Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Methamphetamine-Associated Cardiomyopathy: A Looming Epidemic for A New Generation
*Corresponding author: Leonard Ranasinghe PhD MD, Professor of Emergency Medicine, California Northstate University, Elk Grove, California, USA.
Received: December 04, 2020; Published: December 11, 2020
DOI: 10.34297/AJBSR.2020.11.001612
Abstract
Methamphetamine is a widely used drug of abuse associated with cardiovascular-related events and mortality as discussed in other case studies. There is limited scientific evidence describing the prevalence and presentation of cardiomyopathy due to the use of methamphetamine. Methamphetamine abuse is increasingly problematic, as it is causing early onset cardiomyopathy in generations younger than previously expected. There are few management guidelines for methamphetamine-associated cardiomyopathy (MAC). We present a unique case of a 54-yearold female arriving to the emergency department (ED) with a history of hypertension, obesity, asthma, and congestive heart failure. We made the diagnosis of dilated cardiomyopathy by echocardiogram with additional imaging in consultation with cardiology. Here we discuss epidemiology, pathophysiology, imaging, management, and other issues related to MAC.
Keywords: Cardiovascular, Case Report, Cardiomyopathy, Emergency Medicine, Methamphetamine
Introduction
Methamphetamine in its various forms may be ingested, injected, insufflated, or smoked. It is a highly addictive stimulant that acts on the cardiovascular and central nervous systems. Its mechanism of action involves the release of many neurotransmitters, including dopamine, norepinephrine, and serotonin. It also inhibits re-uptake of these transmitters [1]. Previously, we have associated methamphetamine abuse with several cardiovascular pathologies and complications, including increased heart rate (HR) and blood pressure (BP), which can cause death at high doses [2]. Case studies exist describing the link between abuse and cardiomyopathy, also known as MAC [3]. Retrospective data from 2009 to 2014 collected in a large urban community health system in southern California showed an increased prevalence of MAC in congestive heart failure patients [4]. Cardiomyopathy and heart failure appear to manifest in younger age groups particularly those who abuse methamphetamine, compared to their non-methamphetamine counterparts, with a mean age of 50 compared to 67 years [5]. Despite advances in the understanding of MAC, evidence regarding prevalence, presentation, and management of methamphetamineassociated cardiomyopathy is lacking. Here, we present an early onset presentation of cardiomyopathy linked to methamphetamine abuse.
Case Presentation
A 54-year-old female presented to the emergency department with shortness of breath, general malaise, and cough persisting for three days. On arrival, vital signs were BP: 177/115, P: 94, Resp: 20, BMI: 34.96. The patient complained of dizziness, headache, nausea, and vomiting. She also reported abdominal and shoulder pain exacerbated by excessive coughing. She stated that she lived outside of town and ran out of her Atenolol and Lisinopril two days prior. Past medical history is significant for obesity, hypertension, asthma, and congestive heart failure. The patient reported frequent use of crystal methamphetamine, which she believed contributes to her depression. She denied prior smoking history. She had a previous cholecystectomy and reported that she was allergic to Norco (Hydrocodone and Acetaminophen), describing her reaction as “vomiting.” Her family history is significant for diabetes in a sister.
Physical examination revealed an alert patient in mild distress with intermittent eye closure and mild labial cyanosis. Skin was warm to touch and slightly diaphoretic. Neurological exam was unremarkable. Cardiovascular exam showed a regular rate and rhythm, with normal peripheral pulses, no murmurs, and no edema. Breath sounds were equal bilaterally, with a symmetrical chest wall expansion. There was evidence of decreased aeration to bilateral bases with a faint expiratory wheeze. We ordered the following lab tests: ABG, lactate, troponin, blood cultures, toxicology screening, CBC with differentials, CMP, EKG, CXR, CT angiography, and head CT. We then admitted the patient to the hospital because of her ongoing hypoxia and dyspnea.
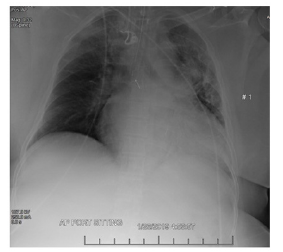
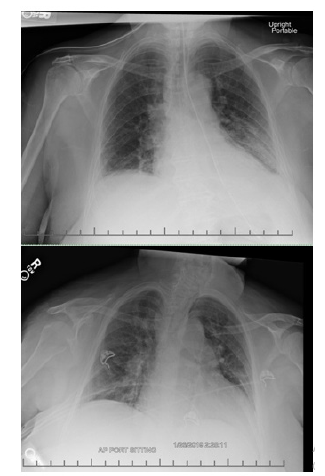
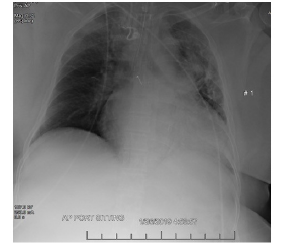
Electrocardiogram revealed possible left atrial enlargement and left ventricular hypertrophy, indicative of progressive left ventricular failure. Her electrocardiogram also showed anterolateral T-wave inversion (Figure 1). On telemetry, she had a normal sinus rhythm with a ventricular rate of 69. Transthoracic echocardiography revealed an estimated left ventricular ejection fraction of 25%. Additional findings include grade III diastolic dysfunction with elevated left atrial pressure, severe left atrial enlargement with severely abnormal left atrial ventricular index, severe right ventricular enlargement with normal function, right ventricular base measuring 5.6 cm, severely enlarged right atrium, severe mitral regurgitation, mitral regurgitation grade severity of 0.5 cm2, moderate to severe tricuspid regurgitation, and her inferior vena cava barely collapsed during inspiration suggesting elevated right atrial and ventricular end-diastolic pressures (Figure 2) Chest x-ray (Figure 3) revealed a nonspecific right infrahilar opacity suggestive of atelectasis or an infiltrative process, in addition to mild-to-moderate cardiomegaly.
The lab report was significant for a beta naturetic peptide of 11,000 and mildly elevated troponin levels. Urine toxicology was positive for methamphetamine. Her Hemoglobin A1C of 8.6% showed poorly uncontrolled diabetes mellitus. Last, because of the patient’s complaint of headache, a non-contrast head CT was unremarkable. Following hospitalization, we diagnosed the patient with congestive heart failure exacerbation, dilated cardiomyopathy, Diabetes mellitus type 2, and methamphetamine use. We administered broad spectrum antibiotics (Ceftriaxone 1 gm IV every 12 hours, and Azythromycin IV 500 mg given once at 250ml/hr over 60 min) due to concern for pneumonia. We treated the patient aggressively with ACE inhibitors, furosemide, and spironolactone. We placed the patient on BiPAP (Bilevel Positive Airway Pressure), Albuterol (10 mg .5% NEB), Atenolol (2gm IV),
The Patient Underwent the Following Procedures:
Myocardial Perfusion Stress Test (Adenosine/Lexiscan Stress test), CT angiography pulmonary, CT chest scan, CXR, ECHO, blood culture, and culture MRSA screen. We counseled the patient on methamphetamine cessation, medication compliance, and follow-up with a cardiologist for long-term treatment. For her Diabetes mellitus, we began sliding scale insulin in the intensive care unit. We later changed her to Metformin which maintained control of her blood sugar. Once the patient improved, we transferred her to a step-down unit for close hemodynamic monitoring. She was to continue her medication regime, including IV diuretics, fluid restriction, and electrolyte repletion. On hospital discharge, we lost the patient to follow-up.
Methamphetamine Associated Cardiomyopathy Presentation, Signs, and Symptoms
We commonly see MAC in young, predominantly male patients, in contrast to alcohol-related and idiopathic cardiomyopathy occurring more often in middle-aged patients [3,6]. Both acute and chronic methamphetamine use has resulted in extensive cardiovascular damage, including but not limited to cardiac ischemia, myocardial infarction, and cardiomyopathy [7,8]. Acute methamphetamine intoxication often elevates heart rate, blood pressure, and respiratory rate, while occasionally causing fever [2]. Signs and symptoms range from asymptomatic to total sympathomimetic crisis involving seizures, metabolic disturbances, and/or cardiovascular collapse. Patients commonly present to the emergency department with aggression, psychiatric disturbances, and chest pain [9]. As a result of heightened sympathomimetic activity induced by methamphetamine use, chronic hypertension is common.
Differential Diagnoses
The differential diagnoses based on their history and nonspecific presentation of cardiorespiratory symptoms include methamphetamine toxicity and heart failure secondary to other cardio toxins, or even exacerbation of a preexisting condition. Shortness of breath and hypoxia can also be signs of asthma exacerbation or pneumonia (if presenting with fever). Other diagnoses include but are not limited to heart failure from myocardial infarction or pulmonary embolism.
Imaging
Routine imaging for methamphetamine associated cardiomyopathy includes chest x-ray and 2dimensional echocardiography. We performed a trans-thoracic echocardiogram at baseline to test for left ventricular ejection fraction (LVEF) and chamber dilatation. We define the diagnosis of cardiomyopathy as LVEF less than 40% per the American Society of Echocardiography, with methamphetamine abusers having more severe dilatation and systolic dysfunction. Many reported cases also have thrombotic complications on echocardiogram [10]. Additional testing may include cardiac magnetic resonance imaging (CMR) with late gadolinium enhancement (LGE) which can identify regional fibrosis, predictive of irreversible MACinduced damage [11]. Fibrotic changes from methamphetamine abuse suggest a longer duration and increased severity of drug use over time [12].
Management
Standard of care for acute methamphetamine-associated toxicity is primarily supportive. Treatment may include a combination of sedation, airway management, and fluid resuscitation. We manage most patients in the ED presenting with behavioral disturbances with sedation, and then discharge them home once stable [6]. However, guidelines for the management of chronic MAC are less well defined, especially given the varying severity and presentation among individuals. Besides supportive care, these patients may receive automatic implantable or wearable cardioverter-defibrillators [3]. There is a link between the cessation of methamphetamine abuse and improved cardiac symptoms and function. However, given the lack of medical therapies available for methamphetamine abuse, treatment of addiction remains a significant obstacle. In comparison, chronic abuse leads to worsening cardiac symptoms and poorer outcomes. A study in California showed a statistically significant improvement in NYHA functional class and LVEF, post methamphetamine cessation [4]. Early detection of MAC, especially in younger individuals, is vital to restoring cardiac function prior to irreversible myocardial damage [3].
We face many challenges in managing methamphetamineassociated cardiomyopathy. This includes identification of patients with MAC, missed because of mistrust of physicians, or the inability to collect a complete history from non-cooperative patients. It can be especially difficult to identify patients with cardiomyopathy in the emergency department, where stabilization and resuscitation are the primary goals. Besides the unexpected diagnosis of cardiomyopathy in a relatively young individual, noncompliance and failure to return for follow-up are additional obstacles. Many individuals addicted to methamphetamine can’t or don’t want to stop. Therefore, counseling and community-based support groups are important keys to maintaining abstinence and helping to avoid relapse. We found that while the opioid epidemic garners greater public awareness, enabling new treatments for addiction and care, for those with methamphetamine addiction research and treatment is still lacking.
Conclusion
Methamphetamine abuse can precipitate an array of complications, including agitation, hyperthermia, metabolic acidosis, hypertension, tachycardia, seizures, psychosis, cardiovascular collapse, and death [13]. Patient behavior is unpredictable and fraught with noncompliance, which commonly results in exacerbation of symptoms. According to the Center for Disease Control and Prevention (CDC), 10,333 drug overdoses occurred in 2017 related to methamphetamine and other psychostimulants [14]. The fight against methamphetamine abuse is taking a back seat to the opioid epidemic, which according to the CDC killed over 47,000 people in the US in 2017 [12]. Although methamphetamine addiction is not as large an epidemic, it is just as deadly and on the rise in many communities throughout the West Coast. Of note, consequences of chronic methamphetamine use are insidious and manifest gradually, whereas opioid overdose is rapid.
According to data collected by the Drug Enforcement Administration (DEA), methamphetamine was the most frequently reported drug of abuse found in cases submitted to state laboratories nationally in 2018 [15]. It is also the drug that is found most frequently in violent crime in the United States according to the National Institute on Drug Abuse [16]. Currently, there are no Food and Drug Administration (FDA) approved pharmacologic treatments for methamphetamine addiction and use is growing among younger populations. The irreversible complications of chronic methamphetamine use emphasize the need for developing treatment for addiction and relapse. A 2012 clinical study found an 88% relapse rate for methamphetamine users [17-19]. We recommend further research to prevent additional cases of MAC similar to our patient and reduce methamphetamine initiation, use, and addiction.
References
- Won S, Hong R, Shohet R, Seto T, Parikh N, et al. (2013) Methamphetamine-associated cardiomyopathy. Clinical Cardiology 36(12): 737-742.
- Erowid (2016) Methamphetamine Dose. The vaults of Erowid.
- Schürer S, Klingel K, Sandri M, Majunke N, Besler C, et al. (2017) Clinical characteristics, histopathological features, and clinical outcome of methamphetamine-associated cardiomyopathy. JACC Heart Fail 5(6): 435-445.
- Sliman S, Waalen J, Shaw D (2016) Methamphetamine-associated congestive heart failure: increasing prevalence and relationship of clinical outcomes to continued use or abstinence. Cardiovasc Toxicol 16(4): 381.
- Thomas I, Nishimura M, Ma J, Dickenson S, AlshawabkehL, et al. (2018) Abstract 12640: Clinical characteristics and outcomes of methamphetamine associated heart failure: an emerging epidemic. Circulation 138(Suppl _1).
- Fauchier L, Babuty D, Poret P, D Casset-Senon, M L Autret, et al. (2000) Comparison of long-term outcome of alcoholic and idiopathic dilated cardiomyopathy. Eur Heart J 21(4): 306-314.
- Turnipseed S, Richards J, Kirk J, Diercks D, Amsterdam E (2003) Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med 24(4): 369-373.
- Hawley L, Auten J, Matteucci M, Decker L, Hurst N, et al. (2013) Cardiac complications of adult methamphetamine exposures. J Emerg Med 45(6): 821-827.
- Isoardi K, Ayles S, Harris K, Finch C, Page C, et al. (2018) Methamphetamine presentations to an emergency department: Management and complications. Emerg Med Australas 31(4): 593-599.
- Ito H, Yeo K, Wijetunga M, Seto T, Tay K, et al. (2009) A comparison of echocardiographic findings in young adults with cardiomyopathy: with and without a history of methamphetamine abuse. Clin Cardiol 32(6): E18-E22.
- Mahrholdt H, Wagner A, Judd R, Sechtem U, Kim R, et al. (2005) Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 26(15): 1461-1474.
- (2019) Center for Disease Control and Prevention. Opioid overdose.
- Derlet R, Rice P, Horowitz B, Lord R (1989) Amphetamine toxicity: experience with 127 cases. J Emerg Med 7(2): 157-161.
- Kariisa M, Scholl L, Wilson N, Seth P, Hoots B, et al. (2019) Drug Overdose Deaths Involving Cocaine and Psychostimulants with Abuse Potential-United States, 2003-2017. Morbidity and Mortality Weekly Report (MMWR) 68(17): 388-395.
- (2019) US Department of Justice DEA, Diversion Control Division. National Forensic Laboratory Information System (NFLIS) 2018 Midyear Report, USA.
- (2019) NIDA Methamphetamine DrugsFacts. National Institute on Drug Abuse Advancing Addiction science.
- Mc Ketin R, Najman J, Baker A, Lubman D, Dawe S, et al. (2012) Evaluating the impact of community-based treatment options on methamphetamine use: Findings from the Methamphetamine Treatment Evaluation Study (MATES). Addiction 107(11): 1998-2008.
- Richardson P, Mc Kenna W, Bristow M, Maisch B, Mautner B, et al. (1996) Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 93(5): 841-842.
- Kish SJ (2008) Pharmacologic mechanisms of crystal meth. CMAJ 178(13): 1679-1682.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.