Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Evaluation of Hepatotoxicity of the Extract of Green Walnut Husks (Juglans regia L.) in Mice by a Metabonomic Approach
*Corresponding author: XiaoMing Sun, School of Clinical Pharmacy (School of Integrative Pharmacy, Institute of Integrative Pharmaceutical Research), Guangdong Pharmaceutical University, Guangzhou, 510006, China.
Received: April 01, 2020; Published: April 16, 2021
DOI: 10.34297/AJBSR.2021.12.001765
Abstract
This work was designed to delineate the comprehensive metabolic changes of hepatotoxicity in mice induced by the extract of green walnut husks (Juglans regia L.) (GWHs). A metabonomic strategy based on high performance liquid chromatography (HPLC) is performed to characterize the metabolic profiling of mice feeding with GWHs extracts at a single dose of 40 mg/kg for ten consecutive days. Investigations on stability and precision of the established metabolic profiles indicated that the method was well controlled and reliable. Acquired chromatographic data sets were subjected to principal component analysis (PCA) and orthogonal partial least square-discriminant analysis (PLS-DA) for differentiating the feeding and the control mice. Six metabolites which have significant contributions to the classification were selected by the variable importance for the projection value. The results indicated that energy metabolism was decelerated, while the creatinine level in serum was significantly decreased. Histopathological slices of liver feeding with GWHs extract showed obviously vacuolization and dilation in the cell sap. We believe that metabonomic approach based on chromatography is helpful to further reveal the toxicity of some medicine.
Introduction
The green walnut husks (Juglans regia L.) are not only the byproduct of walnut production but also a kind of herbal medicine in Chinese for relieving inflammation, clearing away heat and inhibiting cancer [1,2]. Phytochemistry researchers have proved that the naphthoquinones, tetralone, flavonoids, and diarylheptanoids compounds from the Juglans and other natural products have exhibited a broad range of potent biological activities [3-6]. Besides that, the English walnut listed in the FDA Poisonous Plant Database (U.S. Food and Drug Administration) in the year 2000 was reported to be able to cause hyperpigmentation and dermatitis [7,8].
In toxicology study, much time and labor force will be consumed due to a limited number of biochemical indicators are available in general toxicologic evaluation. When the target protein and/or organ responses for toxic syndromes are not clear, some specific analysis cannot be performed. In addition, it has not clarified that the changing discipline of toxic materials, and the regular pattern of endogenous metabolites from the body biochemical process induced by natural products on the toxicity were still not clear [9]. Therefore, this study was designed to investigate the biochemical changes induced by the hepatotoxicity of GWHs through the metabonomics technique.
Metabolic profiling, a branch of metabonomics, has provided an opportunity to obtain new insights in the toxicity by a biochemically based system toxicology approach [10]. Due to the high sensitivity, high throughput, good repeatability and reproducible, nuclear magnetic resonance spectroscopy and/or hyphenated gas chromatography/liquid chromatography-mass spectrometry technologies have been widely used in establishing metabolic profile [11]. Metabolic profiling contains a mass of information concerning the interactions of organisms and natural products and offer promise for identifying early biomarkers that are specific indicators of damage to an organism [12]. In addition, high performance liquid chromatography (HPLC) is generally available to many labs and is of a relative high throughput and selectivity, which also makes it attractive for blood plasma profiling in the primary research [13,14].
The complex data sets obtained in metabonomics are difficult to summarize and interpret without appropriate statistical and visualization tools. The chemometric tools, such as principal component analysis (PCA) [15], partial least squares to latent structures (PLS) [16], and orthogonal PLS (OPLS) [17] are therefore of great importance as they provide efficient and robust methods for modeling, analysis, and interpretation of complex chemical and biological data. OPLS discriminant analysis (OPLSDA) is an efficient approach for modeling of two or more classes, which can handle both the contribution and the correlation to the OPLS model. The present investigation was conducted with the objective of investigating the biochemical changes in mice plasma of GWHs induced hepatotoxicity by HPLC technique coupled with multivariate statistical methods.
Materials and Methods
Chemicals and Herbal materials
Uric acid and cytidine were purchased from Sigma-Aldrich Co. LLC. (USA). Guanosine, adenosine and creatinine were purchased from Chengdu West Chemical Co., Ltd. (China). Methanol and acetonitrile (HPLC grade) were obtained from Shandong Yuwang Industry Co., Ltd. (China). The ehanol (EtOH) and acetic ether (EtOAc) were of technical grade. Ammonium acetate and acetic acid (A.R. grade) was purchased from Sinopharm Chemical Reagent Co. Ltd. (China). The purified water was filtered through 0.22 μm membrane filter before use. Macroporous adsorption resin AB-8 was purchased from Xi’an Sunresin Technology Co., Ltd. (China). Dried green walnut husks (J regia L.) were collected in south Gansu province, China.
Preparation and Characterization of the GWHs Extracts
Dried green walnut husks were extracted for three times with 95% hot EtOH for 6 hours with reflux. Then, the EtOH solution was evaporated to dryness under 0.07 MPa at 50oC. The dry EtOH extract was suspended in H2O and the solution was extracted with EtOAc for five times. Then, the EtOAc solution was evaporated to dryness under 0.07 MPa at 50 oC. The fraction A, B and C from the EtOAc extract were prepared by eluting with elution A (EtOH : H2O = 45:55, v/v), elution B (EtOH : H2O = 60:40, v/v) and elution C (EtOH : H2O = 95:5, v/v) on the macroporous adsorption resin (MAR AB-8). The three fractions were evaporated to dryness under 0.07 MPa at 50 oC, respectively. The powders of EtOAc extract and the three fractions (4 mg/mL) were re-dissolved in 3% tween-80 aqueous solution as the tested drugs, respectively.
Chromatography was performed on an Agilent 1200 series LC system, equipped with a G1311A quaternary pump, a G1315D diode array detector performing the wavelength scanning from 190 to 400 nm, and a G1328B manual injector. The chromatographic separation of analytes were performed on a Sinochrom ODS-BP C18 (Dalian Elite Analytical Instruments Co., Dalian, China) analytical column (250 mm × 4.6 mm i.d., 5 μm). The sample injection volume was 20 μL and the column temperature was at 25°C.
The reference compounds of 1. Juglanin A (JA), 2. Juglanin B (JB), 3. Regiolone (RE) and 4. Sderone (SD) were extracted, isolated and purified from the green walnut husks in our laboratory. The purities of four compounds were determined to be more than 98% by normalization of the peak areas detected by HPLC-DAD methods. The four reference compounds were identified by various spectroscopic methods including intensive 2D NMR and HR-ESI-MS analysis [18,19].
Animal experiments
Male Kunming mice (20.0±2.0 g) purchased from Guangdong Medical Laboratory Animal Center. The animals were housed in cages with wood chip bedding in an animal room with a 12 h lightdark cycle at room temperature (24±2°C) and allowed free access to standard laboratory diet. Forty healthy male mice were divided into four groups. The control group (CG) had been intragastric administrated (i.g.) by 0.9% saline solution for successive 10 days (n = 10). The other three groups had been i.g. fraction A, B and C (group 1, 2 and 3) at dosage of 40 mg/kg for successive 10 days (n = 10), respectively. During the research, the abnormal behaviors, skin and hairs, and the assumption of water and food of mice were observed and recorded.
Preparation of the Plasma samples
The mice administrated with fraction A, B and C were sacrificed on the 11th day. Anticoagulative (Heparin Sodium) blood samples were collected via eyeballs veniplex. After centrifuging, the supernatant was separated and stored at -20oC until pretreatment. Acetonitrile (900 μL) were added to 300 μL plasma sample. Then, the mixture was vortex-mixed for 30s and centrifuged (1,369 × g, 5 min). The supernatants (1 mL) were transferred to Eppendorf tubes and vacuum dehydrated at 37oC for 6 hours. The samples from each group were reconstituted in 130 μL water and were filtered through 0.22 μm millipore filter before injection. All the samples were kept at 4°C during the experiment.
Metabolic profiling analysis of plasma samples
The HPLC-DAD instrument and analytical column for metabolic profiling were the same as in the analysis of the extracts. The sample injection volume was 20 μL and the column was maintained at 25°C. The gradient elution buffers were A (25 mmol/L, pH 4.6, ammonium acetate) and B (methanol), and the flow rate was at 1 ml/min. Gradient consisted that 0.7% (B) in 0-8 min, 0.7-6.3% (B) in 8-18 min, 6.3-28.3% (B) in 18-40 min, and 28.3-64.3% (B) in 40- 60 min. Then B returned to 0.7% for 15 min before next injection. The wavelength of DAD detector was 260 nm.
Data preprocessing and statistical analysis
The peaks were identified by the retention time (tR) and peak areas were integrated by Agilent Chemstation software (version A. 10.02). Because the resolution and integration results of some peaks were not reliable and reproducible, the chromatographic data during the first three minutes were excluded. After peak matching, 27 common peaks were determined and listed as an n × p data set, where n indicates the number of observation and p indicates the number of variables. The data analysis was performed by SIMCA-P+ software version 12.0 (Umetrics AB, Sweden).
In a metabolic fingerprint analysis, the signal response of a metabolite depends on both the concentration and its detection sensitivity. Generally, it is not the absolute concentration change but the relative concentration change of a metabolite that is crucial for SVs discovery. Unit variance (UV) scaling [20] is commonly applied and uses the standard deviation as the scaling factor. All variables have a standard deviation of one and therefore the data is analyzed on the basis of correlations instead of covariance. The UV scaling is expressed as:

where  represents the data after UV scaling, n is the sample
number.
represents the data after UV scaling, n is the sample
number.
PCA was used for dimensionality reduction and cross validation is used to decide how many principle components (PCs) will reproduce the data with “sufficient accuracy”. In addition, groupings, trends and outliers of the data can be readily found by projecting the raw data onto the first 2 or 3 PCs. OPLS-DA was introduced as an improvement of the PLS-DA method to discriminate two or more groups using multivariate data [21]. The advantage of OPLSDA is that single component is used as a predictor for the class, while the other components describe the variation orthogonal to the first predictive component [22]. Cross validation [23] was used to calculate the number of significant PCs. The first M significant components can be used to modeling the elements of X by the formula as:

where the singular value decomposition of the matrix X is defined
by  P is the number of variables and
P is the number of variables and  is a residual term.
is a residual term.
To ensure the robustness to perturbations of the model, crossvalidation predicted variance (Q2) [24] is the commonly used criterion, which is defined as:

where  are the measured, predicted and averaged
(over the entire data set) values of the response variables,
respectively; n is the size of training set.
are the measured, predicted and averaged
(over the entire data set) values of the response variables,
respectively; n is the size of training set.
SVs were selected based on the value of Variable Importance in the Project (VIP) and loading plots, which reflect the importance of the variables in the model. VIP is the sum over all model dimensions of the variable influences. W*C loading plot show both the x-weights (w or w*) and y-weights (c), and thereby the correlation structure between x and y. The formula is expressed as:

where VIP is the sum over all model dimensions of the variable influence, SSY is the sum of squares of the Y matrix and the summation is made over a = 1 to a = A.
Histopathology
After fixed in 10% formalin for 12 h and embedded in paraffin, the liver samples were processed to small sections. Tissue sections were subsequently stained with hematoxylin-eosin (H–E) for light microscope examination.
Results and Discussion
Characterization of the GWHs extracts
The GWHs extracts exhibited both anti-tumor activity and toxicity. Further isolated by eluting from MAR AB-8 was performed to try to find some specific compounds related to toxicity. The four fractions were analyzed at the same level. It can be observed from Figure 1 that three fractions were different in composition and content. Fraction A and B contain compounds JA, JB, RE and SD and other unknown compounds, while fraction D does not contain the four compounds. It can be concluded that the biological activity of four fractions were different.
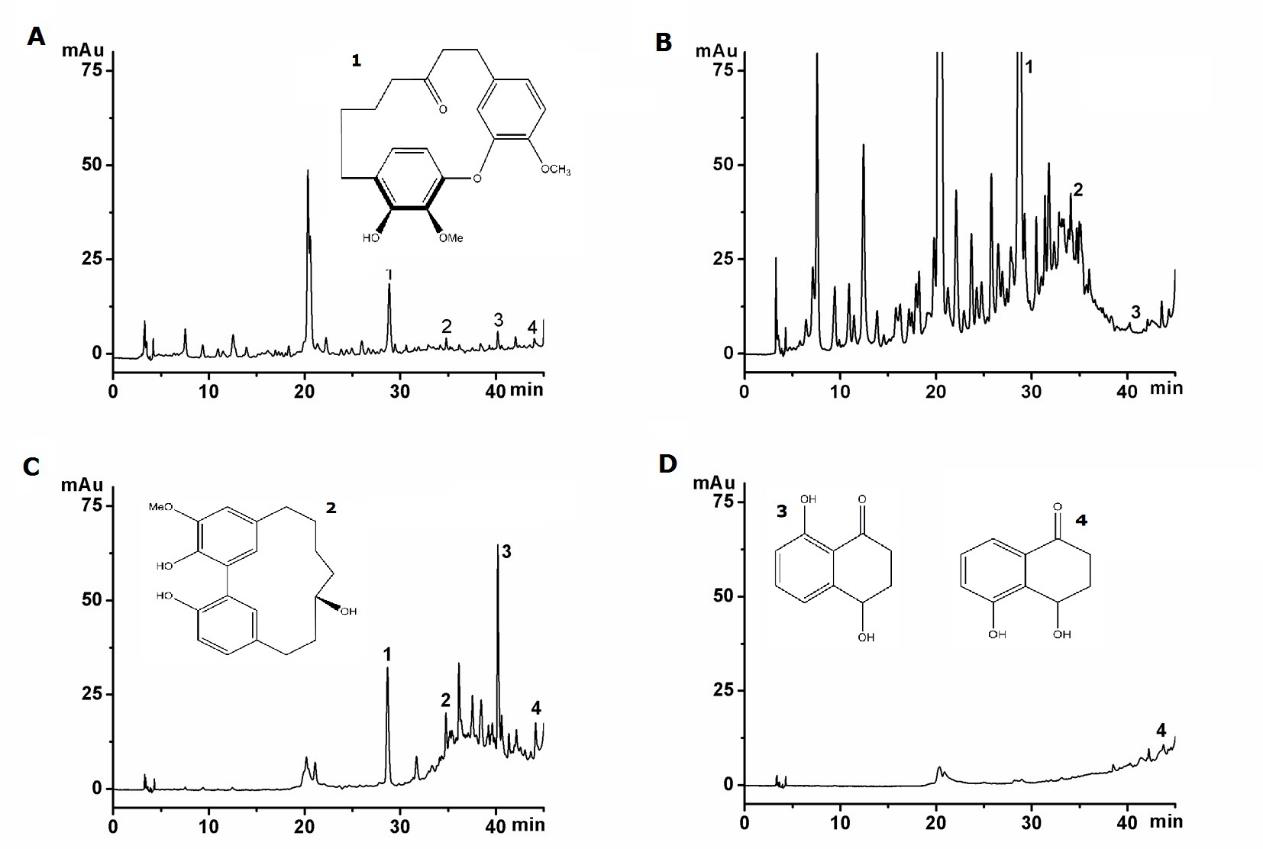
Figure 1: The chromatograms of GWHs extracts. (A) EtOAc extract, (B) extract A, (C) extract B, (D) extract C. The structures of active compounds. (1) Juglanin A, (2) Juglanin B, (3) Regiolone, (4) Sderone.
The gradient elution buffers were A (water and 2% acetic acid) and B (acetonitrile), and the flow rate was at 1 mL/min. Gradient consisted that 0 to 25 min, 5 - 25% B; 25 - 34 min, 25 - 50% B; 34 - 44 min, 50 - 70% B; 44-50 min, 70% B; 50 - 55 min, 70 - 5% B. Then B returned to 5% for 15 min before the next injection. The wavelength of DAD detector was 280 nm.
Histopathology Detection
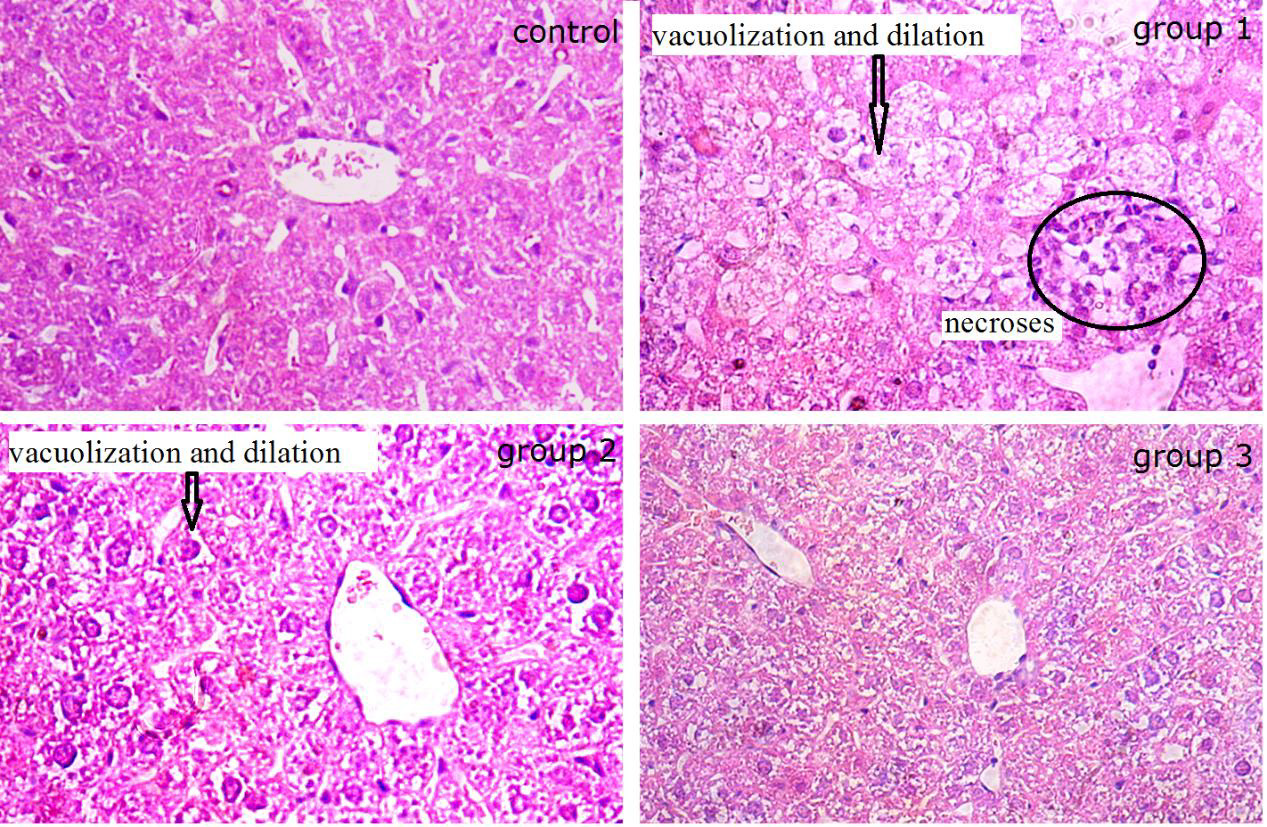
Liver is one of the most vital organs in the organism. It plays important role in the process of drug metabolism. While the GWHs were reported to be toxicity, some histopathology detections of livers of mice administrated with the fractions (A, B and C) were examined. As shown in Figure 2, the liver sections of the healthy mice showed apparently normal structure in liver cells and hepatic cord, and the liver sections of administrated mice revealed a nearly regular morphology of liver cells and hepatic cord in group 3. However, the liver cells from administrated mice showed significant disorder of hepatic cord and narrow of hepatic sinusoid in group 1. At the same time, obvious vacuolization and dilation in the cell sap were observed in liver cells in group 2. Several necroses infiltrated by neutrophilic granulocyte, lymphocytes and mononuclear cell were observed in hepatic lobule. Combined with the pharmacology results, we concluded that the presence of substantial liver damage after the administration of fraction A and B could be confirmed.
Influence of the Extracts on Mice Metabolism
To investigate whether the extracts have some influence on the metabolism of mice, some observations were performed. The results showed that two mice in the group 1 died in the 5th h and 72th h after the i.g. administration, respectively. After dissecting, it was observed that two mice died not from an operation mistake. As shown in Figure 3, the consumption of food and water in group 2 are higher than that of control. However, all the fractions have little influence on the increase of body weight compared with control. In addition, these mice had no significant abnormal behavior and physical signs in the cardiovascular, gastrointestinal, urogenital systems during the research.
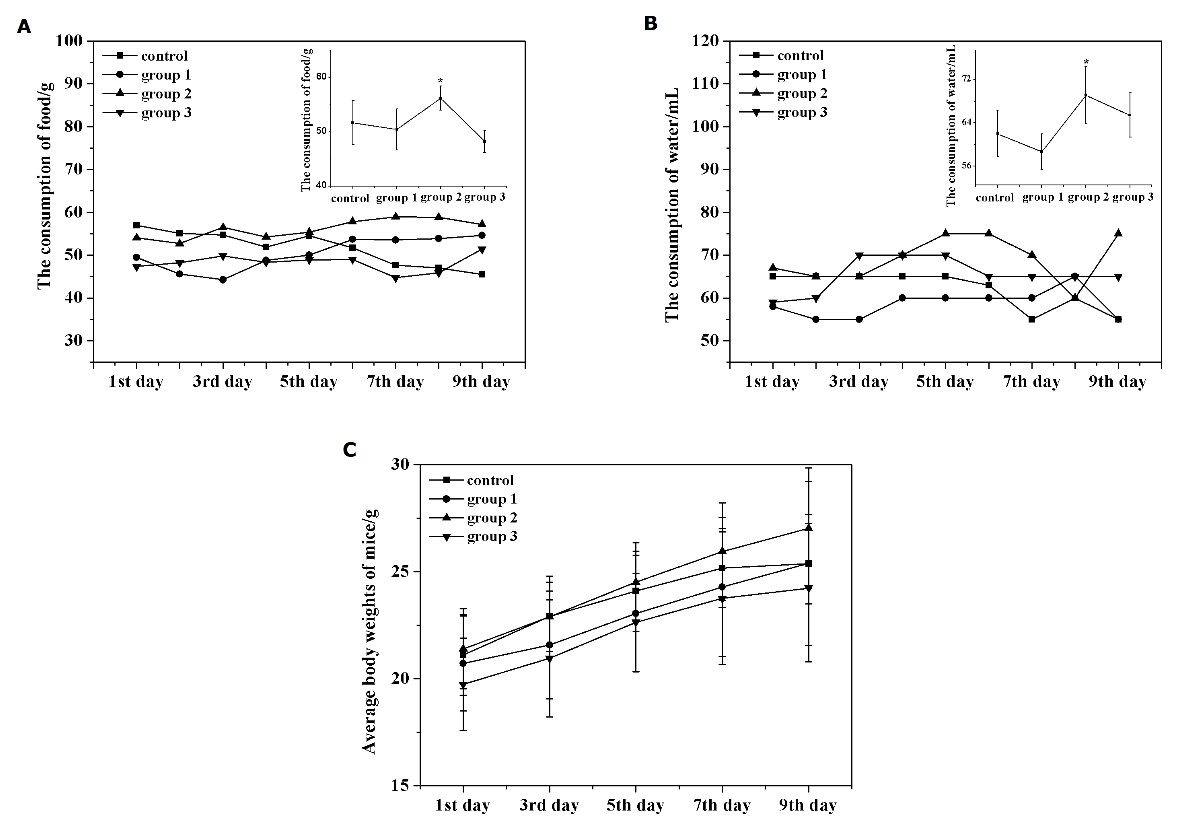
Figure 3: (A) The consumption of food in control and dosed mice. (B) The consumption of water in control and dosed mice. (C) The change of body weight in control and dosed mice.
Biochemical changes of Mice Plasma induced by Extracts
Generally, the toxic effect cannot be detected only by observing the behavior of mice. Besides, the current clinical chemistry markers of toxicity are not necessarily the most sensitive and specific indicators to reflect the progression of the organism damage, especially in the situation of unknowing about the toxic targets. Therefore, metabolic profiling and multivariate statistical analysis may lead us to investigate the biochemical changes induced by the GWHs extracts. Twenty-seven common peaks were matched and used for multivariate statistical analysis. The analytical precisions of the four endogenous compounds (Creatinine, Uric acid, Guanosine, and Adenosine) from the data of intra-daily (six times per day) and inter-daily (twice a day for three consecutive days) determinations are indicated by the relative standard deviations (R.S.D.) for intra-daily and inter-daily determinations are 1.30- 2.77% and 1.95-2.77%, respectively. The stability of the plasma samples, and the R.S.D. values of the peak areas and retention times were 2.67-9.80% and 0.33-3.46% during three consecutive days (n = 6). These results indicate that the conditions for separation and analysis are accurate and reproducible.
HPLC-DAD was used to analyze blood plasma samples of the control and treated groups, and the data of common peaks were used in the PCA and OPLS-DA analysis. In multivariate statistical analysis, the parameters of R2X and R2Y are used to display the fraction of the sum of squares for the selected component. R2X(cum) and R2Y(cum) are the cumulating contribution rate of the selected component of the fraction of explained X-variation and Y-variation, respectively. PCA is a multivariate projection method designed to extract and display the systematic variation in a data set. A scatter plot of the score values provides an overview of the sample grouping and trends. Figure 4 (a) shows the resulting PCA score plot (t[1] vs. t[2]) of the four groups and the contribution rate R2X(cum) of the first two PCs in PCA is 0.422. The goal in metabonomics is to distinguish classes of samples and identify the differences, but the four groups showed an overlapped clustering and could not be discriminated completely by the first two components. To obtain improved model transparency and interpretability, the supervised multivariate projection method OPLS-DA was applied to highlight differences between the control and treated groups. Figure 4 (b) is the score plot which clearly shows the overall distribution of the four groups. The classification of the four groups resulted in one predictive and one orthogonal (1+1) components with the crossvalidated predictive ability Q2(Y) was 0.163 based on 7-fold cross validation. A value of 0.355 of the variance R2(X) is used to account for 0.291 of the variance R2(Y) and the variance related to class separation R2p(X) was 0.19.
To identify SVs, the VIP values and loading plot were used. The preferred selection of metabolites has a high covariance combined with a high correlation and a small confidence interval. Figure 4 (c) shows the loadings where each point represents one peak area/ retention time pair. The loading plot gives an indication of the metabolites that most strongly influence the patterns in the score plot. Six variables with relatively high covariance and correlation regions were selected as SVs. In Figure 4 (d), the VIP values of the six endogenous metabolites are greater than 1, which could reflect the metabolic changes, significantly.
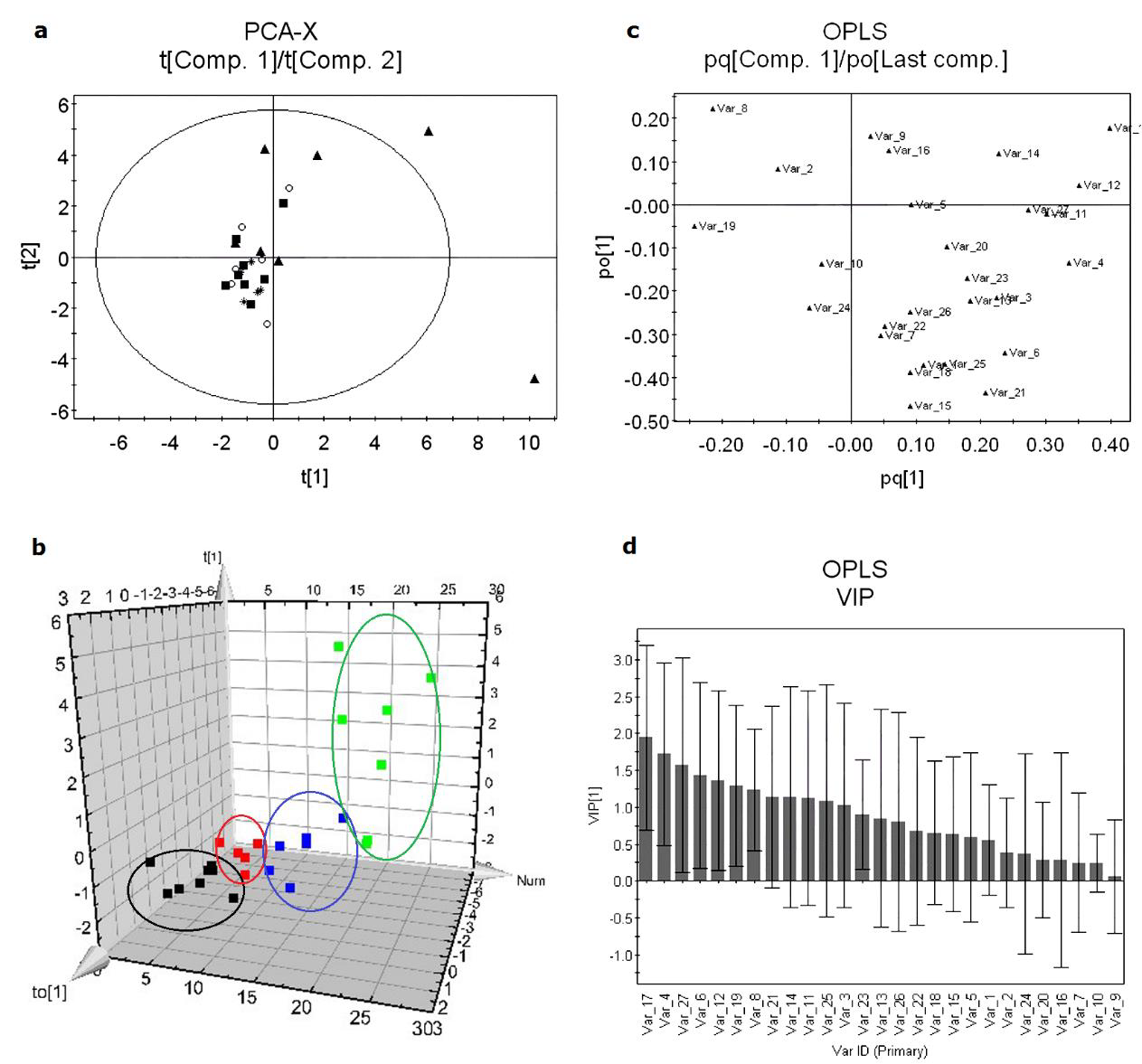
Figure 4: The PCA and OPLS-DA of control and treated samples. (A) Score plot of PCA model. (B) Score plot of OPLSDA model. (C) Loading plot: some SEMs are marked with red square. (D) The VIP values of each variable and value of the first five square marked are greater than 1.
It is far from a trivial scientific problem to evaluate the toxicity of natural products with complex chemical compositions. The biofluids, such as plasma and urine can reflect the changes in a variety of cells, tissues and organs, thus measuring the biochemical changes in biofluid could serve as surrogates for risk assessment. In our study, creatinine, uric acid, guanosine and adenosine in the metabolic profile were identified by spiking and comparing with the standard compounds. Because the levels of guanosine and adenosine in plasma were below the limit of quantification, only creatinine and uric acid were quantified under the optimum condition. The established regression equations, correlation coefficients, linear ranges and detection limits for the two analytes are listed in Table 1. In addition, the level of creatinine in plasma induced by the fraction A is lower than that of control (P < 0.05). According to the assessed metabolic pathway, creatinine is a waste product formed by the slow spontaneous degradation of creatine phosphate. While creatine is charged with energy by the enzyme creatine kinase which transfer the high-energy (~) phosphate bond of ATP to make creatine ~ phosphate in vivo. Creatine and creatine ~ phosphate exist in a reversible equilibrium and creatine ~ phosphate functions as a “battery” that stores the energy of excess ATP [25]. In this study, the GWHs extracts disturb the energy metabolism in mice and the level of creatinine in plasma decreases. In addition, the level of uric acid in plasma is relative higher than that of control in group 1. Systemic administration of uric acid is known to increase serum antioxidant capacity and it can reduce oxidative stress [26,27]. Therefore, the increased level of uric acid in biofluid might be useful to eliminate reactive oxygen species in this research.
The levels of endogenous metabolites have altered as a consequence of GWHs treatment. The fraction A, containing compounds JA, JB, RE, SD and other compounds have a greater influence on the mice metabolism than the other extracts. The pharmacokinetics study proved that JB eliminated rapidly from rats after i.v. administration [28]. RE and SD are dihydroxy-tetralone compounds and the compounds of diastereoisomeric bicyclic ketals showed noticeable antifungal and antibacterial activities [29]. The value of IC50 for the cytotoxicity of RE is 1.16 μmol/L [30]. In this study, the mice plasma presented abnormal level of the endogenous metabolites and morphology in liver induced by fraction A. The results indicate that the biochemical changes induced by GWHs extracts have some relationships with the tetralone and diarylheptanoids compounds.
Conclusion
In this study, we reported an investigation of hepatotoxicity of mice induced by GWHs extracts based on the method of plasma metabolic profiling and chemometric analysis. The biochemical changes are associated with liver damage and the results suggest the involvement of some specific pathways. The formation of creatinine was decelerated, while UA was accelerated in organism. This study indicated that metabolic profiling combined with chemometrics is a promising tool for identifying and characterizing biochemical responses to toxicity.
Conflict of Interest
The authors have declared that there is no conflict of interest.
Acknowledgements
This research was financially supported by the National Natural Sciences Foundation of China (NSFC No. 21205162) and Medical Scientific Research Foundation of Guangdong Province (A2020277).
References
- Ivo Oliveira, Anabela Sousa, Isabel C F R Ferreira, Albino Bento, Leticia Estevinho, et al. (2008) Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol 46(7): 2326-2331.
- Marcia Carvalho, Pedro J Ferreira, Vanda S Mendes, Renata Silva, Jose A Pereira, et al. (2010) Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol 48(1): 441-447.
- Su Lin Lee, Wei Jan Huang, Wan Wan Lin, Shoei Sheng Lee, Chung Hsiung Chen (2005) Preparation and anti-inflamatory activities of diarylheptanoid and diarylheptylamine analogs. Bioorg Med Chem 13(22): 6175-6181.
- J X Liu, D L Di, C Li, XY Huang (2007) Regiolone from the pericarps of Juglans regia L. Acta Cryst E63: 2713-2714.
- Jun Xi Liu, Duo Long Di, Xin Yi Huang, Chen Li (2007) Two new diarylheptanoids from the pericarps of Juglans regia L. Chinese Chem Lett 18(8): 943-946.
- Ning An, Zhong mei Zou, Ze Tian, Xiu zhen Luo, Shi lin Yang, et al. (2008) Diarylheptanoids from the rhizomes of Alpinia offi cinarum and their anticancer activity. Fitoterapia 79(1): 27-31.
- D Bonamonte, C Foti, G Angelini (2001) Hyperpigmetation and dermatitis due to Juglans regia. Contact Dermatitis 44(22): 101-102.
- Iria Neri, Federica Bianchi, Federica Giacomini, Annalisa Patrizi (2006) Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis 55(1): 62-63.
- Xiangfeng Kong, Yuanliang Hu, Rong Rui, Deyun Wang, Xiangrui Li (2004) Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Inter Immunopharma 4(7): 975-982.
- Yanru Liu, Rongqing Huang, Lijun Liu, Jiangnan Peng, Bingkun Xiao, et al. (2010) Metabonomics study of urine from Sprague-Dawley rats exposed to Huang-yao-zi using 1H NMR spectroscopy. J Pharm Biomed Anal 52(1): 136-141.
- Jeremy K Nicholson, John Connelly, John C Lindon, Elaine Holmes (2002) Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov 1(2): 153-161.
- Richard D Beger, Jinchun Sun, Laura K Schnackenberg (2010) Metabolomics approaches for discovering biomarkers of drug-induced hepatotoxicity and nephrotoxicity. Toxicol Appl Pharmacol 243(3): 154-166.
- Jin lan Zhang, Ming Cui, Yun He, Hai lan Yu, De an Guo (2005) Chemical fingerprint and metabolic fingerprint analysis of Danshen injection by HPLC-UV and HPLC-MS methods. J Pharma Biomed Anal 36(5): 1029-1035.
- Baogang Xie, Tao Gong, Rong Gao, Jie Liu, Jiao Zuo, et al. (2009) Development of rat urinary HPLC-UV profiling for metabonomic study on Liuwei Dihuang Pills. J Pharma Biomed anal 49(2): 492-497.
- J E. Jackson (1991) A Users Guide to Principal Components. Wiley, NewYork, USA.
- Barker, W. Rayens (2003) Partial least squares for discrimination. J Chemom 17: 166-173.
- Trygg, S. Wold (2002) Othogonal projections to latent structures (O-PLS). J Chemom 16: 119-128.
- Lijuan Liu 1, Wei Li, Kazuo Koike, Shujie Zhang, Tamotsu Nikaido (2004) New α-Tetralonyl glucosides from the fruit of Juglans mandshurica. Chem Pharm Bull 52: 566-569.
- Junxi Liu, Min Meng, Chen Li, Xinyi Huang, Duolong Di (2008) Simultaneous determination of three diarylheptanoids and an α-tetralone derivative in the green walnut husks (Juglans regia L.) by high-performance liquid chromatography with photodiode array detector. J Chromatogr A 1190(1-2): 80-85.
- Robert A van den Berg, Huub C J Hoefsloot, Johan A Westerhuis, Age K Smilde, Mariet J van der Werf (2006) Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Gen 7: 142-157.
- M Bylesjo, M Rantalainen, O Cloarec, J K Nicholson, E Holmes, et al. (2006) OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J Chemom 20: 341-351.
- Johan A Westerhuis, Ewoud J J van Velzen, Huub C J Hoefsloot, Age K Smilde (2009) Multivariate paired data analysis: multilevel PLSDA versus OPLSDA. Metebolomics 6(1): 119-128.
- H T Eastment, W J Krzanowski (1982) Cross-validatory choice of the number of components from a principle component analysis. Technometrics 24: 73-77.
- A Tropsha, P Gramatica, VK Gombar (2003) The importance of being earnest: validation is the absolute essential for successful application and interpretation of QSPR models. QSAR Comb Sci 22: 69-77.
- M Wyss, D R Kaddurah (2000) Creatine and creatinine metabolism. Physiol Rev 80(3): 1107-1213.
- W S Waring, D J Webb, S R Maxwell (2001) Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharma 38(3): 365-371.
- W S Waring, A Convery, V Mishra, A Shenkin, D J Webb, et al. (2003) Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin sci 105(4): 425-430.
- Xin Yi Huang, Qiu Yan Duan, Jun Xi Liu, Duo Long Di (2010) Determination of a novel diarylheptanoid (Juglanin B) from green walnut husks (Juglans regia L.) in rat plasma by high-performance liquid chromatography. Biomed chromatogr 24(3): 307-311.
- Jin Yan Dong, Li Mei Wang, Hong Chuang Song, Kai Ze Shen, Yong Ping Zhou et al. (2009) Colomitides A and B: novel ketals with an unusual 2,7-dioxabicyclo[3.2.1]octane ring system from the aquatic fungus YMF 1.01029. Chem Biodivers 6(8): 1216-1223.
- Jun Xi Liu, Duo Long Di, Xiao Ning Wei, Yin Han (2008) Cytotoxic diarylheptanoids from the pericarps of walnuts (Juglans regia). Planta Med 74(7): 754-759.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.