Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Non-Invasive Low PEEP Versus High PEEP Ventilation Strategy in Severe COVID-19 Patients: An Observational Case – Control Study
*Corresponding author: Capua MD, Emergency Department - “Maggiore” Hospital of Lodi. Largo Donatori del Sangue, 1. 26900 – Lodi, Italy.
Received: December 14, 2020; Published: January 08, 2021
DOI: 10.34297/AJBSR.2021.11.001646
Abstract
Since the best ventilation strategy of severe covid-19 patients is still uncertain, we conducted a retrospective case–control study to evaluate the outcome of severe covid-19 patients treated in the emergency department (ED) with a non-invasive low positive end-expiratory pressure (PEEP) and early weaning strategy and compared it to a non-invasive high-PEEP strategy. Primary outcome was a composite outcome of ETI rate and/or in-hospital mortality at 7 and 28 days from admission to the ED. We compared 24 severe covid-19 patients treated with non-invasive low-PEEP to 26 sex- and age-matched controls. Main demographic characteristics, p/F ratio, creatinine, procalcitonine, C-reactive protein, d-dimer levels, APACHE II score and NEWS were comparable at admission. The case group received more frequently antivirals (mainly darunavir/cobicistat) and hydroxychloroquine (p = 0.004 and 0.011 respectively). As to primary outcome, at 7 days, 13 cases versus 20 controls experienced ETI or died (p not significant). In the remaining population, at 28 days, 2 out of 18 low-PEEP cases died as opposed to 11 out of 21 high-PEEP controls (11.1% vs 52.4%, p = 0.008; OR 0.11 CI95% 0.02 –0.62). Our analysis of Kaplan-Meier curves confirmed the statistical significance of higher mortality and ETI rate in controls. Although larger trials are needed to confirm our results, this retrospective case-control trial provides a modest evidence that a noninvasive low- PEEP ventilation strategy could reduce ETI rate and mortality at 28 days compared to a high-PEEP approach and be a game changer in the ventilation strategy of COVID-19 patients.
Introduction
The recent diffusion of the novel coronavirus (designated SARS-CoV-2) outbreak is challenging for every emergency physician facing this infection [1]. The pandemic spread of COVID-19 pneumonia has put to the test the healthcare system – in term of resources, infections of healthcare providers and scarce therapeutic strategies [2]. In this setting, intensive care units have had to treat a large number of patients with severe respiratory failures with only limited ICU beds available. Although the clinical characteristics of these patients have been largely investigated [3- 7], there is currently no clear evidence from randomized clinical trials (RCTs) that any potential therapy could improve the outcome in patients with either suspected or confirmed COVID-19, as there is no clinical trial data supporting any prophylactic therapy [8]. Like there is no validated drug therapy, there is great uncertainty about ventilation therapy, as well [9]. It should be taken into account that resources of intensive care units (ICUs) are limited and probably insufficient to manage all severe cases of COVID-19. Nevertheless, the majority of critically ill patients with COVID-19 is classified as acute respiratory distress syndromes (ARDS) and treated with endotracheal intubation (ETI) in ICUs [10].
Since evidence is lacking, there is much debate in literature about the best ventilation technique, both in terms of technique (invasive or non-invasive) and pulmonary recruitment [11]. Some authors argue that COVID-19 is quite similar to typical ARDS, therefore its treatment should not differ from the usual treatment which includes invasive ventilation and recruitment manoeuvres [12]. On the other hand, some evidences have shown that many critical COVID-19 patients fulfil the Berlin criteria of ARDS, both from the clinical and the radiological point of view, but they present an atypical form of the syndrome [13]. Gattinoni et al. suggested, indeed, two different types of SARS- CoV-2 pneumonia. The first one, the so-called type 1 (or type L), shows low elastance, low ventilation to perfusion ratio, low lung weight and low recruitability; the second one, called type 2 or type H, shows opposite characteristics and is more similar to a classical ARDS [14]. Although it is relatively easy to formulate a differential diagnosis with a CT scan, it is not feasible to obtain a CT scan for all severe cases of COVID-19 because of the large number of patients during the SARS-CoV-2 outbreak; moreover, moving a critically ill patient to the radiology department from the emergency department (ED) is not safe due to the possibility of clinical deterioration. Because of the clinical similarity, many type-1 patients are approached as a typical ARDS, namely with high positive end-expiratory pressure (PEEP). A recent retrospective survey from northern Italian ICUs confirmed that most of the patients with severe respiratory failure due to COVID-19 were treated with high PEEP (median 14 cmH2O) [15]. Against this background, we evaluated the outcome of critically-ill COVID-19 patients treated in the ED with a non-invasive low PEEP and an early weaning strategy and compared it to the outcome of a high PEEP non-invasive strategy. The primary outcome of our study is the evaluation of the difference in terms of ETI and in-hospital death at 7 and 28 days from the ED admission.
Methods
We conducted a retrospective case–control study on patients admitted to our ED with severe respiratory failure due to a SARS-CoV-2 infection and treated with a non-invasive low PEEP ventilation strategy versus patients treated with a high PEEP strategy.
Severe respiratory failure has been defined as a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen pressure (p/F) of less than 150 mmHg or the need for a fraction of inspired oxygen (FiO2) greater than or equal to 60% and a PEEP higher than 5 cmH2O to achieve a saturation of at least 94%. Exclusion criteria were a nose swab negative for SARS-CoV-2 or the need for ETI in pre- hospital care.
The low PEEP ventilation strategy followed an internal protocol that provided an initial ventilation administered by a single tube helmet CPAP with a PEEP of 8 to 10 cmH2O and FiO2 60% until 94% of saturation was achieved. Once the patient had reached a saturation between 94% to 98% and a respiratory rate lower that 30 bpm, we started a de-escalation from PEEP. If the patient had constantly adequate saturation and respiratory rate (at least 94% and less than 30 bpm respectively) with a PEEP lower than 7 cmH2O, we used high flow nasal cannula (HFNC) to continue PEEP weaning. Every hour, we evaluated saturation and respiratory rate and downgraded the airflow keeping a saturation range target of 94 – 98% and a respiratory rate of less than 30 bpm. If saturation was permanently higher than 98%, we reduced FiO2 of 10% with a minimum limit of FiO2 administered at 40%. If a patient needed a PEEP higher than 10 cmH2O or a FiO2 constantly higher than 60% to reach a saturation of 94% or if we could not wean the patient from PEEP, they would be considered non-responder and candidate to ETI.
We selected a sex- and age-matched control group, in a ratio 1:1, of patients treated with a ventilation strategy based on ARDS criteria (which had been used before the introduction of the low PEEP protocol). High PEEP (usually higher than 10 cmH2O) was used to, theoretically, improve lung recruitment and reduce the lung opening-closing phenomena [16]. A liberal oxygen therapy was allowed to achieve a saturation at least of 94% [17]. High-PEEP was administered with a helmet single tube CPAP. Weaning from the high-PEEP was based on p/F improvements or a saturation permanently higher than 94%. Every hour, we evaluated saturation and respiratory rate. If the patient showed, in two consecutive records a saturation higher than 94%, we performed an ABG. If the patient showed improvement of p/F (at least higher than 200 mmHg), keeping a respiratory rate of less than 30 bpm and a pCO2 within normal limits, we started a de-escalation from PEEP. When a PEEP of less than 10 cmH2O was reached with a p/F permanently higher than 200 mmHg, we removed the CPAP and used standard oxygen devices (such as Venturi Masks or nasal cannulas). We performed a de-escalation of oxygen (both in CPAP or in venturi mask) if pO2 was higher or equal to 100 mmHg at ABG or if the patient had a saturation permanently higher than 98%. On the other hand, a patient was considered non-responder if a PEEP de-escalation was not achievable after, at least, 24 hours of helmet CPAP or if saturation and/or respiratory rate were lower than 93% and higher than 30 bpm respectively. In that case they were candidate to ETI. We evaluated all the medical records to compare rate and type of comorbidities of every enrolled patient. Moreover, we evaluated the clinical and laboratory status at admission, in particular: the national early warning score (NEWS) and Acute Physiology and Chronic Health Disease Classification System II (APACHE II) score, p/F, creatinine, d-dimer levels, C-reactive protein value, procalcitonin levels. The medical treatment, which was needed for the clinical condition and the viral interstitial pneumonia, was managed by the treating physician and followed the current known standard and internal protocols. We compared the drug therapy administered during the admission, even though the patients were managed by the same medical staff, which followed internal COVID-19 protocols. We investigated the use of antiviral drugs and of heparin, the amount of heparin administered (units/kg/die), ACE- inhibitors, hydroxychloroquine and tocilizumab administration.
The primary outcome was a composite outcome of rate of ETI and/or in-hospital death at 7 and 28 days from admission to the ED. Statistical Package for Social Science (SPSS) version 26 was used for statistical analysis. Differences between the two groups at base line were tested with the use of Student’s t-test or the Fisher’s exact test where appropriate. We performed Kaplan-Meier curves analysis to compare ETI events and in-hospital death in the two groups during the 28 days of observation.
Results
We retrospectively enrolled 27 patients who were consecutively admitted to our ED for severe respiratory failure due to SARS-CoV-2 infection and treated with a low PEEP ventilation strategy. Twentyseven sex- and age-matched patient, previously admitted to our ED and treated with high PEEP and prolonged ventilation, served as a control group. Three patients from the low PEEP population and one patient in the control group were excluded because we failed to find a positive nose swab for SARS-CoV-2 infection in the medical records. The main characteristics of the remaining enrolled patients are summarized in Table 1.
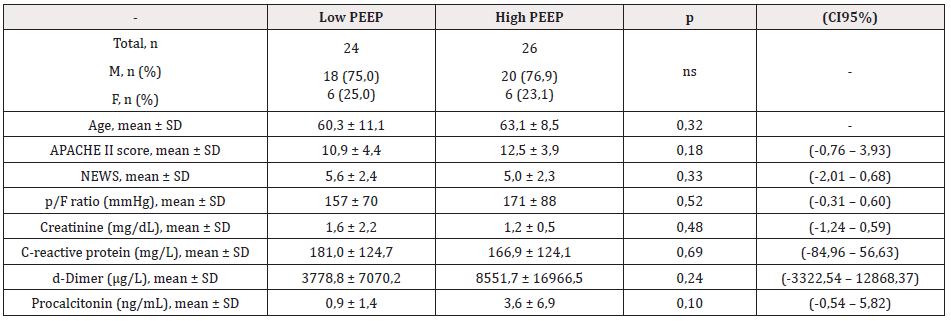
Table 1: This table shows that there is not statistically significant difference in demographic characteristics, main prognostic scores and laboratory values of the two populations compared.
Note: PEEP: Positive End-Expiratory Pressure; n: number; SD: Standard Deviation; APACHE II: Acute Physiology and Chronic Health Disease Classification System II; NEWS : National Early Warning Score; p/F = ratio of partial pressure of arterial oxygen to fraction of inspired oxygen pressure
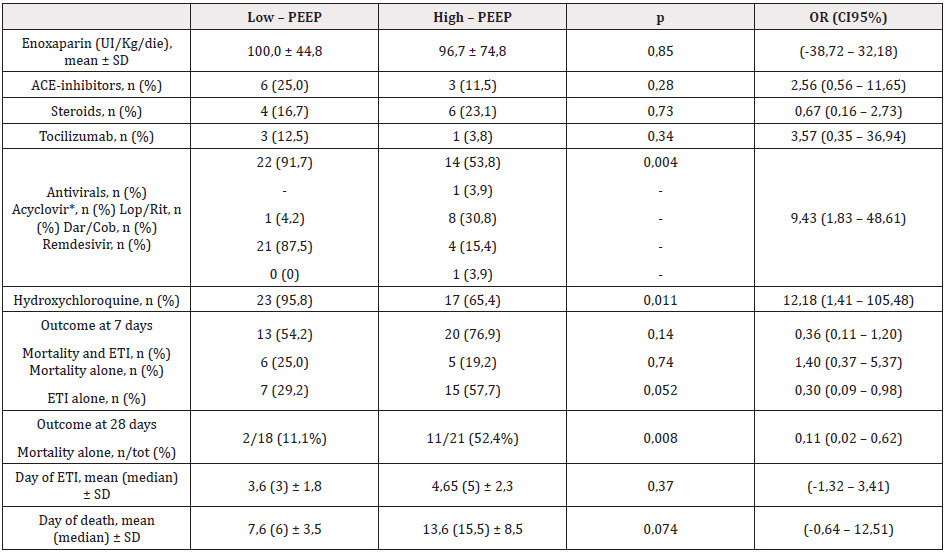
We did not find any statistical difference in mean APACHE II score and NEWS. P/F, creatinine, procalcitonin, C-reactive protein and d-dimer levels at admission were comparable between case and control groups. As to the therapy administered, 6 patients in the low PEEP group received ACE- inhibitors versus 3 controls (25% vs 11,5%; p not significant), 4 cases out of 24 as 6 controls out of 26 received steroids, 3 cases out of 24 were treated with tocilizumab versus 1 control out of 26 (12,5% vs 3,8% respectively; p not significant). As to the antiviral therapy, 22 out of 26 patients in the low PEEP strategy group received antivirals (1 patient lopinavir/ ritonavir 400 +100mg bid; 21 patients darunavir/cobicistat, 800+150mg qd) while only 14 controls were treated with antivirals (1 patient acyclovir 400mg bid as prophylaxis after chemotherapy; 8 patients lopinavir/ritonavir 400 + 100mg bid; 4 patients darunavir/ cobicistat 800 + 150mg qd; 1 patient remdesivir 100mg qd) with a statistically significant difference (91,7% vs 53,8%; p = 0,004; OR 9,43 CI95% 1,83 – 48,61). Hydroxychloroquine was administered in 23 out of 24 patients in the low PEEP group versus 17 controls (95,8% vs 65,4%, p 0,011; OR 12,18 CI95% 1,41 – 105,48).
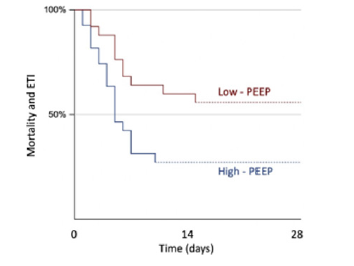
At 7 days from admission, 13 out of 24 patients in the low PEEP group had experienced endotracheal intubation or died as opposed 20 out of 26 controls (54,2% vs 76,9%; p = 0,136). When analysed separately, 6 cases died within 7 days from admission versus 5 controls (25% vs 19,2%; p not significant), while 7 out of 24 cases experienced ETI compared to 15 out 26 controls (29,2% vs 57,7%; p = 0,052; OR 0,30 CI95% 0,09 – 0,98). At 28 days from admission, 2 patients out of 18 remaining patients of the low PEEP group died compared to 11 out of 21 remaining high PEEP controls (11,1% vs 52,4%, p = 0,008; OR 0,11 CI95% 0,02 – 0,62). Only one more patient experienced ETI in the high PEEP group (Table 2). Kaplan-Meier curves showed a statistically significant difference in ETI rate (log rank z = 2,99; p = 0,0028) but not in in-hospital death over the 28 days (log rank z = 1,68; p = 0,094) (Figure 1). When we analysed together ETI rate and mortality, we found again a significant difference between the two groups (log-rank z = 2,51; p = 0,012) (Figure 2). Furthermore, we compared only patients who experienced ETI and there was no difference in results as, at 28 days, only 1 patient out of 7 died in the low PEEP group versus 11 patients out of 16 in the high PEEP one (14,3% vs 68,8%, p 0,027; OR 0,076 CI95% 0,007 – 0,807). In the same way, when we compared only patients that had received antiviral therapy, we found no difference in ETI rate and in-hospital death at 7 days, while there was still a statically significant difference in in-hospital death at 28 days (11,1% vs 53,8%, p 0,017; OR 0,107 CI95% 0,017 – 0,668).
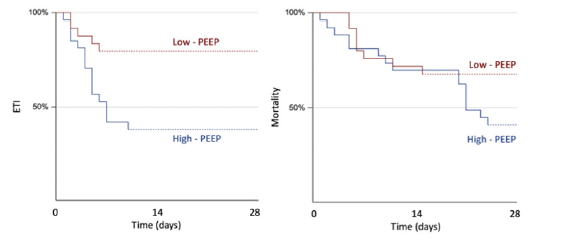
Figure 1: On the left the Kaplan-Meier curves showing a significant difference in endotracheal intubation (ETI) rate during the 28 days of observation between the low PEEP and the high PEEP groups (log rank z = 2,99; p = 0,0028). On the right the Kaplan-Meier curves of in-hospital death during the 28 days that do not differ between the low PEEP and the high PEEP group (log rank z = 1,68; p = 0,094). Note*: PEEP = positive end-expiratory pressure.
Discussion
Since the beginning of the SARS-CoV-2 outbreak, treatment and management of COVID-19 patients have noticeably improved. Nonetheless, there is currently, to our knowledge, no trial that compares the role of different ventilation strategies in fighting a disease that shows a peculiar respiratory failure. The present retrospective case-control study compares a non-invasive low PEEP ventilation and a high PEEP ventilation in a small series of severe respiratory failures due to a SARS-CoV-2 infection and provides a modest evidence that low PEEP could reduce ETI rate and mortality at 28 days. Although we also found a reduction of almost 50% in ETI rate at 7 days, this difference did not achieve statistical significance due to the small sample size. These results are in line with recent findings on this new disease and with a pathophysiology that leads to a unique clinical pattern.
As Gattinoni and colleagues have suggested, COVID-19 patients with severe respiratory failure can have two pneumonia patterns, called type 1 (or L) and type 2 (or H). Although type 1reminds in many respects of a typical ARDS, it stands out for some others, while type 2 is more similar to a typical ARDS. Despite the two types sharing some clinical and arterial blood gas (ABG) characteristics – mainly a severe respiratory failure with severe hypoxemia and similar p/F –, they differ in pathophysiology mechanisms, pulmonary compliance and potential pulmonary recruitment, and thus in the management of these patients and, perhaps, in their outcome [14].
Our low PEEP and early weaning ventilation strategy has the advantage of improving clinical parameters of patients at ED admission, avoiding the risk related to a high PEEP ventilation, e.g. hemodynamic instability, and perhaps, as recently theorized, the evolution from type 1 to type 2 COVID-19 pneumonia. Indeed, some authors have theorized that a typical ARDS approach, namely high PEEP ventilation, associated to the patient self-inflicted lung injury (P-SILI) resulting from the high negative intrathoracic pressure, could lead from a type 1 covid-19 pneumonia to a type 2, causing low pulmonary compliance, fluid retention with high lung weight and a possible worse outcome [13]. Our low PEEP approach has shown a better outcome in terms of mortality and ETI rate, allocating precious ICU beds to the treatment of type 2 pneumonia that may benefit from a higher PEEP, pulmonary recruitment and protective ventilation like a classical ARDS. These results are concordant to those of Mauri et al. that found large variability in pulmonary recruitment in a small series of intubated COVID-19 patients. Mauri et al. conclude that COVID-19 ARDS probably needs personalized mechanical ventilation settings [18]. The worthwhile application of HFNC in our series of severe respiratory failures hints at the atypical features of this ARDS. While in mild typical ARDS, indeed, HFNCs have shown to reduce ETI rate [19], they have been rarely applied to severe patients because of the risk of high failure rate [20]. In our trial, we found that even patients at the lowest p/F could be managed with HFNC up to a complete weaning from PEEP. Similar conclusions have been described in a recent review in which the authors state that some severe COVID-19 ARDS can be treated with HFNC and this is inconsistent with the stratified treatment strategies of ARDS caused by other factors [21].
Our trial presents several limitations. There could be some bias due to the retrospective design of the study. Moreover, the small series of patients enrolled does not allow a good statistical power.
Both the case and control groups are relatively young (mean age 60 and 63 respectively) and present few comorbidities; therefore, they do not correctly represent the COVID-19 population at higher risk of in-hospital death. Furthermore, we found a statistical difference in antiviral and hydroxychloroquine administration between cases and controls, as the low PEEP group have received more frequently an antiviral treatment, particularly darunavir/cobicistat, and hydroxychloroquine. Even though two recent trials – one prospective RCT and one observational– have investigated a different protease inhibitor (lopinavir/ritonavir) and hydroxychloroquine, respectively, in addition to the usual treatment of COVID-19 patients and found no difference [22,23], we cannot exclude that our results could be partially due to the association of darunavir/cobicistat and hydroxychloroquine with the low PEEP ventilation strategy. Moreover, as the measurement of the oesophageal pressure swings is unfeasible in non-invasive ventilated patient in the ED, we used saturation and respiratory rate, as well as clinical evaluation and tolerance to weaning, to monitor respiratory effort improvement, trying to avoid P-SILI. This approach is not standardized and deserves a stronger validation before its implementation in clinical practice. We are aware that prospective larger trials are needed to confirm our data; should this be the case, the low PEEP and early weaning ventilation strategy associated to parametric and clinical monitoring of respiratory distress could be the game changer in this pandemic emergency. We could treat type-1 COVID-19 severe respiratory failures, which represent up to 70% of the severe patients, with non-invasive ventilation, allocating the ICU beds to the treatment of type 2 which, on the other side, could benefit of ETI, protective ventilation and lung recruitment.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. (2020) A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 382(8): 727-733.
- Phelan AL, Katz R, Gostin LO (2020) The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA 323(8): 709-710.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382(18): 1708-1720.
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. 323(11): 1061-1069.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223): 497-506.
- Zhou F, Yu T, Du R, Fan G, Liu Y et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229): 1054-1062.
- Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, et al. (2020) Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 368: m606.
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 232(18): 1824-1836.
- Meng L, Qiu H, Wan L, Ai Y, Xue Z, et al. (2020) Intubation and Ventilation amid the COVID-19 Outbreak: Wuhan's Experience. Anesthesiology 132(6): 1317-1332.
- Yang X, Yu Y, Xu J, Shu H, Xia J, et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8(5): 475-481.
- Rahmanzade R, Rahmanzadeh R, Tabarsi P, Hashemian SM (2020) Non-Invasive versus Invasive Ventilation in COVID-19: One Size Does Not Fit All! Anesth Analg 131(2): e114-e115.
- Wilcox SR (2020) Management of respiratory failure due to covid-19. BMJ 369: m1786.
- Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, et al. (2020) Covid-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 201(10): 1299-1300.
- Gattinoni L, Chiumello D, Rossi S (2020) COVID-19 pneumonia: ARDS or not? Crit Care 24(1): 154.
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, et al. (2020) Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 232(16): 1574-1581.
- Caironi P, Cressoni M, Chiumello D, Ranieri M, Quintel M, et al. (2010) Lung opening and closing during ventilation of acute respiratory distress syndrome. Am J Respir Crit Care Med 181(6): 578-586.
- Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, et al. (2020) Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N Engl J Med 382(11): 999-1008.
- Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, et al. (2020) Potential for Lung Recruitment and Ventilation-Perfusion Mismatch in Patients with the Acute Respiratory Distress Syndrome from Coronavirus Disease 2019. Crit Care Med 48(8):1129-1134.
- Ou X, Hua Y, Liu J, Gong C, Zhao W (2017) Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ 189(7): E260-E267.
- Wang K, Zhao W, Li J, Shu W, Duan J (2020) The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care 10(1):37.
- Li X, Ma X (2020) Acute respiratory failure in COVID-19: is it "typical" ARDS? Crit Care 24(1): 198.
- Cao B, Wang Y, Wen D, Liu W, Wang J, et al. (2020) A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med 382(19): 1787-1799.
- Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, et al. (2020) Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 382(25): 2411-2418.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.