Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Effectiveness, Safety and Tolerability of Intravaginal Prasterone for the Treatment of Genitourinary Syndrome in Postmenopausal Women in Spain: The Estip-Es Study
*Corresponding author: Silvia P Gonzalez, HM Gabinete Velázquez, Madrid, Spain.
Received: May 10, 2021; Published: May 20, 2021
DOI: 10.34297/AJBSR.2021.12.001817
Abstract
Genitourinary syndrome of menopause has a significant and negative impact on quality of life. The objective of this study was to evaluate the effectiveness, safety, and tolerability of prasterone for the treatment of genitourinary syndrome in clinical practice in Spain. We performed a prospective, observational, and multicenter study in adult postmenopausal women. Female Sexual Function Index (FSFI) questionnaire and Visual Analog Scale (VAS) were administrated and level of satisfaction was analyzed at baseline and after 30±7 days. The study included 184 postmenopausal women with genitourinary syndrome. The FSFI increased from 15.7±6.3 to 19.9±5.38 (P<0.01), with significant improvements in all items. The level of satisfaction with intravaginal prasterone was high (completely/moderately satisfied, 63.5%). Only 6.5% of patients reported side effects. Treatment with intravaginal prasterone in postmenopausal women with genitourinary syndrome, was associated with significant improvements in sexual function and urinary symptoms, and reduction in genital dryness, burning, irritation and pain. The drug was well tolerated, with high satisfaction rates after one month of treatment, suggesting that intravaginal prasterone should be considered a first-line therapy for the management of this population in clinical practice.
Keywords: Dehydroepiandrosterone; Genitourinary Syndrome; Menopause; Prasterone
Abbreviations: VAS: Visual Analog Scale; FSFI: Female Sexual Function Index; DHEA: Dehydroepiandrosterone; n: Absolute Frequency; %: Relative Frequency
Introduction
Menopause is a normal mid-life event associated with a decrease in ovarian synthesis of estrogen, progesterone and dehydroepiandrosterone (DHEA), leading to a group of genital and urinary symptoms known as genitourinary syndrome of menopause, which is usually chronic and progressive without treatment and has a significant and negative impact on quality of life. This syndrome affects up to 50% of menopausal women and is characterized by genital symptoms (dryness, burning, and irritation), sexual symptoms (lack of lubrication, discomfort, pain, and impaired function) and urinary tract involvement (urgent urination, dysuria, and recurrent urinary tract infection). Women may experience some or all of these signs and symptoms [1-5] Despite the high frequency of genitourinary syndrome of menopause, many women do not seek medical advice because of embarrassment, believe that the symptoms are normal with ageing, or think that no effective or safe treatments are available [6].
The first goal in genitourinary syndrome is the improvement in and relief of symptoms. The many currently available options include numerous adjunctive therapies such as moisturizers, lubricants, and laser therapy, as well as systemic hormonal therapies, low-dose vaginal estrogen therapies, and selective estrogen receptor modulators, such as ospemifene [7]. Despite these treatments, many women remain unsatisfied for a variety of reasons [8-10]. Furthermore, while low-dose vaginal estrogen preparations effectively alleviate the symptoms of genitourinary syndrome, potential systemic absorption of vaginal estrogens may increase systemic exposure to estradiol. Additionally, declining androgen levels cannot be restored by vaginal estrogens [11,12] of note, early discontinuation of these therapies is common [10].
Prasterone (DHEA) is an endogenous precursor steroid hormone that is metabolized into both androgens and estrogens. It has been approved as an intravaginal insert for the treatment of moderate to severe dyspareunia caused by vulvovaginal atrophy secondary to menopause. Additionally, it has proven effective for the treatment of other types of sexual dysfunction that are secondary to menopause [13-15]. As a result, prasterone not only minimizes the potential risks associated with estrogen-based therapy, but also activates the vaginal estrogen and androgen receptors that are necessary for the normal functioning of the vagina (intracrinology), without inducing systemic side effects [15]. However, although data from clinical trials show that prasterone can improve some symptoms of vulvar and vaginal atrophy compared with placebo, data from real-life patients are lacking [16-18]. In addition, given potential cultural differences in the perception of genitourinary symptoms and disparities in treatment options between regions, country-specific approaches may be required [19]. Therefore, data on the role of prasterone in the management of patients with genitourinary syndrome and on the level of satisfaction with treatment and discontinuation rates in clinical practice are warranted. The objective of this study was to evaluate the effectiveness, safety, and tolerability of prasterone for the treatment of genitourinary syndrome in clinical practice in Spain. The clinical profile of women with genitourinary syndrome treated with intravaginal prasterone was also analyzed. Furthermore, the impact of genitourinary syndrome on physical status and sexual functioning was examined.
Methods
We performed a suitable table form for solving the problem that suspected covid cases may affect by confirmed covid cases when the PCR test is not ready in fast covid ward. This form includes the symptoms and histories from the patient which every single question or mark in the form has a score. This data gathered from novel covid-19 papers which focused on symptom percentage in suspected and confirmed covid cases. So, by this method and defining new score for suspected and confirmed covid cases we can predict which case has fewer chance for confirmed positive covid test. Besides the form another part of this method is that we should divide the fast covid ward into two parts: first part for admitting the patients with score near confirmed covid cases and second part for admitting patients with higher chance of negative PCR test. In the second part all protocols must apply including wearing suitable masks for patients with poor consciousness and proper air condition. Overall, by separating suspected patients with less possibility of positive test and those with high possibility of positive test and applying preventive protocols the chance of transmission the disease from confirmed cases to suspected cases will decrease and the result will be lower mortality rate. It also helps healthcare providers for categorizing the patients into proper ward for admission and know the prognosis level of each one.
The study followed the principles of the Declaration of Helsinki and was approved by the local participating Institutional Review Boards. In order to evaluate the impact of intravaginal prasterone on genitourinary syndrome after 30±7 days of treatment, the short Female Sexual Function Index (FSFI) and the visual analog scale (VAS) were administered, and the level of satisfaction was analyzed. The FSFI is a 19-item multidimensional self-reported instrument developed to assess female sexual function. For this study, a validated short version with 7 items was used. Each item ranged from never (score 0) to always (score 5). Higher scores indicate better sexual function. A score between 0 and 20 suggests that sexual dysfunction may be present. A difference of more than 3 points between the baseline and study end questionnaires was interpreted as considerable clinical improvement, a difference between 2 and 3 points as moderate clinical improvement, and a difference of 1 point as mild clinical improvement [20]. A 19-item VAS was administered to assess the impact of intravaginal prasterone on genitourinary syndrome. Each item ranged from 0 (absence of discomfort) to 10 (extreme discomfort). As a result, there was an inverse relationship between the score and the improvement in symptoms. Thus, higher scores indicate more discomfort. A difference of more than 3 points between the baseline and study end questionnaires was interpreted as considerable clinical improvement, a difference of 2 to 3 points as moderate clinical improvement, a difference of 1 point as mild clinical improvement, and a difference <1 point as absence of clinical improvement. The level of satisfaction with intravaginal prasterone at 30-45 days was determined using a Likert type scale to rate these answers from 1 to 6, where 1 was completely satisfied and 6 completely unsatisfied. In addition, the incidence and severity of side effects during the study period were also assessed. At baseline, biodemographic data (age, weight, height, being in a stable relationship), medical history (smoking, diabetes, thyroid disease), obstetric/gynecologic history (breast cancer, time since menopause, vaginal birth, instrumental birth, cesarean, episiotomy), and baseline treatments (vaginal hormonal therapy, vaginal moisturizer/lubricants, oral contraceptive, antidepressant treatment, antihistamine drugs, anti-estrogen therapy, hormone replacement therapy, retinoids, chemotherapy, radiotherapy) were recorded. In addition, the FSFI and VAS questionnaires were completed by the patients. A gynecological examination was performed during a routine visit to confirm the presence of genitourinary syndrome, including the determination of vaginal pH and the level of tropism in routine cytology, if necessary. The follow-up visit was at 30±7 days after inclusion. The patient’s medical history, obstetric/gynecologic history, and treatments were re-evaluated. The FSFI and the VAS questionnaires were completed by the patients. The presence of side effects and satisfaction with treatment during the study period were also evaluated.
Statistical Analysis
Categorical variables were expressed as absolute frequency (n) and relative frequency (%). Continuous variables were expressed as mean and standard deviation. Categorical variables were compared using the chi-square test or the Fisher exact test when appropriate. When two means were compared, the t test or the Mann-Whitney test was used, as applicable. The changes in the scores of the FSFI and VAS questionnaires during follow-up were evaluated in the overall study population and in the subgroup of patients on vaginal moisturizer/lubricants or on vaginal hormonal therapy. Missing data or lost values were not imputed to avoid information bias. Missing data for important variables were controlled for using filters when collecting data from the case report form. Statistical significance was set at P<0.05. Data were analyzed using the statistical package SPSS (v24.0 or higher).
Results
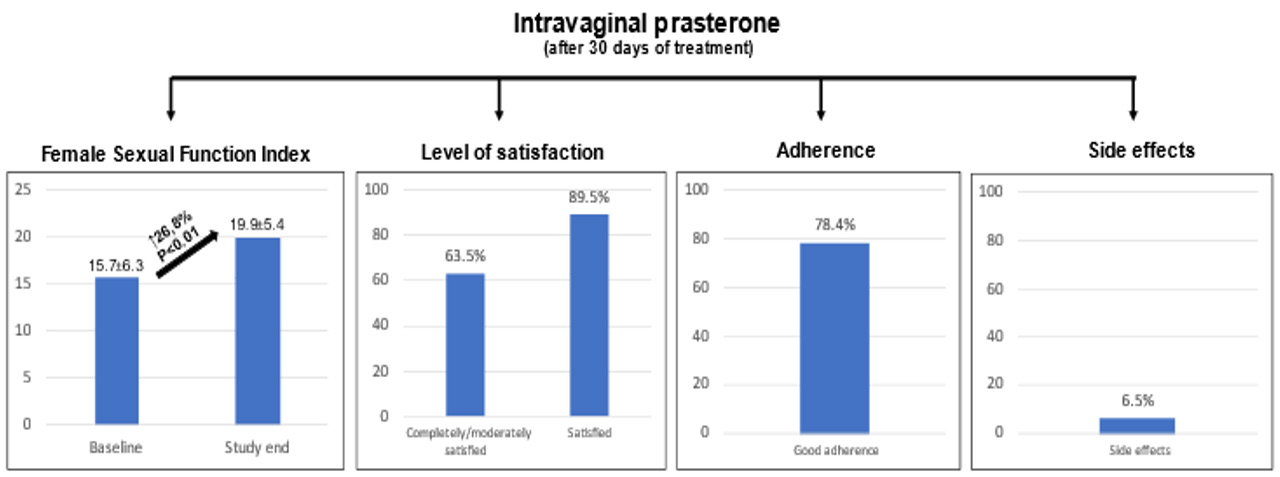
The study population comprised 184 postmenopausal women with genitourinary syndrome treated with intravaginal prasterone at various centers throughout Spain. Mean age was 57.98±6.06 years, mean time since menopause was 7.56±6.23 years, 6.1% were smokers, and 6.1% had diabetes. In addition, 56.1% had had at least one previous delivery, 65.2% had had an episiotomy, and 21.6% had had a cesarean delivery. With regard to treatment, 42.9% of patients were taking vaginal hormonal therapy and 32.1% vaginal moisturizer/lubricants. Except for less frequent use of vaginal hormonal therapy among patients taking vaginal moisturizer/lubricants (27.1% vs 42.9%; P=0.02), no significant differences were observed in the baseline clinical characteristics of this subgroup of patients compared with the overall study population. The FSFI was lower in the subgroup of patients on vaginal hormonal therapy (13.63±5.77 vs 15.72±6.33; P=0.01), as was the proportion of patients taking oral contraceptives (8.3% vs 24.2%; P=0.02) and antihistamine drugs (8.3% vs 21.2%; P=0.02). In addition, a trend towards reduced use of vaginal moisturizer/ lubricants (20.3% vs 32.1%; P=0.05) compared with the overall study population was also observed for this group, with no other significant differences between the groups (Supplementary Table 1). Table 1 shows changes in FSFI during the study period in the overall study population and in patients on vaginal moisturizer/lubricants and vaginal hormonal therapy. In the overall study population, the FSFI increased from 15.7±6.3 to 19.9±5.38 (mean difference, 4.2; P<0.01), leading to a marked improvement, which was observed for all items, albeit with variable intensity. In the subgroup of patients on vaginal moisturizer/lubricants, the FSFI rose from 17.55±6.89 to 21.52±6.09 (mean difference, 3.97; P<0.01), and in the subgroup of patients receiving vaginal hormonal therapy, the FSFI rose from 13.63±5.77 to 19.70±4.40 (mean difference 6.07; P<0.01). Table 2 shows changes in the VAS score during the study period in the overall study population and in patients on vaginal moisturizer/ lubricants and vaginal hormonal therapy.
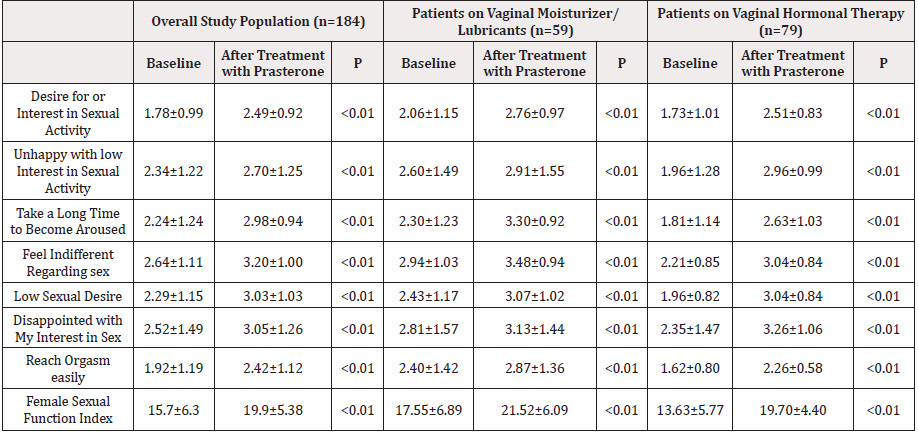
Table 1: Female Sexual Function Index at baseline and after treatment with progesterone in the overall study population and in patients on vaginal moisturizer/lubricants and vaginal hormonal therapy.
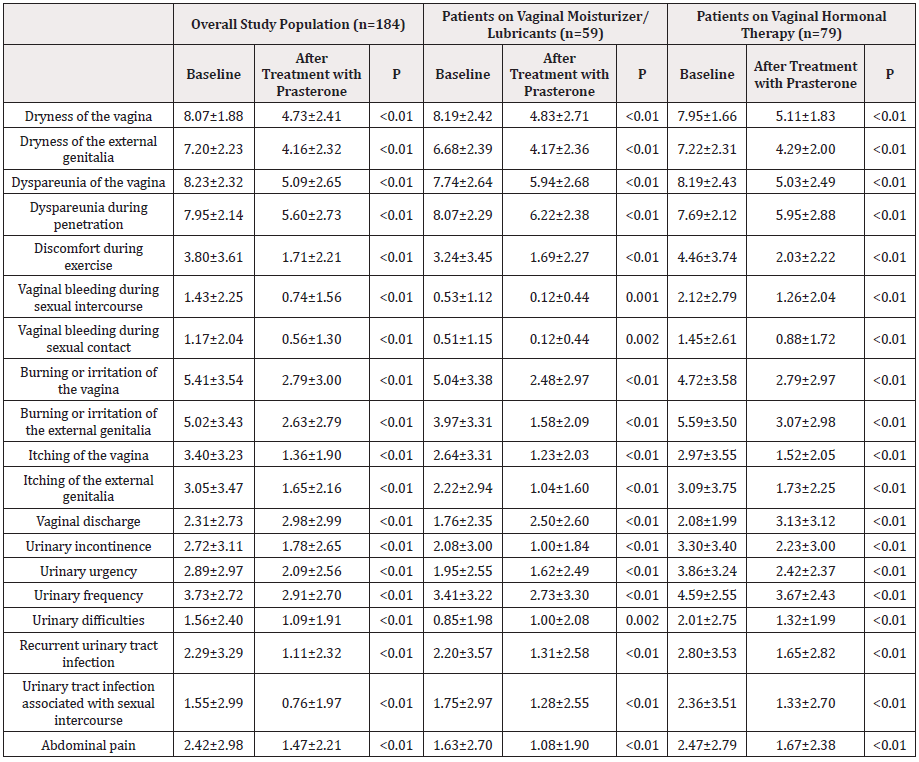
Table 2: Satisfaction, side effects, and adherence during treatment with prasterone in the overall study population.
In the overall study population, the only item that worsened was vaginal discharge, in which the score increased slightly but significantly (2.31±2.73 to 2.98±2.99, P<0.01). The score for the remaining 18 items improved significantly, with different levels of intensity, depending on the item. Among patients on vaginal moisturizer/lubricants, a significant decrease (improvement) was observed in 17 items during the study period, with different levels of intensity, depending on the item. The only items in which the score increased (worsened) were vaginal discharge and urinary difficulties (1.76±2.35 to 2.50±2.60 [P<0.01] and 0.85±1.98 to 1.00±2.08 [P=0.002], respectively). Among patients on vaginal hormonal therapy, scores decreased significantly for all items, with different levels of intensity depending on the item, except for vaginal discharge, in which there was a significant increase (worsening), from 2.08±1.99 to 3.13±3.12 (P<0.01). The level of satisfaction with intravaginal prasterone was high in the overall study population (completely/moderately satisfied, 63.5%; completely/moderately/ slightly satisfied, 89.5%), as well as in the subgroup of patients on vaginal moisturizer/lubricants (54.5% and 90.9%, respectively) and in the subgroup of patients on vaginal hormonal therapy (62.9% and 87.0%, respectively). Satisfaction was higher in the subgroup of patients on vaginal moisturizer/lubricants than in the overall study population (P=0.032). In addition, although intravaginal prasterone was safe (only 6.5% of patients reported side effects during follow-up), adverse events were more common in the subgroup of patients on vaginal moisturizer/lubricants (16.9% vs 6.5%; P=0.02). Similarly, adherence to treatment was high during follow-up, as 78.4% of patients remained on prasterone at the end of the study, with no significant differences between the groups. Side effects were the main reason for withdrawal (Supplementary Table 2 & Figure 1).
Discussion
This study of a wide sample of postmenopausal women with genitourinary syndrome showed that in clinical practice, treatment with intravaginal prasterone was associated with significant improvements in sexual function and in most symptoms of genitourinary syndrome after only one month of treatment. In addition, the tolerability and safety profile was excellent. Our study population comprised almost 200 postmenopausal women with genitourinary syndrome from medical centers throughout Spain. Mean age was 58 years, mean time since menopause was 7.5 years, 43% of patients were taking vaginal hormonal therapy, and 32% vaginal moisturizer/lubricants at baseline. In addition, the clinical profile of patients did not differ greatly according to whether they were using vaginal hormonal therapy or vaginal moisturizer/lubricants. MUMENESP, a cross-sectional study of Spanish postmenopausal women seen in a routine clinical setting, showed that approximately 54% of patients had vaginal dryness, 55% dyspareunia, and one third were taking systemic hormonal therapy or phytotherapy at some time in their lives [21]. As a result, the clinical profile of the patients included in our study may be representative of postmenopausal women seen in clinical practice in Spain.
Genitourinary syndrome of menopause is a progressive condition that has a negative impact on women’s quality of life [22]. Of note, despite current treatments, many symptomatic women are disappointed with the available therapeutic options [23]. The management of genitourinary syndrome should be individualized and should consider not only formulation, route of administration, and timing of therapy, but also efficacy and safety [12]. Various clinical trials have shown that, compared with placebo, intravaginal prasterone is effective in the treatment of dyspareunia secondary to vulvovaginal atrophy in women with genitourinary syndrome of menopause and may be also effective among women with other symptoms of genitourinary syndrome, such as decreased lubrication, decreased sexual desire, decreased sexual satisfaction, impaired ability to achieve orgasm and pain [17,22]. Thus, in a 12-week placebo-controlled clinical trial analyzing intravaginal administration of DHEA, pain during sexual activity decreased by 1.42 severity score units from baseline, and moderate to severe vaginal dryness improved by 1.44 severity score units. In addition, gynecological evaluation revealed that vaginal secretions, epithelial integrity, and epithelial surface thickness had also improved, with no increase in serum steroid levels [22].
A systematic review of 14 clinical trials assessing the efficacy of vaginal DHEA in women with vulvovaginal atrophy showed that sexual dysfunction improved with treatment regardless of the level of dyspareunia at baseline [23]. Our data not only confirmed that the results of clinical trials can be translated into clinical practice in Spain, but also provided valuable information about the use of prasterone in real-life conditions after one month of treatment [22]. Overall, there was an increase of 4 points in the FSFI, which represented an intense improvement according to the protocol. This improvement was recorded for all items of the FSFI, indicating that the positive effects of prasterone were consistent in all aspects of sexual function. In addition, the positive impact of prasterone was observed not only in the overall study population, but also in the subgroup of patients on vaginal moisturizer/lubricants, and, more intensely, in those women on vaginal hormonal therapy at baseline, suggesting that the double effect of prasterone on both androgens and estrogens may be more comprehensive and potent than local estrogen-based therapy. However, this possibility should be analyzed in a specific study. Clinical trials have also shown that treatment with prasterone improves many symptoms of genitourinary syndrome of menopause, such as vaginal dryness and vulvovag¬inal irritation or itching [13,14]. Our study showed that in clinical practice, intravaginal prasterone improved the vast majority of symptoms associated with genitourinary syndrome, such as vaginal dryness or dryness of the external genital, burning, itching, dyspareunia, vaginal bleeding, and urinary symptoms. This also occurred in patients on vaginal moisturizer/lubricants or vaginal hormonal therapy at baseline. With regard to side effects, various clinical trials have shown that the most common adverse event was discharge at the application site, with less than 1% of patients discontinuing treatment owing to this side effect [24-26]. Other adverse events reported in clinical trials with prasterone include urinary tract infections, weight fluctuations, vaginal discharge, and sinusitis [13,14, 21-24]. Our study showed that in clinical practice, treatment with intravaginal prasterone was safe, with only 6.5% of patients exhibiting side effects during followup (i.e.blisters on the face, hair loss, constipation, leukorrhea, and dizziness). In addition, no systemic effects are expected with DHEA therapy, as intravaginal prasterone is used locally, and the drug does not pass to the bloodstream [13,14]. In our study, the favorable safety and efficacy profile led to a high adherence rate in clinical practice, thus confirming the results of clinical trials [21].
In contrast to other therapies that are underutilized, mainly owing to concerns about potential side effects or low efficacy [15] our study showed that in real-life patients, the use of intravaginal prasterone was associated with high levels of satisfaction, suggesting that it can be considered a first-line choice for the treatment of genitourinary syndrome of menopause in clinical practice. This study is subject to a series of limitations. First, the lack of control group reduced the possibility of reaching definitive conclusions about the efficacy of intravaginal prasterone in clinical practice. However, the prospective design of the study, as well as the meticulous data collection, may reduce this potential bias. In addition, our data were consistent with those obtained from clinical trials. As our study was performed in Spain and the therapeutic approach of genitourinary syndrome may be country-specific, our results can only be extended to those populations with a similar clinical profile, cultural breakdown, and health care system. In conclusion, in postmenopausal women with genitourinary syndrome treated in Spain, intravaginal prasterone was associated with significant improvements in sexual and urinary symptoms, as well as with reduced genital dryness, burning, irritation, and pain. Furthermore, the drug was well tolerated, with high rates of satisfaction, after only one month of treatment. Therefore, intravaginal prasterone is an effective and safe alternative and should be considered a firstline therapeutic choice for the management of postmenopausal women with genitourinary syndrome in clinical practice.
Contributors
All authors contributed extensively to the work presented in this paper. All authors have contributed significantly to the conception, design, or acquisition of data, or analysis and interpretation of data. All authors have participated in in drafting, reviewing and/or revising the manuscript and have approved its submission.
Competing Interest
Authors declare no conflict of interest.
Funding
The authors received no funding from an external source.
References
- Keskinen R, Ekholm P, Yli-Halla M, Hartikainen H (2009) Efficiency of different methods in extracting selenium from agricultural soils of Finland. Geoderma 153(1-2): 87-93.
- Sippola J (1979) Selenium content of soil and timothy (Phleum pratense) in Finland. Annales Agriculturae Fenniae 18: 182-187.
- Yläranta T (1983) Selenium in Finnish Agricultural sois. Ann Agr Fenniae 22: 122-136.
- Yli-Halla M (2005) Influence of selenium fertilization on soil selenium status. Agrifood Research Reports 69. Proceedings Twenty Years of Selenium Fertilization
- Koljonen T (1973) Selenium in certain metamorphic rocks. Bulletin of the Geological Society of Finland 45(2): 107-117.
- Biotite.
- Boyle JR, Voigt GK, Sawhney BL (1974) Chemical weathering of biotite by organic acids. Soil Science. The Williams & Wilkins Co 117(1): 42-45.
- Yläranta T (1980) Farmland Selenium Plant Selenium. Bulletin No. 11, Agricultural Research Institute. Earth Research Institute. Helsinki 1980.
- Wang D, Sippola J (1990) Selenium in soil extracts and plants determined by fluorometry. Annales Agriculturae Fenniae 29(2): 51-156.
- Yläranta T (1982) Volatilization and leaching of selenium added to soils. Annales Agricultirae Fenniae 21:103-114.
- Olson 0E, Cary EE, Allaway WH (1976) Fixation and volatilization by soils of selenium from trimethylselenonium. [Agron J 68: 839-843. in Yläranta T (1982) Volatilization and leaching of selenium added to soils. Annales Agricultirae Fenniae, 21: 103-114.
- Wen H, Carignan J (2007) Reviews on atmospheric selenium: Emissions, speciation and fate. Atmospheric Environment 41 (34): 7151-7165.
- Wu X, Låg J (1988) Selenium in Norwegian Farmland Soils. Acta Agriculturae Scandinavica, Vol 38(3): 271-276.
- Lahermo P, Tarvainen T, Hatakka T, Backman B, Juntunen R, et al. (2002) A thousand wells - the physico-chemical quality of Finnish well waters in 1999. Summary: One thousand Wells - the physical-chemical quality of Finnish well waters in 1999. Geological Survey of Finland, Research Report - Geological Survey of Finland, Report of Investigation 155. (Permission for benefiting map ‘Regional distribution of oxygen isotope composition (δ18O ‰) of water from ring wells and boreholes, Fig.56’, is p. 76, in GTK / 332 / 11.0 3.02 / 2020 (Nov 17, 2020).
- Hartikainen H (2005) Occurence and chemistry of selenium in Finnish soils on p. 22 in Agrifood Research Reports 69. In: Eurola M (Edt.). Proceedings Twenty Years of Selenium Fertilization, Helsinki, Finland.
- Kurki M (1982) On the fertility of Finnish fields III. Viljavuuspalvelu Oy. Helsinki 1982. Pariset Oy ISBN 951-99399-0-3: 181.
- Koivunen Kalevi, Eurofins Viljavuuspalvelu oy (personal communication)
- Official Statistics of Finland: Farm Register [printed product]. ISSN = 0784-8404. 1988. Agriculture and forestry 1990:2. Table 2.7.: Land use on farms by Agricultural Advisory Centre according to municipality 31.12.1988 (“Maatilojen maankäyttölajit maatalouskeskuksittain ja kunnittain 31.12.1988”). Helsinki: National Board of Agriculture".
- Jones A (2014) Soil Atlas of Europe, European Soil Bureau Network, European Commission, 2005, 128.
- Tikkanen M, Oksanen J (2002) Late Weichselian and Holocene shore displacement history of the Baltic Sea in Finland. Fennia, International Journal of Geography 180: 1-2.
- Töysä T, Associations of Humus Content and pH of Mineral Soilswith Silicate Weathering Factors and Carbon Capture.| Biomed J Sci & Tech Res | BJSTR. MS.ID.005655.
- Yläranta T (1990) The selenium content of some agricultural crops and soil before and after the addition of selenium to fertilizers in Finland. Annales Agriculturae Fenniae 29(2): 131-139.
- Eurofins, personal communication.
- Mäkelä-Kurtto R, Sippola J (2002) Monitoring of Finnish arable land: changes in soil quality between 1987 and 1998. Agricultural and Food Science in Finland 11 (4): 273-284.
- Yläranta T (1983.b) Effect of added selenite and selenate on the selenium content of italian ryegrass (Lolium multiflorum) in different soils. Annales Agriculturae Fenniae 22(3): 139-151.
- Wang D, Alfthan G, Aro A, Soveri J (1993) Anthropogenic emissions of Se in Finland. Applied geochemistry 8: 87-93.
- Backman B (2004) Geological Survey of Finland - Bulletin 401. Groundwater quality, acidification and recovery trends between 1969 and 2002 in South Finland. Geologian Tutkimuskeskus. Geological Survey of Finland. Espoo 2004. ISBN 951-690-895-0. ISSN 0367-522X. Vammalan Kirjapaino Oy 2004.
- Larsen EH, Lobinski R, Burger-Meÿer K, Hansen M, Ruzik R, et al. (2006) Uptake and speciation of selenium in garlic cultivated in soil amended with symbiotic fungi (mycorrhiza) and selenate. Anal Bioanal Chem 385(6): 1098-108.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.