Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Activity of 2,3-Dimethylquinoxaline against Madurella mycetomatis
*Corresponding author: Abdelbagi Alfadil Mousa, Department of Microbiology and Medical Parasitology, College of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
Received: January 06, 2021; Published: January 18, 2021
DOI: 10.34297/AJBSR.2021.11.001654
Abstract
Objective: Madurella mycetomatis is the most prevalent causative species of the eumycetoma, a chronic progressive neglected tropical disabling infectious disease of the subcutaneous tissue. It carries a significant social and economic burden to the endemic areas. Unfortunately, the available treatment is challenging, requires prolonged therapy, resulting in low cure rates and a high incidence of recurrence. Screening new compounds for antifungal activity against Madurella mycetomatis is needed.
Design: In vitro: the susceptibility of a Madurella mycetomatis isolate toward the 2,3-dimethylquinoxaline was determined. In vivo: the efficacy of 2,3-dimethylquinoxaline 1% topical gel against the same strain was evaluated in the BALB/c mouse eumycetoma model (n=6; 6-8 weeks old).
Results: 2,3-Dimethylquinoxaline inhibited the growth of Madurella mycetomatis at MIC 312 µg/ml (1.9 mM). The infected mouse neck's granuloma disappeared after 14 days of the treatment with 2,3-dimethylquinoxaline 1 % gel.
Conclusion: 2,3-Dimethylquinoxaline showed good antifungal activity and worth further optimization towards developing a new eumycetoma treatment.
Keywords: 2,3-Dimethylquinoxaline; Eumycetoma; Madurella mycetomatis; Mycetoma; Quinoxaline
Introduction
Mycetoma is a chronic progressive inflammatory disease [1]. It is classified as either eumycetoma caused by fungi or actinomycetoma caused by bacteria [1]. Mycetoma was first reported in Madura's Indian town and was initially called Madura foot [2]. It commonly affects young adults in developing countries after a traumatic inoculation of the causative microorganism into subcutaneous tissue. Mycetoma is endemic in tropical and subtropical areas at the “mycetoma belt,” which includes ten countries distributed over three continents [3]. It commonly involves the extremities, gluteal, and back region. It is characterized by a painless mass with multiple discharge grains. It often spreads to involve deep structures and bone, resulting in deformity and loss of function [1].
Madurella mycetomatis is the most common causative species of eumycetoma and accounts for 70% of infections [4]. Morphologic features of grains associated with Madurella mycetomatis are black, hard, and relatively large in diameter (1-2 mm) [5]. Clinical improvement is judged by mass size reduction, while the cure is considered when the mass disappears and confirmed clinically, cytologically, and radiologically [6].
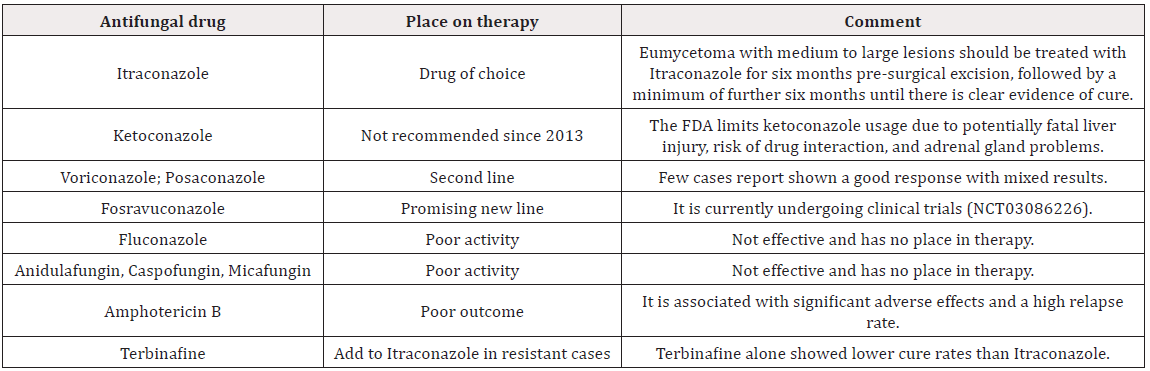
Treatment of eumycetoma should be started as early as possible to avoid amputation and disability [7]. Unfortunately, there is a minimal option for eumycetoma management [7]. Eumycetoma treatment usually requires more than 12 months with a low cure rate (25.9%) and a high recurrence rate (50%) [8]. The current antifungal therapy mainly helps to localize the disease for easily surgical excision. Itraconazole replaced ketoconazole as the standard gold therapy after concerns of adrenal insufficiency and liver injury [9,10]. Posaconazole was found to be a clinically successful antifungal therapy for refractory cases (Table 1) [11].
The current treatment is unsatisfactory and involves long-term antimicrobial therapy and aggressive surgical excisions [12]. Drug discovery and development often create additional challenges for being a neglected tropical disease. Efforts are needed to discover a new treatment for Madurella mycetomatis. There is a need for an affordable and more effective therapy for endemic developing countries.
Quinoxalines as privileged scaffold occupy a prominent place in chemistry due to their broad therapeutic potential and relatively simple chemical synthesis [13-18]. Some quinoxaline derivatives have antifungal activity but have not been tested against Madurella mycetomatis [15]. This study aimed to evaluate the activity of 2,3-dimethylquinoxaline (DMQ) against Madurella mycetomatis [19].
Materials and Methods
DMQ 1% topical gel formulation
All chemicals were of analytical grade. DMQ was obtained from Sigma–Aldrich (D184977). Initially, 2 gm of 3% hydroxypropyl methylcellulose (HPMC) was added to 50 ml of distilled water and allowed to soak overnight. On the second day, 10 ml of glycerol was added to the soaked HPMC and allowed for further soaking for 8 hours. On the other side, 1 gm of DMQ was dissolved in 5 ml of 99% alcohol then diluted by 35 ml of distilled water. Finally, the dissolved DMQ was added by gently stirring the soaked HPMC glycerol until the homogenous gel was formed. The gel was protected from light and stored at 4˚C.
Madurella mycetomatis clinical Isolate
Madurella mycetomatis strain was isolated by direct culture of the black grains obtained by fine-needle aspiration from a patient seen at Omdurman teaching hospital, Sudan. The isolate was cultured on SDA with Chloramphenicol (Becton Dickinson) for two weeks at 37˚C. The culture was examined every four days for the growth rate, the colonial configuration, and the pigmentation presence. Identification of the isolate was done by morphology and confirmed by polymerase chain reaction (PCR).
In vitro susceptibility testing
Microdilution assay was used as described previously to determine the susceptibility of Madurella mycetomatis towards 2,3-dimethylquinoxaline [20]. The isolate was cultured in RPMI1640 medium (Gibco) with MOPS (Sigma) at 37˚C for 7 days, then harvested by centrifugation, homogenized by sonication, and adjusted to obtain transmission of 70% at 660nm. 2,3-Dimethylquinoxaline was tested at concentrations range from 4.8 µg/ml to 2.5 mg/ml (0.03 to 15.8 mM). The assay were perfomed in duplicate and repeated in triplicate.
In vivo susceptibility testing
Male BALB/c mice (n=6; 6-8 weeks old) were used as described previously [21,22]. Mice have inoculated with Madurella mycetomatis 140 mg (0.4 ml) subcutaneously in the neck. The site of inoculation was examined daily for two weeks for the development of the lesion. When the lesion was formed, different specimens were taken, and wet preparation was done. The tissue section was prepared for a histopathology examination. After confirmation of Madurella mycetomatis infection, the lesion was applied topically by 1% DMQ gel. The lesions of the mice were followed up daily.
Ethical statement
The study was supported by grant G-1256-140-1440 from the Deanship of Scientific Research (DSR) at King Abdul-Aziz University (KAU). The Biomedical Ethics Committee approved the experimental protocol at KAU and the National Committee of Bioethics (NCBE) (Registration No. HA-02-J-008). Informed consent obtained from the patient prior to obtain the clinical isolate. The procedure was performed following NCBE and with the 1964 Helsinki Declaration and its amendments. Animal handling was performed in strict compliance with the ethical guidelines for treating animals as defined by KAU and NCBE.
Results and Discussion
An inhibition of Madurella mycetomatis growth was observed with concentrations of 5, 2.5, 1.2, 0.6, and 0.3 mg/ml (15.8, 7.9, 3.9, 1.9 mM). Minimum inhibition concertation (MIC) of DMQ against Madurella mycetomatis was documented as 312 µg/ml (1.9 mM).
All infected mice (n=6) with Madurella mycetomatis developed a well-defined granuloma on their necks (Figure 1A). For all treated mice (n=3) with DMQ 1 % gel for 14 days, the granuloma disappeared, leaving a mild scar (Figure 1B).
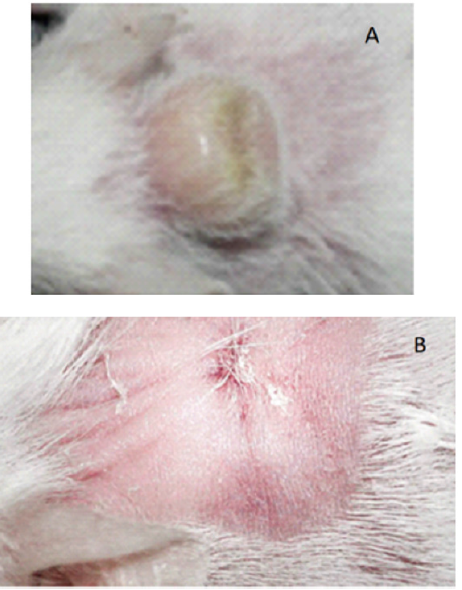
Figure 1: Mouse model of eumycetoma-Madurella mycetomatis. A) The granuloma developed in infected mouse model. B) The improvement after treatment with DMQ 1% gel.
Madurella mycetomatis known to produce translationally controlled tumor protein (TCTP) [23]. This protein has a vital role in controlling the cell cycle and stress response [24]. TCTP gene expression is considered a virulent factor with an anti-apoptotic effect that stabilizes cells from programmed cell death. Quinoxalines have been shown to induce programmed cell death in treated cells [25]. Further studies are essential to explicate how precisely fungal cell death happened and how other antifungal drugs can interact for possible enhancement of the treatment outcome.
Minimal reports addressed DMQ pharmacology and pharmacokinetic. The current application of DMQ is in the laboratory to determine the amount of diacetyl in foods and beverages [26]. Few reports indicate that DMQ is a competitive hepatic P-450 enzyme inducer [27,28].
Given the properties and uses of approved quinoxalines, one of their most prominent characteristics is their ability to reach target tissues at an appropriate concentration (Table 2) [29]. In contrast, amphotericin B has minimal or no value in Madurella mycetomatis, although it has excellent in vitro activity, explained by the limited drug distribution into the infected tissues [30].
The results obtained from this study revealed a promising activity of DMQ against Madurella mycetomatis and merited further optimization. The antifungal effects probably rely on a new mechanism of action.
Disclosure Conflict of interest
No conflict of interest associated with this work.
Acknowledgments
We are grateful to the DSR at KAU for the financial support (G- 1256-140-1440), the king Fahd medical research center (KFMRC) for their valuable collaboration, and Omdurman teaching hospital for providing the Madurella mycetomatis clinical isolate.
Contribution of Authors
The authors will bear all liabilities on claims relating to the content of this article. Alfadil, Ali, and Alsamhan conceived and designed the study. Alsamhan and Ali performed the literature review and retrieved all data. Alkreathy, fatani, Alfadil, and Ali contributed to the supply of reagents and materials. Alfadil, Ali, and Alsamhan performed the experiments. Alfadil, Alsamhan, and Abdulmajed collected and analyzed the data. Alsamhan, Abdulmajed, Alfadil, and Ali wrote the manuscript.
References
- Zijlstra EE, Van De Sande WW, Welsh O, El Sheikh Mahgoub, Goodfellow M, et al. (2016) Mycetoma: a unique neglected tropical disease. The Lancet Infectious Diseases 16(1): 100-112.
- Ghosh L, Dey N, Panja D (1950) Madura foot (mycetoma). The Indian Medical Gazette 85(7): 288-291.
- Fahal A, Van De Sande W (2012) The Epidemiology of mycetoma. Current Fungal Infection Reports 6(4): 320-326.
- Nenoff P, Van de Sande W, Fahal A, Reinel D, Schöfer H (2015) Eumycetoma and actinomycetoma–an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. Journal of the European Academy of Dermatology and Venereology 29(10): 1873-1883.
- Ahmed AO, Van Leeuwen W, Fahal A, Van de Sande W, Verbrugh H, et al. (2004) Mycetoma caused by Madurella mycetomatis: a neglected infectious burden. The Lancet infectious diseases 4(9): 566-574.
- Wankhade AB, Ghadage DP, Mali RJ, Bhore AV (2012) Mycetoma foot due to Madurella mycetomatis. Annals of Tropical Medicine and Public Health 5(4): 352-354.
- Abbas M, Scolding PS, Yosif AA, Rahman RFE, EL-Amin MO, et al. (2018) The disabling consequences of Mycetoma. PLoS neglected tropical diseases 12(12): e0007019.
- Zein HA, Fahal AH, Mahgoub ES, El Hassan TA, Abdel-Rahman ME (2012) Predictors of cure, amputation and follow-up dropout among patients with mycetoma seen at the Mycetoma Research Centre, University of Khartoum, Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene 106(11): 639-644.
- Welsh O, Al-Abdely HM, Salinas-Carmona MC, Fahal AH (2014) Mycetoma medical therapy. PLoS neglected tropical diseases 8(10).
- Scolding P, Fahal A, Yotsu RR (2018) Drug therapy for Mycetoma. Cochrane Database of Systematic Reviews (7).
- Negroni R, Tobón A, Bustamante B, Shikanai-Yasuda MA, Patino H, et al. (2005) Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Revista do Instituto de Medicina Tropical de São Paulo 47(6): 339-346.
- Sow D, Ndiaye M, Sarr L, Kanté MD, Ly F, et al. (2020) Mycetoma epidemiology, diagnosis management, and outcome in three hospital centres in Senegal from 2008 to 2018. Plos one 15(4): e0231871.
- Adkins JC, Balfour JA (1998) Brimonidine. Drugs & aging 12(3): 225-241.
- Cohen DE (2019) Clinical Development of Viekira Pak to Mavyret. Topics in Medicinal Chemistry 32: 347-367.
- Jampilek J (2014) Recent advances in design of potential quinoxaline anti-infectives. Current medicinal chemistry 21(38): 4347-4373.
- Jiménez-Ruiz C, Berlin I, Hering T (2009) Varenicline. Drugs 69(10): 1319-1338.
- Robertson MN, Barr E (2019) Development of ZEPATIER®. Topics in Medicinal Chemistry 32: 369-407.
- Stamm LM, McHutchison JG (2019) The Clinical Development of Sofosbuvir/Velpatasvir/Voxilaprevir (SOF/VEL/VOX, Vosevi®). Topics in Medicinal Chemistry 32: 317-345.
- Woźniak K, Krygowski T, Kariuki B, Jones W (1990) Structure of 2, 3-dimethylquinoxaline. Acta Crystallographica Section C: Crystal Structure Communications 46(10): 1946-1947.
- Ahmed AO, Van de Sande WW, Van Vianen W, Van Belkum A, Fahal AH, et al. (2004) In vitro susceptibilities of Madurella mycetomatis to itraconazole and amphotericin B assessed by a modified NCCLS method and a viability-based 2, 3-Bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) assay. Antimicrobial agents and chemotherapy 48(7): 2742-2746.
- Ahmed AO, Van Vianen W, Ten Kate MT, Van de Sande WW, Van Belkum A, et al. (2003) A murine model of Madurella mycetomatis eumycetoma. FEMS Immunology & Medical Microbiology 37(1): 29-36.
- Van de Sande W, Van Vianen W, Ten Kate M, Fahal A, Bakker-Woudenberg I (2015) Amphotericin B but not Itraconazole is able to prevent grain formation in experimental Madurella mycetomatis mycetoma in mice. British Journal of Dermatology 173(6): 1561-1562.
- Van de Sande WW, Janse D-J, Hira V, Goedhart H, Van der Zee R, et al. (2006) Translationally controlled tumor protein from Madurella mycetomatis, a marker for tumorous mycetoma progression. The Journal of Immunology 177(3): 1997-2005.
- Bommer U-A, Thiele B-J (2004) The translationally controlled tumour protein (TCTP). The international journal of biochemistry & cell biology 36(3): 379-385.
- Zamudio-Vázquez R, Ivanova S, Moreno M, Hernandez-Alvarez MI, Giralt E, et al. (2015) A new quinoxaline-containing peptide induces apoptosis in cancer cells by autophagy modulation. Chemical science 6(8): 4537-4549.
- Shibamoto T (2014) Diacetyl: occurrence, analysis, and toxicity. Journal of agricultural and food chemistry 62(18): 4048-4053.
- Beraud M, Gaillard D, Derache R (1975) Effect of ingestion of derivatives of quinoxaline on the enzymatic detoxication activity of hepatic microsomes in the rat. Archives internationales de pharmacodynamie et de therapie 218(2): 328-337.
- Hahnemann B, Legrum W, Koss G, Netter K (1989) Interactions of heterocyclic Maillard products with the hepatic microsomal monooxygenase system. Xenobiotica 19(11): 1319-1326.
- Ajani OO (2014) Present status of quinoxaline motifs: Excellent pathfinders in therapeutic medicine. European journal of medicinal chemistry 85: 688-715.
- Kloezen W, Parel F, Brüggemann R, Asouit K, Helvert-van Poppel M, et al. (2018) Amphotericin B and terbinafine but not the azoles prolong survival in Galleria mellonella larvae infected with Madurella mycetomatis. Medical mycology 56(4): 469-478.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.