis.
Materials and Methods
Study Design/Intervention
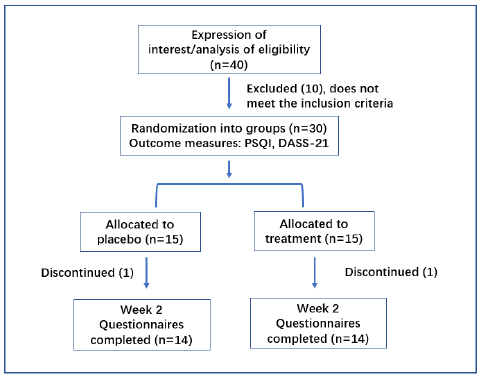
The study was a randomized, double-blind, placebo-controlled design for 2 weeks (Figure 1). Volunteers were randomized into two groups – placebo (P-group) or treatment (T-group). The participants consumed either one stick powder of or placebo 30 to 60 minutes before they go to bed.
Test Materials
The food/supplement called Night CalmTM was kindly provided by Shanghai TopVita Company. One sachet of powder for the treatment group (Night CalmTM) contained GABA (100 mg), saffron extract and tart cherry extract. One sachet of powder for the placebo group only contained GABA (100 mg). The identical appearance, odors and flavors of both kinds of sachets were realized by fillers (e.g fruit powders with no sleep-aid function).
Cohort
The 30 participants (mean age 35 years old, ranging from 29-68), BMI (mean BMI 22, ranging from 18.5-29.9) were divided into 2 groups. 15 were randomly assigned to P-group (placebo) and 15 to T-group (treatment). Of these initial 30 participants, 28 completed the 2-week study: 14 from the P-group, and 14 from the T-group. One subject in the P-group dropped out of the study for the reason that he did not feel obvious improvement. The other one dropped out in the T-group because she was busy with her errands and gave up this participation. Those participants have been evaluated by Insomnia Severity Index (ISI) and have overall rating from 8-21 (subthreshold insomnia to moderate severity insomnia). The exclusion criteria include clinical insomnia (severe) - evaluated by ISI with overall score from 22-28; hypertension, diabetes or any diseases that need medical treatment.
Food Intake
The participants were suggested to take no caffeine products during the study while this is not compulsory. The daily calorie intake and activity were assessed and it was found no baseline variance. The subjects were asked to keep the daily dietary intake and activity at the day of the randomization and during the course of the study. No dietary or activity interventions were recommended.
Outcome measures
Primary Outcome Measures
The Pittsburgh Sleep Quality Index (PSQI) is a self-rated questionnaire which assesses sleep quality and the severity and impact of disturbances [12]. The questionnaire includes subject sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleep medication and daytime dysfunction. Each of the components scored 0 (no difficulty) to 3 (severe difficulty). The global score (range 0 to 21) is produced by summation of the component scores. Higher scores indicate worse sleep quality.
Secondary Outcome Measures
The Depression, Anxiety and Stress Scale - 21 Items (DASS-21) is a self-report scales designed to measure the emotional states of depression, anxiety and stress [13]. DASS-21 contained three subscales with 7 items in each subscale with similar content. The depression scale assesses dysphoria, hopelessness, devaluation of life, self-deprecation, lack of interest/involvement, anhedonia and inertia. The anxiety scale assesses autonomic arousal, skeletal muscle effects, situational anxiety, and subjective experience of anxious affect. The stress scale is sensitive to levels of chronic non-specific arousal. It assesses difficulty relaxing, nervous arousal, and being easily upset/agitated, irritable/over-reactive and impatient. Scores for depression, anxiety and stress are calculated by summing the scores for the relevant items.
Adverse Events
The safety and tolerability of the administration of treatment powder were evaluated at day 14 through an online questionnaire querying adverse effects such as vomiting, nausea, diarrhea that might be associated with the supplement. During the whole study, the participants were requested to report instantly to the investigator once they experienced any uncomfortable feelings.
Statistical analysis
Statistical analyses of this clinical study were completed using IBM Statistical Package for Social Sciences (SPSS) at an alpha level of 0.05. An independent-samples t-test was used to compare demographic variables between placebo group and treatment group. To evaluate primary and secondary outcome measures, a two-way analysis of variance (ANOVA) was used to compare withingroup changes and group changes over time.
Results
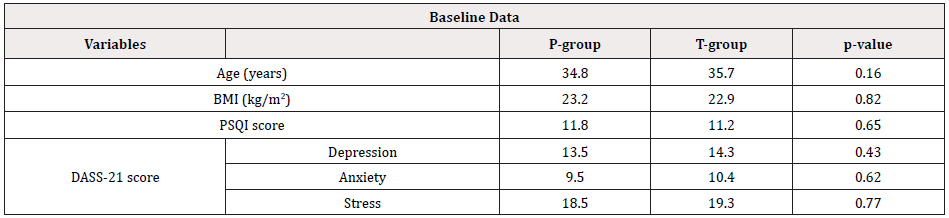
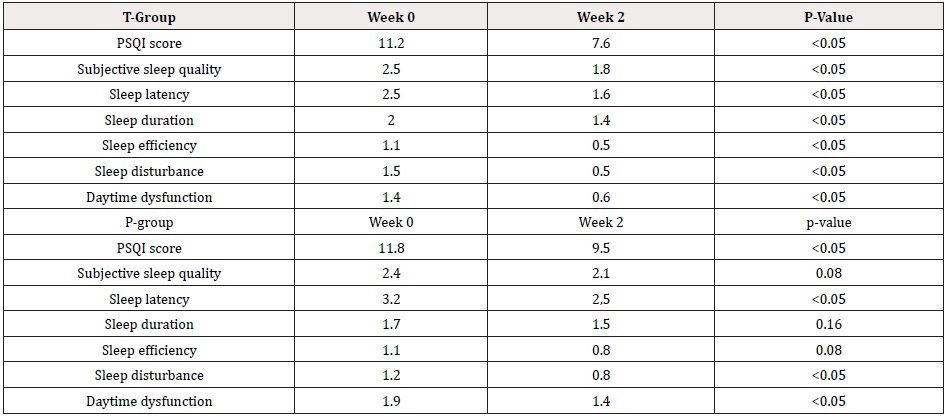
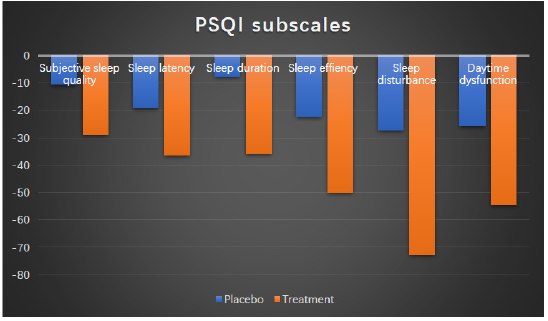
The baseline variables for placebo and treatment groups are shown in Table 1. There are no differences between the two groups from aspects of age, BMI, PSQI score and DASS-21 score. The results of PSQI scores for the placebo and treatment groups are shown in Table 2. For the treatment group, PSQI total score and all subscales have improved with statistical significance (p<0.05). For the placebo group, even though the sleep quality for the subjects has recovered given improved PSQI and subscale scores, several of the subscale scores do not reach statistical significance (Table 1 & 2).
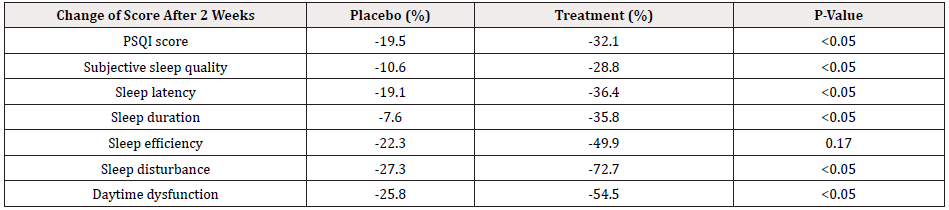
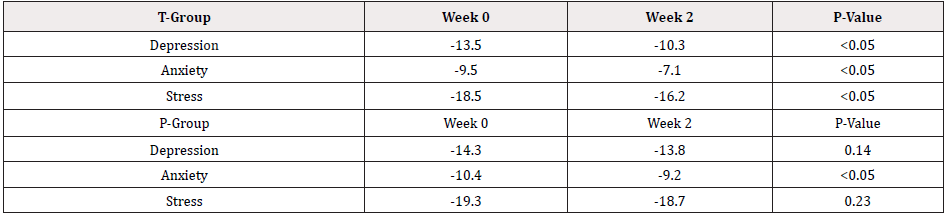
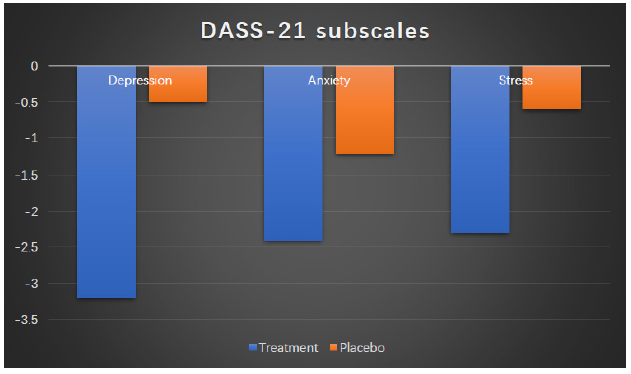
The changes of PSQI scores for placebo and treatment groups are manifested in percentage change between week 2 and week 0. The detailed information is shown in Table 3 and Figure 2. The changes for T-group outweigh much more than those for P-group and all differences among changes reach statistical significances except for sleep efficiency (Table 3 & Figure 2). The mood scores reflected by DASS-21 questionnaires are shown in Table 4 and Figure 3. After two weeks supplementation of Night CalmTM, the scores of depression, anxiety and stress have decreased with statistical significances. On the other hand, the DASS-21 scores in the placebo group improve with a lesser content than those in treatment groups while only the improvement in anxiety subscale reach a statistical significance (Table 4 & Figure 3).
Adverse events
In our study, there was no significant adverse events reported by participants or withdrew due to adverse feeling during the course of the study.
Discussion
Poor sleep has caused a lot of health issues that lead to tremendous socioeconomic loss. In China, there are over 300 million people that suffer from poor sleep and the estimated market value has surpassed billions of dollars. To develop a sleep-aid food product with advanced functionality and originality will undoubtedly be welcomed by these individuals. In this study, we aim to testify the functionality of a novel formulated food product that consists of clinically demonstrated ingredients including GABA, saffron extract and tart cherry extract. We aim to demonstrate that this formula outweigh GABA used alone in terms of sleep quality recovery and mood improvement as GABA has been widely used but the efficacy is not satisfied.
It is observed from our study that there are improvements of PSQI scores and DASS-21 scores for both T-group and P-group. However, it is obvious that the sleep quality recovery and mood improvement in T-group are much better than that in the P-group considering the improvement extent and statistical significances. This food comprehensively considers three target pathways that dominate and impact during the natural sleep process of human beings. The first crucial target pathway is tryptophan–melatonin/serotonin, significant sleep nutrients that regulate the sleep and wake cycle in a collaborative manner. It is found that over degradation of tryptophan by the enzyme indoleamine 2,3-dioxygenase (IDO) to produce kynurenine has occurred in individuals with poor sleep [14]. As a raw material for sleep nutrients, insufficient tryptophan leads to the dysregulated melatonin secretion for those who have abnormal circadian rhythms [15].
They usually suffer from a longer sleep latency and also a feeling of fatigue and drowsiness after wake-up because melatonin fades more slowly [16]. The diversified polyphenols especially procyanidin B-2 in the tart cherry have found to be effective in inhibiting the activity of IDO [17]. In this sense, the bioavailability of tryptophan is enhanced for serotonin and melatonin synthesis, which would recover the circadian rhythms. On the other hand, the natural plant-based melatonin in tart cherry also contributes to the circadian rhythms recover in a synergistic manner although the melatonin content of the tart cherry powder is less than 200 ng/g. It was observed that individuals with insomnia (older than 50 years) increased sleep time by 84 minutes on polysomnography and sleep efficiency increased by 4.6% based on the Pittsburgh Sleep Quality Index [17]. In another randomized, double-blind, placebo-controlled, crossover study, tart cherry effectively improved total sleep time and sleep efficiency for young subjects (average age of 26.6 years) [18].
The second mechanism is according to the theory that sleep occurs once the neurons that are in charge of wakefulness are inhibited [19]. GABA, produced by traditional fermentation process, is a neuron transmitter inhibitor that once binds the GABA receptors, will facilitate to open the channels of chlorine ions in the targeted neurons, leading to the inhibition and tranquilization of those neurons [20]. Various animal and clinical studies have demonstrated the efficiencies of GABA to shorten the sleep latency, prolong the sleep time and improve the sleep efficiency [21-22]. It is observed that individuals with sleep disorders also suffer from mood disorders or even depression. A total of 512,891 adults aged 30–79 years from China Kadoorie Biobank (CKB) were involved in a study to probe into the association of sleep behaviors and depression. It was found individuals with long sleep duration (9h) and short sleep duration (6h) have increased odd ratio of having depression by 1.32 and 0.56 respectively [23]. Actually, the complex relationship between sleep and stress should be critically considered, since the neuroendocrine response derived from stress activate the hypothalamic-pituitary-adrenal (HPA) axis, intervening the sleep homeostasis [24]. The third mechanism lies in the inhibitory effect of reabsorption of neuron transmitter in sleep improvement that saffron can function. It is found crocins and safranal act as reuptake inhibitors of dopamine, norepinephrine, and serotonin, which are critical for regulation and normalization of sleep and mood [25-26]. Four high-quality clinical studies have demonstrated the efficacies of saffron extracts in treating moderate and major depression and anxiety for people at various ages [27-30]. In a 28-day, parallel-group, double-blind, randomized controlled trial, health individuals from 18-70 administered with 28 mg/d saffron extract had greater sleep quality improvements according to measurements by Insomnia Severity Index, Restorative Sleep Questionnaire and the Pittsburgh Sleep Diary [31].
Limitations and Futuristic Directions
In our study, questionaries including PSQI and DASS-21 were used to evaluate the sleep quality recovery and mood improvement respectively. In the futuristic study, it is important that instruments as polysomnography and actigraphy should be included as to validate these self-reported assessments. However, these assessments should not be underestimated as they are capable of predicting sleep-associated morbidity and mortality [32-33]. The main aim of our study has been verified that this formula Night CalmTM generates more positive results than those from GABA used along while these findings remain to be investigated using a larger sample size and diversified populations. On the other hand, the gut-microbiota brain axis has been proposed, and it is concluded gut-microbiota ecosystem can significantly impact on the brain and sleep health [34]. Futuristic investigations could be focused on whether the gut microbiota transformation induced by this Night CalmTM would benefit sleep. It is found that polyphenols in the tart cherry could function as prebiotics for lactobacillus that might produce neurotransmitters for sleep and mood improvement [35].
Conflicts of Interest
The authors declared that they have no conflicts of interest to this work.
References
- Stoyan D, Tanja L, Cécile G, Anja TR, Michael S, et al. (2019) Gαs-coupled receptor signaling and sleep regulate integrin activation of human antigen-specific T cells. J Exp Med 216(3): 517-526.
- Huang T, Mariani S, Redline S (2020) Sleep Irregularity and Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol 75(9): 991-999.
- Oyetakin-White P, Suggs A, Koo B, Matsui MS, Yarosh D, et al. (2015) Does poor sleep quality affect skin ageing? Clin Exp Dermatol 40(1): 17-22.
- Cappuccio FP, D Elia L, Strazzullo P, Miller MA (2010) Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 33(2): 414-420.
- Cedernaes J, Schönke M, Westholm JO, Mi J, Chibalin A, et al. (2018) Acute sleep loss results in tissue-specific alterations in genome-wide DNA methylation state and metabolic fuel utilization in humans. Sci Adv 4(8): eaar8590.
- Chattu V, Manzar M, Kumary S, Burman D, Spence D, et al. (2018) The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 7(1): 1.
- Wei S, Smits M G, Tang X, Kuang L, Meng H, et al. (2019) Efficacy and safety of melatonin for sleep onset insomnia in children and adolescents: a meta-analysis of randomized controlled trials. Sleep Medicine 68: 1-8.
- Kim S, Jo K, Hong KB, Han SH, Suh HJ, et al. (2019) GABA and l-theanine mixture decreases sleep latency and improves NREM sleep. Pharm Biol 57(1): 65-73.
- Pigeon WR, Carr M, Gorman C, Perlis ML (2010) Effects of a Tart Cherry Juice Beverage on the Sleep of Older Adults with Insomnia: A Pilot Study. J Med Food 13(3): 579-583.
- Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, et al. (2012) Cherry consumption and decreased risk of recurrent gout attacks. Arthritis Rheum 64(12): 4004-4011.
- Gohari AR, Saeidnia S, Mahmoodabadi MK (2013) An overview on saffron, phytochemicals, and medicinal properties. Pharmacogn Rev 7(13): 61-66.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, et al. (1989) The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Research 28(2): 193-213.
- Lovibond SH, Lovibond PF (1995) Manual for the Depression Anxiety & Stress Scales. (2nd ) Sydney: Psychology Foundation.
- Dantzer R, O’Connor JC, Freund GG, Rodney W Johnson, Keith W Kelley, et al. (2008) From inflammation to sickness and depression: when theimmune system subjugates the brain. Nat Rev Neurosci 9(1): 46-56.
- Arendt J (2000) Melatonin, Circadian Rhythms, and Sleep. N Engl J Med 343(15): 1114-1116.
- Depner CM, Melanson EL, Eckel RH, Snell-Bergeon JK, Perreault L, et al. (2019) Ad libitum Weekend Recovery Sleep Fails to Prevent Metabolic Dysregulation during a Repeating Pattern of Insufficient Sleep and Weekend Recovery Sleep. Curr Biol 29(6): 957-967.
- Losso Jack N, Finley John W, Namrata Karki, Ann G Liu, Alfredo Prudente, et al. (2018) Pilot study of the tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am J Ther 25(2): e194-e201.
- Howatson G, Bell PG, Tallent J, Middleton B, Mc Hugh MP, et al. (2012) Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur J Nutr 51(8): 909-916.
- Baghdoyan HA, Lydic R (2012) The Neurochemistry of Sleep and Wakefulness. Basic Neurochemistry pp. 982-999.
- Behar KL (2009) Gaba synthesis and metabolism. Encyclopedia of Neuroscience 433-439.
- Yamatsu A, Yamashita Y, Pandharipande T, Maru I, Kim M, et al. (2016) Effect of oral γ-aminobutyric acid (GABA) administration on sleep and its absorption in humans. Food Science and Biotechnology 25(2): 547-551.
- Ebert B, I Storustovu S, Mortensen M, Frølund B (2002) Characterization of GABAAreceptor ligands in the rat cortical wedge preparation: evidence for action at extrasynaptic receptors? Br J Pharmacol 137(1): 1-8.
- Sun X, Zheng B, Lv J, Guo Y, Bian Z, et al. (2018) Sleep behavior and depression: Findings from the China Kadoorie Biobank of 0.5 million Chinese adults. J Affect Disord 229: 120-124.
- Nader N, Chrousos GP, Kino T (2010) Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab 21(5): 277-286.
- Georgiadou G, Tarantilis PA, Pitsikas N (2012) Effects of the active constituents of Crocus Sativus L.crocins, in an animal model of obsessive-compulsive disorder. Neurosci Lett 528(1): 27-30.
- Ettehadi H, Mojabi SN, Ranjbaran M, Jamal Shams, Hedayat Sahraei, et al. (2013) Aqueous extract of saffron (Crocus sativus) increases brain dopamine and glutamate concentrations in rats. J Behav Brain Sci 3(3): 315-319.
- Kell G, Rao A, Beccaria G, Clayton P, Inarejos-García AM, et al. (2017) affron® a novel saffron extract (Crocus sativus L .) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement Ther Med 33: 58-64.
- Lopresti AL, Drummond PD, Inarejos-García AM, Prodanov M (2018) affron®, a standardised extract from saffron (Crocus sativus L.) for the treatment of youth anxiety and depressive symptoms: A randomised, double-blind, placebo-controlled study. J Affect Disord 232: 349-357.
- Lopresti AL, Smith SJ, Hood SD, Drummond PD (2019). Efficacy of a standardised saffron extract (affron®) as an add-on to antidepressant medication for the treatment of persistent depressive symptoms in adults: A randomised, double-blind, placebo-controlled study. J Psychopharmacol 33(11): 1415-1427.
- Lopresti AL, Drummond PD (2017) Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: A randomised, double-blind, placebo-controlled study. J Affect Disord 207: 188-196.
- Lopresti AL, Smith, SJ, Metse AP, Drummond PD (2020) Effects of saffron on sleep quality in healthy adults with self-reported poor sleep: a randomized, double-blind, placebo-controlled trial. J Clin Sleep Med 16(6): 937-947.
- Bei B, MilgromJ, Ericksen J, Trinder J (2010) Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep 33(4): 531-538.
- Kronholm E, Laatikainen T, Peltonen M, Sippola R, Partonen T, et al. (2011) Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med 12(3): 215-221.
- Morais LH, Schreiber HL, Mazmanian SK (2020) The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol.
- Mayta-Apaza AC, Pottgen E, De Bodt J, Papp N, Marasini D, et al. (2018) Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J Nutr Biochem 59: 160-172.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.