Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Progesterone-Oocyte Index as a Predictor of the Final Outcome of an IVF Cycle
*Corresponding author: Bárbara Romero Guadix, Servicio de Obstetricia y Ginecología, Hospital Universitario Virgen de las Nieves, Avenida de las Fuerzas Armadas, 2, 18014, Granada, Spain.
Received: October 22, 2020; Published: January 20, 2021
DOI: 10.34297/AJBSR.2021.11.001660
Abstract
Aim: To compare the serum levels of progesterone (P) on the day of human chorionic gonadotropin (hCG) administration and the progesterone-oocyte index (POI) on the day of follicular puncture, in relation to the clinical pregnancy rate obtained in cycles of in vitro fertilization (IVF).
Material and method: Between March 2011 and December 2014, a retrospective observational study was performed at a third-level hospital, during which 1691 IVF cycles were analysed. A new variable, the progesterone-oocyte index (POI), is calculated for each cycle included in the study by dividing the serum P level (ng/ml) on the day of hCG administration by the total number of oocytes obtained by follicular puncture, and the pregnancy-prediction accuracy of the serum P level on the day of hCG administration was compared with that of the POI.
Results: The adjusted odds ratio for the probability of clinical pregnancy was 1.49 (95% CI: 1.22-1.84) for progesterone in blood and 28.5 (95% CI: 9.4-86.3) for the POI (p <0.001). ROC curves were plotted. The lower the POI, the higher the clinical pregnancy rate, when the analysis was performed for blood progesterone levels, with an area under the curve of 0.53 and 0.597 respectively (p<0.001). Both variables were divided into deciles, and the clinical pregnancy rate was established for each case. Progesterone levels in blood had only limited predictive value, while the POI presented an inverse linear relationship with clinical pregnancy rates.
Conclusions: The POI is a valid parameter and a more reliable measure than progesterone levels in blood for determining the final results of an IVF cycle.
Keywords: In vitro fertilization; Assisted reproduction techniques; Progesterone; Oocyte; Progesterone-oocyte index
Introduction
Historically it was thought that during the process of controlled ovarian stimulation in a cycle of assisted reproduction, the elevation of serum progesterone (P) levels prior to the exogenous administration of human chorionic gonadotropin (hCG) [1] was associated with an early increase in luteinising hormone, thus provoking “premature luteinisation” [2], together with an independent impact on endometrial gene expression [3], and thus decreased receptivity of the endometrium and lower overall rates of implantation [4-6].
This supposed inverse relationship led to an increase in the number of delayed frozen embryo transfers performed, which raised the cost of in vitro fertilisation-embryo transfer (IVF-ET) treatment and made it less acceptable to patients than a fresh transfer [7].
For this reason, in recent years, many studies have attempted to clarify the possible relationship between P levels and the final results of an IVF cycle. However, widely-differing results have been obtained, mostly due to population differences and insufficient methodological rigour [8-11].
To resolve this question, some studies have sought to establish the relationship between follicular response and serum P levels on the day of hCG administration [5,9,11].
In the present study, we compare the serum levels of P on the day of hCG administration and the progesterone-oocyte index (POI) on the day of follicular puncture, in relation to the clinical pregnancy rate obtained in IVF-ET cycles.
Materials and Methods
Study population
This retrospective noninterventional observational study was carried out at the assisted reproduction unit of a third-level hospital. The study population was based on a cohort of patients receiving the usual clinical attention; no inclusion/exclusion criteria were applied with respect to the baseline characteristics of these patients; the study was carried out from March 2011 until December 2014, and a total of 1691 IVF-ICSI cycles were analysed.
The patients were given a controlled ovarian stimulation treatment using an agonist (n=1008) or a GnRH antagonist (n=683), according to their baseline characteristics. Ovarian stimulation was performed with recombinant follicle stimulating hormone receptor (FSHR, Gonal®, Laboratorios Serono, Madrid, Spain or Puregon®, Organon, Barcelona, Spain), with highly purified human menopausal gonadotropin (hMG, Menopur®, Ferring Pharmaceuticals, Geneva, Switzerland) or with the two combined.
Serum oestradiol and P, and an ultrasound test (follicular growth) were performed every 48 hours to determine the evolution of the cycle. Final oocyte maturation was achieved with the administration of 250 mcg of human chorionic gonadotropin (hCG, Ovitrelle ®; Merck SL) 36 hours before follicular puncture when ultrasonography revealed follicles measuring 17 mm or larger.
IVF or ICSI was performed according to the sperm parameters identified in each case. ET was performed 48-72 hours after oocyte collection (d+2 or d+3). The number of embryos transferred (maximum 2 embryos) was determined taking into account the patient’s clinical history and the embryo quality, and in accordance with Clinical Practice Guidelines.
Calculations and statistical analysis
The POI was calculated for each cycle included in the study, by dividing the total serum P (ng/ml) level on the day of hCG administration by the total number of oocytes obtained by follicular puncture. The data were fitted to logistic regression models, and odds ratios were calculated for the probability of clinical pregnancy, both in the general population, and adjusted for age, protocol (GnRH agonist/antagonist) and cycle parameters. The area under the curve (ROC) was calculated to determine statistical significance.
Two different logistic regression models were obtained, one for serum P levels and the other for the POI, to determine whether one model was superior to the other as a predictor of clinical pregnancy. In order to stratify the sample in a homogeneous way, both models were divided into deciles. The clinical pregnancy rate was then calculated for each one, and the values above which this rate decreased significantly were determined.
All data were compiled and processed using Microsoft Excel software, and the statistical analysis was performed using SPSS 21.0.
Results
Data were obtained and analysed from 1691 IVF-ICSI cycles, carried out in patients with a mean age of 34.2 ± 3.9 years. The overall clinical pregnancy rate obtained was 31.2%.
A cycle of ovarian stimulation with the GnRH agonist was performed in 1008 patients (59.6%), and with an antagonist in the remaining 683 patients (40.4%). The overall pregnancy rates obtained were 31.8% and 30.2%, respectively (the difference is not statistically significant).
The mean level of P in blood on the day of hCG administration was 0.96 ± 0.59 ng/ml. The mean number of oocytes obtained by follicular aspiration was 8.6 ± 4.9. The mean POI was 0.16 ± 0.19. The mean serum oestradiol value was 2262 ± 1445 pg/ml.
According to the univariate analysis performed of the individual factors related to the results of an IVF-ET cycle, the younger the patient, the greater the quantity of oocytes collected by follicular puncture, the lower the POI and the lower the serum P value, the higher the overall pregnancy rate (p<0.001) (Table 1).
The adjusted odds ratio (OR) for the probability of clinical pregnancy was 1.49 (95% CI: 1.22-1.84) for P in blood and 28.5 (95% CI: 9.4-86.3) for the POI (p<0.001). After adjusting for the pituitary suppression protocol, a value of OR=1.34 (95% CI: 1.05-1.71) was obtained for P and OR=34.91 (95% CI: 7.22-168-83) for the POI in the subgroup in which a GnRH agonist protocol was used (p<0.001). In the GnRH antagonist subgroup, OR=1.96 (95% CI: 1.34-2.87) was obtained for P and OR=23.30 (95% CI: 4.96-109.50) for the POI (p<0.001).
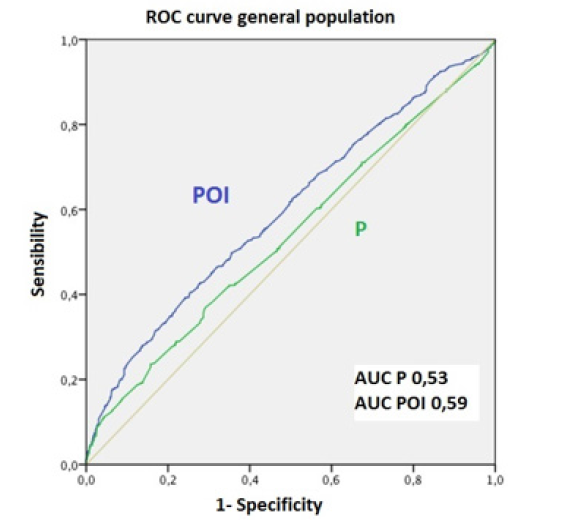
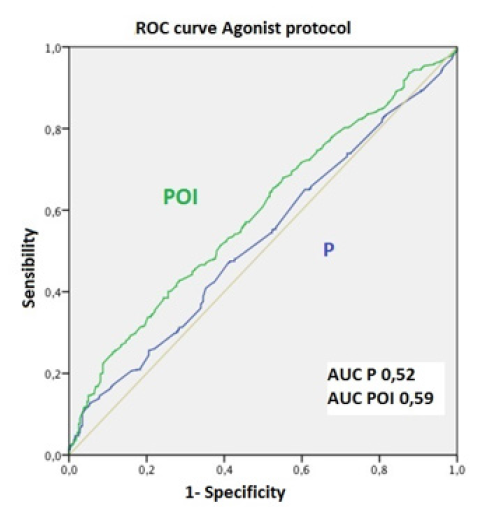
The pregnancy prediction capacity of blood P levels and of the POI was established using ROC curves. The lower the POI, the higher the rate of clinical pregnancy observed; however, this relationship was not so apparent in the analysis of blood P levels. Thus, the area under the curve (AUC) was 0.53 and 0.6 respectively (p<0.001). Similar results were obtained when adjusted by protocol type: for the agonist protocol, AUC=0.55 for P in blood and AUC=0.60 for the POI; and for the antagonist protocol, AUC=0.52 for P in blood and AUC=0.59 for the POI (p<0.001) (Figure 1).
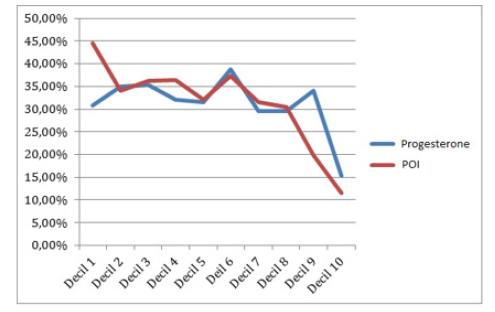
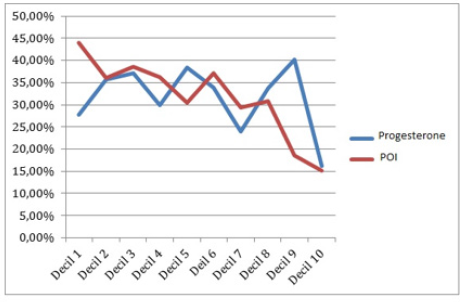
The clinical relevance of blood P levels and of the POI on the results of the cycle were further compared by dividing these two variables into deciles, and then establishing the clinical pregnancy rate for each one (Table 2). The results obtained show that blood P levels had limited predictive value, being associated with a low rate of clinical pregnancy from the ninth decile (>1.6 ng/ml). The POI values presented a linear inverse association with clinical pregnancy rates from the eighth decile (>0.2 ng/ml/oocytes). This association was more significant than that found for blood P levels (Figure 2).
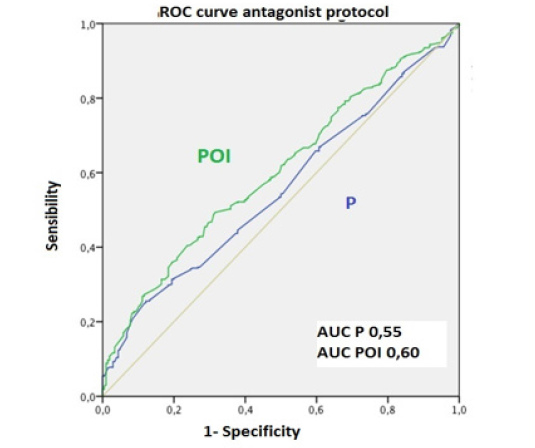
Figure 1C: P: Progesterone. POI: progesterone-oocyte index. AUC: area under the curve. Figure 1: ROC curve for the whole sample.
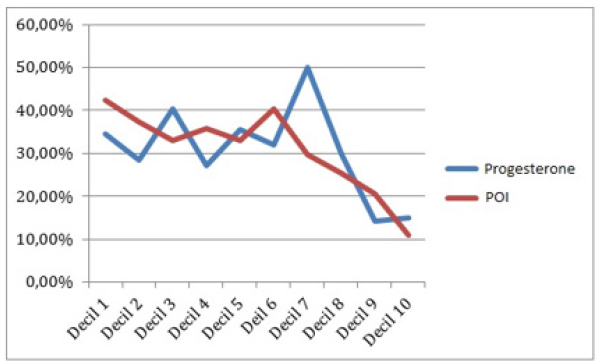
Figure 2C: Comparison of global gestation rates by dividing the sample into deciles. It can be seen that from the decile 9 of P (> 1.2 ng / ml) and from the decile 8 of POI (> 0.2) the percentage of clinical gestation is lower.
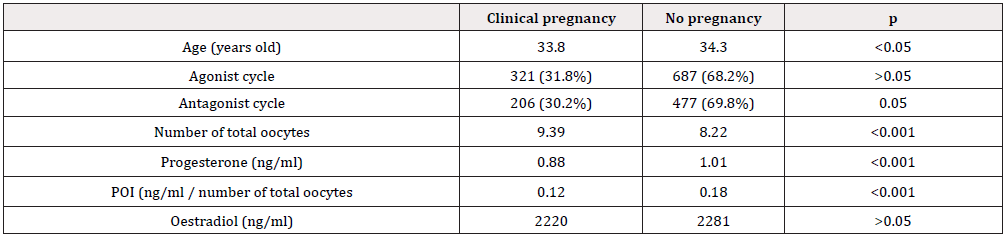
Table 1: Univariate analysis of individual factors related to the final results of an IVF cycle. (POI: progesterone-oocyte index).
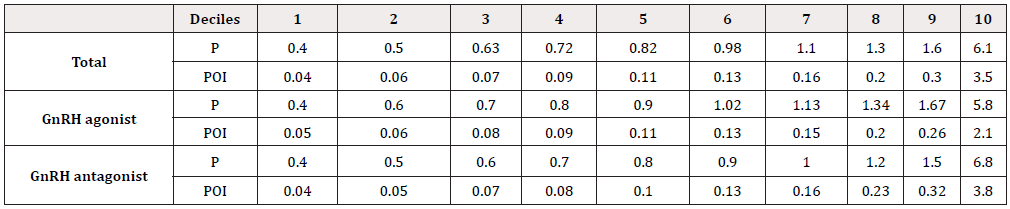
Table 2: Blood P levels and POI split by deciles in the overall sample and according to the pituitary suppression cycle used (GnRH agonist vs GnRH antagonist). (P: Progesterone. POI: progesterone-oocyte index).
The clinical relevance obtained for each decile was then determined, according to the pituitary suppression protocol used (GnRH agonists vs. GnRH antagonists) (Table 2). The results obtained were similar to those for the sample as a whole. In the agonist subgroup, there was a decrease in pregnancy rates for blood P levels from the ninth decile (>1.67 ng/ml) and for the POI from the eighth decile (>0.2 ng/ml/oocytes) (p<0.001). In the antagonistic subgroup, clinical pregnancy rates were lower for blood P levels from the last two deciles (>1.2 ng/ml) and for the POI, from the seventh decile (>0.23 ng/ml/oocytes) (p<0.001) (Figure 2).
Discussion
The role played by elevated serum P in the late follicular phase and its impact on the final results of an IVF cycle remains an open question. There is increasing evidence that supraphysiological hormonal elevations can alter endometrial receptivity, decreasing overall pregnancy rates in a fresh ET cycle. However, oocyte quality is not affected by this hormonal elevation [12]. Thus, it would be possible to identify the type of patients who would benefit from delayed frozen embryo transfer versus fresh ET [13]. However, it is still a challenge to determine the threshold value of serum P above which the overall pregnancy rate is affected [14].
Studies by Griesinger9 and Kyrou5 reported the clinical irrelevance of P elevation in patients with a high oocyte response to a stimulation cycle. These authors suggested that the blood P level considered should be the total quantity of P secreted by each follicle, including those with a diameter <14 mm. No clear relation was observed with poor results from the stimulation cycle.
More recent studies have considered the elevation of P in the late follicular phase under two scenarios. In the first, there is an increase in the secretion of P by each follicle recruited, which alters follicular development and is associated with lower rates of clinical pregnancy. In the second, additional follicles are recruited, but there is no change in the amount of P secreted by each one, and hence there is no negative impact on follicular development or on the final results of the IVF cycle. This is the case of most patients who achieve a high response to the stimulation cycle [11,15].
As a complement to the latter hypothesis, we calculated a new parameter, the POI, calculated by dividing the serum P level (ng/ml) on the day of hCG administration by the total number of oocytes obtained by follicular puncture. However, we did not use the number of follicles measured by transvaginal ultrasonography prior to the administration of hCG, as has been done by other study groups [11,15] for several reasons. Firstly, due to the well-documented limitations of measuring follicular development by 2D ultrasound scanning and also because of possible interobserver differences, which can lead to the underestimation of the follicles that are actually contributing to the total secreted quantity of P in blood. Secondly, because one of our study goals was to obtain a valid non-invasive marker enabling rapid decision-making in a patient with elevated serum levels of P at the time of ET (fresh vs. delayed).
Inclusion/exclusion criteria were not applied in selecting patients for this study because we wished to obtain a new parameter that would allow us to make decisions in response to elevations in serum P in any type of patient, regardless of the oocyte response to the stimulation cycle. Thus, patients with large elevations of serum P due to the recruitment of additional follicles and not to an increase in the amount of P secreted by each follicle (high response) would benefit from a fresh transfer, which would reduce the costs of assisted reproduction and alleviate the anxiety that can lead patients to cancel ET.
Our study, of 1691 cycles, shows that the POI is a better predictor of the pregnancy rate than are serum P levels, in the analysis of sensitivity vs. specificity in the ROC curve, where the POI has a larger AUC. Only from values >1.67 ng/ml (ninth decile) of serum P does the possibility of pregnancy decrease. On the other hand, the POI presented an inverse linear relationship with the pregnancy rate for all values and ranges of the sample. Any increase in the POI was associated with a proportional decrease in the pregnancy rate [16].
In order to eliminate as possible confounding factors the individual and clinical characteristics of each patient, the sample was subdivided according to the protocol used: GnRH agonist or antagonist. In this secondary analysis, the ROC curve response in each of these groups was similar to that of the sample analysed as a whole (Figure 1), thus reinforcing our conclusion that the pregnancy rate is better predicted by the POI than by blood P levels.
In conclusion, the POI reflects the mean amount of P secreted by each follicle, differentiating new follicular recruitment from an increase in P production by each follicle. In the future, the POI may be considered a potential marker of oocyte and endometrial quality.
Unlike the serum P level, the POI has an inverse linear relationship with the rate of clinical pregnancy and is not affected by individual factors such as the pituitary suppression protocol used.
In short, reference to the POI would improve the profitability of an IVF-ET cycle. If the patient has a low POI, fresh ET can be performed. On the contrary, if the POI is high, the cycle should be suspended, and a delayed ET performed.
References
- Ubaldi F, Camus M, Smitz J, Bennink HC, Van SA, et al. (1996) Premature luteinization in in vitro fertilization cycles using gonadotropin-releasing hormone agonist (GnRH-a) and recombinant follicle-stimulating hormone (FSH) and GnRH-a and urinary FSH. Fertil Steril 275-280.
- Van Vaerenbergh I, Fatemi HM, Blockeel C, Van Lommel L, In't Veld P, et al. (2011) Progesterone rise on hCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online 263-271.
- Bosch E, Labarta E, Crespo J, Simon C, Remohi J, et al. (2010) Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod 2092–2100.
- Smitz J, Andersen AN, Devroey P, Arce JC (2007) Endocrine profile in serum and follicular fluid differs after ovarian stimulation with HP-hMG or recombinant FSH in IVF patients. Hum Reprod 676-687.
- Kyrou D, Popovic-Todorovic B, Fatemi HM, Bourgain C, Haentjens P, et al. (2009) Does the estradiol level on the day of humanchorionic gonadotrophin administration have an impact on pregnancy rates in patients treated with rec-FSH/GnRH antagonist? Hum Reprod 24(11): 2902-2909.
- Labarta E, Martinez-Conejero JA, Alama P, Horcajadas JA, Pellicer A, et al. (2011) Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the folicular phase: a functional genomics analysis. Hum Reprod 1813-1825.
- Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, et al. (2007) Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update 13(4): 343-355.
- Kolibianakis EM, Venetis CA, Bontis J, Tarlatzis BC (2012) Significantly lower pregnancy rates in the presence of progesterone elevation in patients treated with GhRH antagonists and gonadotrophins: a systematic review and meta-analysis. Curr Pharm Biotechnol 13(3): 464-470.
- Griesinger G, Mannaerts B, Andersen CY, Witjes H, Kolibianakis EM, et al. (2013) Progesterone elevation does not compromise pregnancy rates in high responders: a pooled analysis of in vitro fertilization patients treated with recombinant follicle-stimulating hormone/gonadotropin-releasing hormone antagonist in six trials. Fertil Steril 100(6): 1622-1628.
- Dai W, Bu ZQ, Wang LL, Sun YP (2015) The relationship between the changes in the level of progesterone and the outcome of in vitro fertilization-embryo transfer. Syst Biol Reprod Med 61(6): 388-397.
- Hamdine O, Macklon NS, Eijkemans MJ, Laven JS, Cohlen BJ, et al. (2014) Elevated early follicular progesterone levels and in vitro fertilization outcomes: a prospective intervention study and meta-analysis. Fertil Steril 102(2): 448-454.
- Shufaro Y, Sapir O, Oron G, Ben Haroush A, Garor R, et al. (2015) Progesterone-to-follicle index is better correlated with in vitro fertilization cycle outcome than blood progesterone level. Fertil Steril 103(3): 669-674.
- Maheshwari A, Bhattacharya S (2013) Elective frozen replacement cycles for all: ready for prime time? Hum Reprod 28(1): 6-9.
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C (2014) Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril 102(1): 3-9.
- Barnhart KT (2014) Introduction: Are we ready to eliminate the transfer of fresh embryos in in vitro fertilization? Fertil Steril 102(1): 1-2.
- Roque M, Valle M, Sampaio M, Geber S, Checa MA (2015) Ratio of progesterone-to-number of follicles as a prognostic tool for in vitro fertilization cycles. J Assist Reprod Genet 32(6): 951-957.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.