Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Factors Influencing Bacterial Biofilm Formation and Development
*Corresponding author: Ghazay F Alotaibi, Department of Environment and Marine Biology, Saline Water Desalination Technologies Research Institute, Saudi Arabia, Email: DAlotaibi@swcc.gov.sa.
Received: September 30, 2020; Published: May 21, 2021
DOI: 10.34297/AJBSR.2021.12.001820
Abstract
Bacterial communities attached to surfaces and established a protected mode of growth within extracellular matrix substances are defined as a bacterial biofilm. Formation of the biofilm occurs naturally as a result of balancing between a variety of chemical, physical and biological processes. Biofilms form on different surfaces, such as inert materials and living tissues or cells after the surface bacterial adhesion followed by the growth and production of extracellular polymeric substances (EPS). In this article, biofilm formation from the initial attachment of bacterial cells to the substratum, physiological changes within the microbe, multiplication of adhered cells to form microcolonies, and finally biofilm maturation is reviewed. The review article will also highlight factors involved in biofilm formation. Hopefully, this article will serve as a supportive document for further research into the molecular nature of biofilms.
Keywords: Biofilm Formation, Hydrodynamic Conditions, Adhesion, Extracellular Polymeric Substances.
Introduction
Bacterial biofilms are aggregations of cells attached to surfaces and surrounded by a matrix of extracellular polymeric substances (EPS) [1-4]. On Earth, over 99% of bacteria are thought to live in structured biofilm communities [5,6]. The mostly self-produced extracellular polymeric matrix that encases the microorganisms promotes survival in hostile environments, including tolerance to antibiotics and provides structure to the biofilm [7]. Biofilms can exist in both natural and anthropogenic environments [8]. Biofilms may also form on a wide variety of surfaces, including inert or living materials, such as tissues or cells [9,10].
Establishment and development of bacterial biofilms are known to be dynamic and complex processes regulated by intrinsic biological properties and also by many environmental factors, since changes in the environment often trigger the formation of biofilm [11-13]. Biofilm-associated bacteria demonstrate distinct features from their free-living planktonic counterparts.
i. Intercellular signalling systems, such as QS, in which cells
produce signalling molecules that regulate the development of
the biofilm.
ii. Cyclic nucleotide second messengers, such as the bacterial
second messenger c-di-GMP, which regulates biofilm formation
and dispersal by controlling flagellar motility, attachment and
extracellular polysaccharide production.
iii. Biofilm-associated proteins, which form a scaffold and
builds the biofilm matrix [14].
The composition of biofilms depends on environmental factors, such as temperature, pH and nutrient availability [14- 17]. Although the composition may not be identical, the major components are typically water, bacterial cells and their secreted EPS [18,19]. Biofilms are complex highly hydrated structures since they incorporate large amounts of water by hydrogen bonding [9,20,21]. Hence, within the biofilm matrix, water is the primary component up to 97% of the mass and a biofilm is considered as an absorbent and porous structure that has water channels and pores [22,23]. The water channels allow the distribution of nutrients, oxygen and even microorganisms through fluid circulation [24,25]. Extracellular polymeric substances constitute about 1-2% while microbial cells account for about 2-5% of the biofilm matrix mass [26].
Biofilm Formation Process
Biofilm formation is the net result of several physical, chemical and biological processes [5,27]. Biofilm formation and development can be viewed as a multi-step process [28,29]. These steps include an initial reversible attachment of planktonic microorganisms to a pre-conditioned surface, a transition from reversible to irreversible attachment during the formation of biofilm through the production of EPS [10]. The development of microcolonies into a mature biofilm then occurs followed by cells dispersion from the biofilm into the surrounding environment (Figure 1).
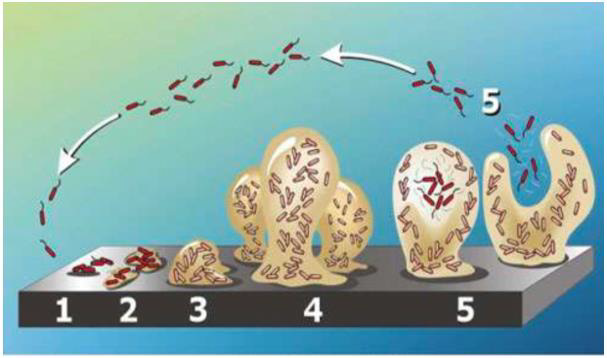
Figure 1: The five stages of biofilm development. Stage 1: Free-floating (planktonic) bacteria adhere to the surface. Stage 2: Bacterial cells aggregate form microcolonies, secrete extracellular polymeric substances (EPS) and the attachment becomes irreversible. Stage 3: A biofilm is formed and matures, and cells form multi-layered clusters. Stage 4: The growth of three-dimensional and further maturation of the biofilm, providing protection against the external environment. Stage 5: The biofilm reaches a critical mass, and planktonic bacteria disperse to colonize other surfaces. Image from [30].
Initial Reversible Attachment
Biofilm formation begins with the adhesion of single cells to material surfaces that are exposed to an aqueous medium and formation of a conditioning layer [21]. Adhesion is known to be a critical step in biofilm formation; once the bacteria attach to the surface, the chances of further transport of other free-floating microbes increases resulting in coaggregation and the creation of multiple layers [31]. The conditioning layer is an organic monolayer which forms on surfaces and acts as a docking place for the first reversibly attached cells; however, the strength of the biofilm is dependent upon the cohesiveness of the conditioning film [5,31]. This layer can be formed within minutes of exposure to an aqueous medium (e.g., water) and then proceed to grow for several hours [21].
Thus, the existence of external appendages on the bacteria, such as flagella, pili and fimbriae can overcome the repulsive physical forces and reach the bulk lattice of the conditioning layer stimulating chemical reactions and consolidating the bacteria–surface bond [5,38]. Adhesion can also be affected by the availability of nutrients in the surrounding medium [35]. After the initial interaction has developed between the bacterial cells and the substratum, many interaction forces can influence this reversible adhesion, such as van der Waals attraction forces, electrostatic forces and hydrophobic interactions, and fluid shear forces can often easily remove the bacterial cells at this stage [35]. Electrostatic forces tend to favor repulsion since many bacteria and inert surfaces are negatively charged [9].
Irreversible Attachment
In this stage, microorganisms are irreversibly attached to the surface and synthesize EPS [35,39]. Secretion of EPS by bacteria reaches a certain level, forming a strong interaction between the microbe and the surface [14]. During this stage, planktonic microorganisms can stick to each other or different species of surface-bound organisms, forming aggregates on the substratum and the adhesion becomes irreversible in the absence of physical or chemical intervention, thus the bacterial cells become attached firmly to the surface [9].
Biofilm Architecture Development
After an initial lag phase, a rapid increase (the exponential growth phase) in population can be observed which depends on the nature of the environment, both physically and chemically [40]. The irreversibly attached cells start growing and dividing using the nutrients in the conditioning film and in the surrounding fluid to form microcolonies and produce the further polymer (EPS) which helps anchor the cells to the surface and stabilize the colony from environmental fluctuations [35]. In this stage, both physical and chemical processes contribute to the initial adhesion ends, biological processes start to dominate, production of polysaccharide intercellular adhesion (PIA) polymers and the presence of divalent cations interact to form stabler bonding [40]. Besides, surface appendages production is inhibited in sessile cells since motility is restricted and no longer necessary; however, the expression of genes that are responsible for cell surface proteins production and secreted products increases concurrently [5].
Maturation
During this stage, the attached small colonies grow into a mature biofilm, with the characteristic three-dimensional biofilm structure, through reproduction and by accumulating debris and new planktonic bacteria from the surrounding environment [14,41]. In this stage, cell to cell and cell to substratum attachment depends on the EPS [14]. At high cell density, cell signalling mechanisms that use a range of different signal types known as quorum sensing are used by the biofilm; however, QS is important for biofilm maturation processes since bacteria monitor cell density and regulate collective behaviour [5,14,42].
Although quorum sensing is typically thought to mediate intraspecies communication, there is evidence that interspecies interaction also occurs [43]. Gram-positive and gram-negative bacteria use different types of QS systems that involve the production, detection, and response to extracellular signalling molecules called autoinducers, including specific peptides for gram-positive bacteria and acylated homoserine lactones for gram-negative bacteria (Figure 2) [44]. The population of bacteria regulate their gene expression by producing and responding to these autoinducers [42]. Once mature, the biofilm has three layers: a joining film binding the biofilm to the surface; a base film composed of a dense layer of bacteria; and a surface film from which freefloating bacteria can arise and spread [45].
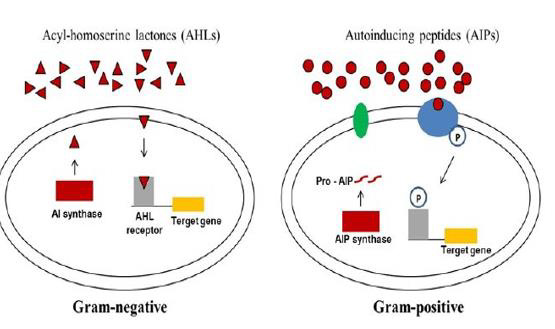
Figure 2: Quorum sensing systems in bacteria.
Gram-negative bacteria produce acylated homoserine lactones (AHLs) that upon reaching threshold concentrations enter the cells and activate the
cognate AHL receptor and induce the expression of QS-regulated genes. Gram-positive bacteria secrete mature autoinducing peptides (AIPs) that
interact with a transmembrane histidine kinase receptor activating target gene expression through autophosphorylation of the cognate transcriptional
regulator. Image from [46].
Dispersal
Dispersal is the final stage of the biofilm process where attached cells detach and disperse to colonize a new niche [47]. Biofilm cells can be dispersed either by shedding of daughter cells from actively growing cells or detachment can arise due to various factors, such as nutrient limitation, fluid dynamics and shear effects of the bulk fluid, secretory proteins and catabolite repression [14,47]. The detachment stage consists of sloughing, erosion and abrasion [21]. Erosion refers to the continuous removal of single cells or small biofilm fragments [48]. Sloughing is the loss of large particles of biofilm biomass (Figure 3) [48]. This loss is due to nutrient and dissolved oxygen depletion at the base of the biofilm or to a sudden increase in nutrient concentration in the bulk liquid [21].
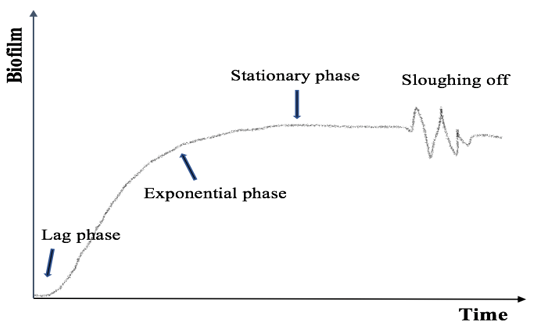
Figure 3: Biofilm accumulation through time.
After an initial lag phase, the growth of bacterial population increased rapidly, otherwise described as the exponential growth phase. The biofilm
tends to reach a maximum thickness that may vary from a few microns to some millimeters. In the stationary phase, the rate of bacterial cell growth
is equal to the rate of bacterial cell death. Shear stress is actively releasing surface bacteria for colonization of new substrates. Figure based on [49].
Abrasion is a loss of biofilm by suspended particles [48]. Any released cells may be transported to new locations and then restart the process of biofilm formation [47]. Detachment from biofilms is thought to be a key reason for the spread of pathogens [14].
Factors Involved in Biofilm Formation
The formation of biofilm is a dynamic and complex process which includes initial attachment of bacterial cells to the substratum, physiological changes within the microbe, multiplication of adhered cells to form microcolonies and finally biofilm maturation [50]. Biofilm-associated bacteria demonstrate distinct features from their free-living planktonic counterparts, such as different physiology and high resistance to immune system and antibiotics that render biofilm a source of chronic and persistent infections [51]. It is known that the change of phenotype from planktonic to the sessile form occurs in response to changes in environmental conditions [52]. These environmental factors, such as nutrient level, temperature, pH, ionic strength can influence biofilm formation [53].
Various factors can influence bacterial adhesion; cell surface properties, such as hydrophobicity, flagellation, and motility, surface properties, such as hydrophobicity and roughness and environmental factors, such as temperature, pH, availability of nutrients and hydrodynamic conditions (Table 1) [54]. Cell surface properties, specifically the presence of extracellular appendages, such as fimbriae, flagella, the interactions involved in cell-to-cell communication and EPS production, such as surface-associated polysaccharides or proteins possibly provide a competitive advantage for one organism in a mixed microbial community [21,55].
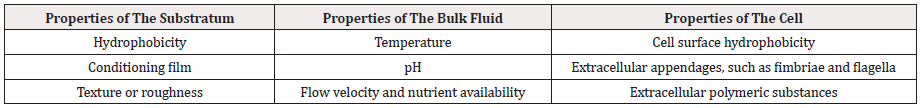
Table 1: Important variables in bacterial cell attachment and biofilm formation.
Information based on [21].
Bacteria with hydrophobic properties are more likely to attach to surfaces than hydrophilic bacteria; however, the attachment of biofilm will occur readily on surfaces which are rough, hydrophobic, and coated by surface conditioning films [17,21]. The physicochemical properties of the substratum, such as texture (rough or smooth), hydrophobicity and charge can also be modified by environmental conditions, such as pH, temperature, and nutrient levels [17]. In aquatic environments, the rate of microbial attachment can be increased with increasing the velocity of the flow, water temperature or nutrient concentration, providing these factors do not exceed critical levels [56].
Cyclic-di-GMP
The bacterial second messenger c-di-GMP plays a central role in the formation of biofilm [57]. The molecule was originally identified as an allosteric activator of cellulose synthesis in Gluconacetobacter xylinus [58]. Since then, it has emerged as an important molecule that controls the switch from a motile, planktonic lifestyle to a sessile, biofilm-associated existence [59]. Its role in regulating the transition from a motile to a settled state has been observed in several bacteria, including but not limited to P. aeruginosa, Salmonella enterica and Vibrio cholerae [43]. Many bacteria can produce c-di-GMP, which has been shown to regulate a wide range of functions, including bacterial adhesion and biofilm formation, EPS production, bacterial motility and control of virulence [60,61]. Cyclic-di-GMP was also found to affect a wide array of other fundamental bacterial behaviors, such as cell cycle proliferation, development, fimbrial synthesis, type III secretion, RNA modulation and stress response [43].
The intracellular levels of c-di-GMP are regulated by the antagonistic activity of diguanylate cyclases (DGCs) and phosphodiesterases (PDEs) enzymes that catalyze the synthesis and hydrolysis of this molecule [62]. Cyclic di-GMP is synthesized from two molecules of GTP by DGCs and degraded into 5′-phosphoguanylyl-(3′-5′)-guanosine (pGpG) or to two molecules of GMP by PDEs [63]. Diguanylate cyclases share a conserved domain containing the amino acid motif GGDEF, whereas PDEs include one of two conserved domain families: one defined by the EAL motif and the other by an HD-GYP motif [64]. High levels of c-di-GMP are found to be associated with the adhesion to surfaces, production of EPS, and formation of bacterial biofilm or a sessile lifestyle, whereas low levels can increase bacterial motility, promote biofilm disassembly and lead to the activation of virulence pathways [62,63,65].
GGDEF and EAL domains may be found individually or together as hybrid proteins that harbor both domains; however, hybrid proteins usually have either PDE or DGC activity only, although in some cases both functions can be present [62]. Proteins containing GGDEF and EAL domains or HD-GYPs are generally modular in nature, with the enzymatic domain associated with various aminoterminal sensory domains; these sensory domains respond to environmental or host-derived signals to regulate the downstream enzymatic activity [43].
In the biofilm formation process, the intracellular levels of c-di-GMP play a role in the regulation of multiple stages [57]. For example, in P. aeruginosa, reversible attachment, the first step of biofilm development, is regulated by flagellar movement which is regulated by FleQ, a transcriptional regulator of flagellar gene expression; however, binding of c-di-GMP to FleQ results in a conformational change which reduces the bacterial swimming motility [63]. The production of extracellular polysaccharides, such as Pel and Psl in P. aeruginosa and other biofilm matrix biopolymers during biofilm maturation were also found to be regulated by c-di-GMP [57]. Cyclic di-GMP can also be involved in controlling biofilm dispersion [63]. For example, exposure to nitric oxide (NO)- releasing compounds in P. aeruginosa biofilms can lead to dispersal since the exposure increases PDE activity, resulting in decreased c-di-GMP levels in the exposed biofilms [57].
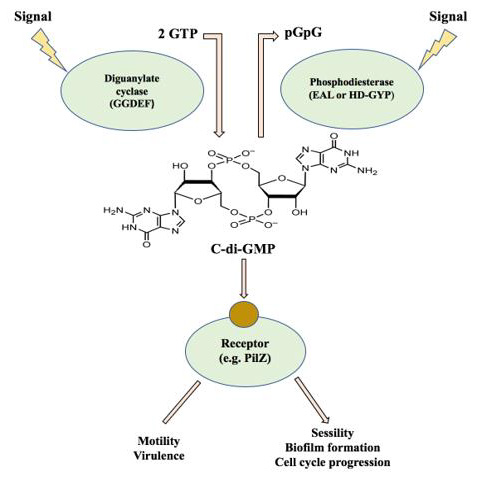
Figure 4: The c-di-GMP signalling module.
Intracellular c-di-GMP is generated from two GTP molecules by
diguanylate cyclases, containing GGDEF motifs, and is degraded to
linear diguanylate by phosphodiesterases containing either EAL or
HD-GYP domains. Cyclic di-GMP is afterwards bound by a variety
of effectors, including PilZ domain- containing proteins, and act on
targets affecting motility, virulence, and biofilm formation. Figure based
on [107].
Cyclic di-GMP signalling consists of enzymes needed for the synthesis and degradation of this molecule, effector proteins associated with binding c-di-GMP, and target elements that are eventually the output of the control module (Figure 4). Thus, DGCs and PDEs sense internal or external signals and translate them into c-di-GMP levels, which then regulate the function of c-di-GMP binding molecules, resulting in a change of the physiology and behaviour of the cell [64].
Hydrodynamic Conditions
Biofilms in different environments are exposed to a variety of hydrodynamic conditions that can have an effect on biofilm matrix [66]. These conditions affect biofilm formation by changing nutrient and oxygen supply and by applying shear forces, which can affect the cells’ attachment to surfaces [12]. The transport rate of the bacterial cells, nutrients and oxygen from the bulk fluid to the biofilm were also found to be determined by fluid hydrodynamics [67]. Besides, these conditions can influence the physical properties of biofilm, such as the density and strength, which in turn might affect the diffusion of nutrients and signals within the biofilm [68].
There is considerable evidence that shear stress has effects on the growth rate, biofilm structure, EPS production, mass transfer and metabolic/genetic behaviour of biofilm [54,69,70]. For example, analysis of microbial populations that developed under a given wall shear force has shown that shear stress slows down P. putida biofilm maturation [71]. Quorum-sensing in P. aeruginosa biofilm was also found to be influenced by hydrodynamic conditions [72]. In P. aeruginosa biofilm, high shear stress caused by high flow velocity was found to induce cell detachment [73].
Detachment usually occurs when external shear forces are higher than the inner strength of the matrix that joins the biofilm together [74]. Two mechanisms can lead to cell detachment from the biofilm, either increase of the external shear forces or decrease of the internal strength (e.g., through hydrolysis of the polymeric biofilm matrix) [75]. High flow rate is known to cause two phenomena of opposite nature: it supports the transport of nutrients to the surface, contributing to the growth of the cell in the microbial layer and exopolymers production; on the other hand, with increasing flow velocity the shear rates also rise which cause additional erosion and detachment of biofilm parts, and then decrease in the amount of biomass attached to the solid support [12]. However, the detachment of the biofilm under hydrodynamic forces leads to viable biomass reduction, which also decreases EPS secretion; thus, higher shear stresses can result in denser and thinner biofilm [76,77]. Stronger adhesion and lower detachment rates have been observed for cells that are grown under high shear conditions [78].
Environmental Conditions
In aquatic habitats, such as a river, the structure and function of biofilms can be affected by various environmental factors that control these ecosystems; physical (light penetration, temperature and water current), chemical (pH, nutrient availability and toxicant effects), as well as biological factors, including community composition (bacteria, algae and fungi), relative contribution of autotrophs and heterotrophs biomass thickness and grazing [79,80]. Environmental conditions can influence both bacterial properties (mediated by changes in gene regulation and/or cell surface physicochemical properties) and surface properties (mainly through physicochemical changes) [17]. They can also control the concentration of the second messenger c-di-GMP which regulates biofilm-related factors, such as cell appendages, surface proteins, EPS, and cell motility [61].
The environmental pH can have a major effect on the formation of biofilms [53]. Microbial adhesion to surfaces, which is the first step in biofilm formation, has been shown to be influenced by this factor [54,81]. In bacteria, such as Staphylococcus epidermidis, pH is considered an important determinant in the primary adhesion to surfaces [82]. Besides, the production of bacterial biofilm slime has been shown to be dependent on the pH of the medium, which can affect the activity of enzymes, since each enzyme has an optimal pH [83]. For example, S. aureus biofilm formation was lower at highly acidic (pH 3) and alkaline (pH 12) pH compared to pH 7 [84].
It is known that the optimum pH for polysaccharide secretion depends on the individual species; however, it is around pH 7 for most bacteria [85]. Exopolysaccharide production plays a role in biofilm protection against environmental stress factors, such as pH [18]. Thus, bacterial cells within the biofilm can withstand pH changes compared to the free-floating cells [86]. For example, under highly acidic conditions, the bacterial biofilm’s gel-like structure can help in reducing the rapid diffusion of ions and allows for the development of a pH gradient within the extracellular matrix [108]. However, under alkaline conditions, poorly structured and very thin biofilm, as a result of impairment of biofilm maturation, have also been observed, along with adhesion inhibition for some bacteria, such as S. aureus and S. epidermidis [82].
The formation of bacterial biofilm can also be affected by temperature [53,87]. The optimal temperature for bacterial growth is associated with a raise in the intake of the nutrient [88]. It is known that nutrient metabolism depends on the presence and reaction rates of enzymes that regulate the development of various physiological and biochemical systems in bacteria and as a result, the optimum temperature enhances bacterial growth, resulting in a rapid formation of biofilm [85,88]. In contrast, when the temperature is removed from the optimum, bacterial growth can be decreased, due to a decline in reaction rates, and as a consequence, the biofilm development might be affected [5]. In addition to enzymes, environmental temperature can affect the physical properties of the compounds within and surrounding the cells [5].
For bacteria, such as S. aureus, increasing the growth temperature from 20 to 37°C was found to increase their hydrophobicity and adhesion to surfaces was enhanced [89]. Also the presence of bacterial cell surface appendages, such as flagella, pili and fimbriae that help the bacteria to adhere to surfaces has been shown to be dependent on temperature [5]. For example, a decrease in temperature reduces the adhesive properties of an aquatic Pseudomonad due to a reduction in bacterial surface polymers [5].
Bacterial EPS properties, such as the viscosity of the polysaccharides can also be influenced by temperature [17]. It was found that the increase in EPS temperature creates a gel-like substance which gradually increases in strength until a critical point after which the gel forms a solution [17]. Thus, lower temperatures can lead to more uniform properties of the polysaccharides that often increase the possibility of bacterial biofilm adhesion [5]. On the other hand, in some microbes, high temperatures were found to increase the adherent nature of the biofilm to the surface [90].
Bacterial attachment to submerged surfaces and subsequent biofilm development can be dependent on oxygen accessibility [91]. Oxygen availability can also determine bacterial energy production with a possible influence on biofilm formation; for example, bacterial biofilm metabolic activity can be decreased as a result of poor supply of oxygen reduction [17]. Thus, oxygen supply is considered a key environmental factor which can have an impact on biofilm composition and development [92,93]. Lower oxygen availability usually triggers active dispersal, which is critical for the biofilm life cycle [61]. For example, at the base of a biofilm, bacterial cells were found to receive limited oxygen compared to those at the surface enhancing detachment from the deeper layers of a biofilm [35]. Also, sloughing was observed within biofilm grown under oxygen limitation [94]. In some microbes, such as E. coli, the presence of oxygen is required for the formation of biofilm, since lack of oxygen can be a detachment signal [95]. For other bacteria, such as P. aeruginosa, biofilm has been shown to form when grown anaerobically [96].
The transition between planktonic and sessile bacterial lifestyles can be affected by nutrients since the bacterial response to form a biofilm or to remain in suspension depends on the nutritional status [85]. Availability of nutrient in the surrounding medium has been found to influence the bacterial attachment to the surfaces; thus, an increase in nutrient concentration increases the microbial attachment rate [35,56]. Besides, biofilm development and dispersal of cells from the biofilm can be affected by nutrient levels [14,47]. Also, changes in the essential nutrient availability have been shown to have an impact on bacterial physiology in growing biofilms [97].
Several studies have addressed the effect of nutrient levels on the formation of bacterial biofilm. For example, in drinking water distribution systems, high nutrient concentration increased cell numbers within biofilms [98,99]. In a paper mill water stream the rate and quantity of P. putida biofilm increased with increasing nutrient levels [100]. In some microbes, the addition of glucose as carbon source to the medium has been found to enhance the formation of biofilm, such as E coli [97] P. putida [100]. However, the addition of glucose to different media was found to hinder biofilm formation in several species of Enterobacteriaceae family, such as K. pneumoniae, Citrobacter freundii, and Salmonella enterica [101].
There is some information about the effect of glucose levels on biofilm formation, but little is known about the impact of changing nitrogen concentrations in the same process. Rochex and Lebeault, (2007) have shown that rate and extent of P. putida biofilm accumulation increased with nitrogen concentration from carbon/nitrogen=90 to carbon/nitrogen=20. In contrast, depletion of nitrogen led to the active detachment of Pseudomonas fluorescens biofilm, similar to that observed under glucose limitation [102]. However, variation in peptone and yeast extract concentration, which are good nitrogen sources had no significant impact on E. coli biofilm formation [12].
In most natural environments, bacteria–surface association leads to biofilm formation, which is the prevailing microbial lifestyle [103]. Bacteria in these environments are exposed to a variety of abiotic stresses, such as osmolarity; however, planktonic and biofilm bacteria, by inducing stress response genes, might become more tolerant to these environmental stresses [23]. In bacteria, such as Lactobacillus rhamnosus, Listeria monocytogenes and Shigella boydii formation of biofilm has been found to be associated with high osmolarity [14]. For other microbes, an increase in NaCl concentrations inhibited biofilm formation, such as Salmonella species [1], Sinorhizobium meliloti [104], S. aureus [105], Enterococcus faecalis and P. aeruginosa [106].
Conclusion
Bacteria have the ability to grow in both a free form (planktonic lifestyle) or as biofilms attached to various surfaces. Biofilms are complex communities of microorganisms attached to surfaces and enclosed in a matrix of extracellular polysaccharide matrix (EPS). Thus, biofilms seem to play an essential role in bacterial survival under natural and harsh conditions and protect the bacterial cells from antimicrobial agents and toxic compounds. The quantity of EPS varies depending on the age of biofilms, the type of microbes present, and the environmental conditions. As the biofilm ages, the number of EPS tends to increase. Bacterial biofilms can form on all kinds of surfaces, including glass, plastic, wood, metal, soil particles, medical implant materials, tissues, and food products. The attachment and bacterial biofilm-forming abilities depend on numerous factors, such as inherent biological characteristics and environmental factors. Biofilm-associated bacteria are known to differ significantly from their free-living planktonic counterparts.
References
- Karaca B, Akcelik N, Akcelik M (2013) Biofilm-producing abilities of Salmonella strains isolated from Turkey. Biologia 68: 1-10.
- Yin W, Wang Y, Liu L, He Jin (2019) The microbial “protective clothing” in extreme environments. Int J Mol Sci 20(14): 3423.
- Caruso G (2020) Microbial Colonization in Marine Environments: Overview of Current Knowledge and Emerging Research Topics. Journal of Marine Science and Engineering 8(2): 78.
- Marvin W, Bangera G, Roger EB, Matthew RP, GAIL M T, et al. (2001) Gene expression in Pseudomonas aeruginosa biofilms. Nature 413(6858): 860-864.
- Trevor Roger Garrett, Manmohan Bhakoob, Zhibing Zhang (2008) Bacterial adhesion and biofilms on surfaces. Progress in Natural Science 18: 1049-1056.
- Tasneem U, Yasin N, Nisa I, Faisal Shah, Ubaid Rasheed, et al. (2018) Biofilm producing bacteria: A serious threat to public health in developing countries. Journal of Food Science and Nutrition 1(2): 25-31.
- Limoli DH, Jones CJ, Wozniak DJ (2015) Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr 3(3): 10.
- Beściak G, Surmacz Górska J (2011) Biofilm as a basic life form of bacteria. In Proceedings of a Polish–Swedish–Ukrainian seminar. Krakow Poland pp. 17–19.
- Dunne W (2002) Bacterial adhesion: seen any good biofilms lately? Clinical Microbiology Reviews 15(2): 155-166.
- Muhammad MH, Idris AL, Fan X, GUO Y, YU Y, et al. (2020) Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front Microbiol 11.
- Rühs P, Böni L, Fuller G, Inglis R, Fischer P, et al. (2013) In-situ quantification of the interfacial rheological response of bacterial biofilms to environmental stimuli. PLOS One 8: e78524.
- L C Gomes, J M R Moreira, J S Teodósio, J D P Araújo, J M Miranda, et al. (2014) 96-well microtiter plates for biofouling simulation in biomedical settings. Biofouling 30(5): 535-546.
- Matthew W Cowle, Gordon Webster, Akintunde O Babatunde, Bettina N Bockelmann-Evans, Andrew J Weightman, et al. (2020) Impact of flow hydrodynamics and pipe material properties on biofilm development within drinking water systems. Environ Technol 41(28): 3732-3744.
- Zhao X, Zhao F, Wang J, Zhong N (2017) Biofilm formation and control strategies of foodborne pathogens: food safety perspectives. RSC Advances 7: 36670-36683.
- Marić S, Vraneš J (2007) Characteristics and significance of microbial biofilm formation. Periodicum Bilogorum 109(2): 115-121.
- Marwan Abdallah, Corinne Benoliel, Djamel Drider, Pascal Dhulster, Nour-Eddine Chihib, et al. (2014) Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Archives of microbiology 196(7): 453-472.
- Pagán R, García-Gonzalo D (2015) Influence of Environmental factors on bacterial biofilm formation in the food industry: a review. Journal of Postdoctoral Research 3(6): 3-13.
- Pablo C Bogino, María de las Mercedes Oliva, Fernando G Sorroche, Walter Giordano (2013) The role of bacterial biofilms and surface components in plant bacterial associations. Int J Mol Sci 14(8): 15838-15859.
- Wickramasinghe NN, Hlaing MM, Ravensdale JT, Coorey R, CHANDRY PS, et al. (2020) Characterization of the biofilm matrix composition of psychrotrophic, meat spoilage pseudomonads. Sci Rep 10(1): 16457.
- Neu TR, Lawrence JR (1997) Development and structure of microbial biofilms in river water studied by confocal laser scanning microscopy. FEMS Microbiology Ecology 24: 11-25.
- Donlan RM (2002) Biofilm: microbial life on surfaces. Emerg Infect Di 8(9): 881-890.
- Mercedes Berlanga, Ricardo Guerrero (2016) Living together in biofilms: the microbial cell factory and its biotechnological implications. Microbial cell factories 15(1): 1-11.
- Vatansever C, Turetgen I (2018) Investigating the effects of different physical and chemical stress factors on microbial biofilm. Water SA 44(2): 308-317.
- Vasudevan R (2014) Biofilms: microbial cities of scientific significance. Journal of Microbiology & Experimentation 1(3): 84-98.
- Choudhary P, Singh S, Agarwal V (2020) Microbial Biofilms. In Bacterial Biofilms Intech Open.
- Sutherland IW (2001) The biofilm matrix an immobilized but dynamic microbial environment. Trends Microbiol 9(5): 222-227.
- Qureshi N, Annous BA, Ezeji TC, Karcher P, Maddox I, et al. (2005) Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb Cell Fact 4: 24.
- Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbio 56: 187-209.
- Muhsin J, UFAQ T, Tahir H, Saadia A (2015) Bacterial biofilm: its composition, formation and role in human infections. The Journal of Microbiology and Biotechnology 4: 1-14.
- Karaguler T, Kahraman H, Tuter M (2017) Analyzing effects of ELF electromagnetic fields on removing bacterial biofilm. Biocybernetics and Biomedical Engineering 37(2): 336-340.
- Perni S, Preedy EC, Prokopovich P (2014) Success and failure of colloidal approaches in adhesion of microorganisms to surfaces. Advances in Colloid and Interface Science 206: 265-274.
- L Boulange-Petermann, C Jullien, P E Dubois, T Benezech, C Faille, et al. (2004) Influence of surface chemistry on the hygienic status of industrial stainless steel. Biofouling 20(1): 25-33.
- Santina Carnazza, Cristina Satriano, Salvatore Guglielmino, Giovanni Marletta (2005) Fast exopolysaccharide secretion of Pseudomonas aeruginosa on polar polymer surfaces. Journal of Colloid and Interface Science 289(2): 386-393.
- Gharechani M, Moosavi H, Forghani M (2012) Effect of surface roughness and materials composition. Journal of Biomaterials and Nanobiotechnology 3(04): 541.
- Kumar CG, Anand SK (1998) Significance of microbial biofilms in food industry: a review. Int J Food Microbiol 42(1-2): 9-27.
- Chavant P, Martinie B, Meylheuc T, Marie-Noëlle Bellon-Fontaine, Hebraud M, et al. (2002) Listeria monocytogenes LO28: surface physicochemical properties and ability to form biofilms at different temperatures and growth phases. Appl Environ Microbiol 68(2): 728-737.
- Lisa Gorski, Jeffrey D Palumbo, Robert E Mandrell (2003) Attachment of Listeria monocytogenes to radish tissue is dependent upon temperature and flagellar motility. Applied and environmental microbiology 69(1): 258-266.
- Boakye YD, Osafo N, Danquah CA, Adu F, Agyare C, et al. (2019) Antimicrobial Agents: Antibacterial agents, anti-biofilm agents, antibacterial natural compounds, and antibacterial chemicals. Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods 75.
- Jefferson KK (2004) What drives bacteria to produce a biofilm. FEMS Microbiol Lett 236(2): 163-173.
- Protima Banerjee, Madhulika Singh, Varsha Sharma (2015) Biofilm formation: a comprehensive review. International Journal of Pharma Research and Health Sciences 3(2): 556-560.
- Sehar S, Naz I (2016) Role of the biofilms in wastewater treatment. Microbial Biofilms-Importance and Applications. In Tech Open.
- Rabin N, Zheng Y, Opoku-Temeng C, Du Y, Bonsu E, et al. (2015) Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Medicinal Chemistry 7(4): 493-512.
- Srivastava D, Waters CM (2012) A tangled web: regulatory connections between quorum sensing and cyclic-di-GMP. The Journal of Bacteriology 194(17): 4485-4493.
- Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2(11): a012427.
- Silverstein A, Donatucci CF (2003) Bacterial Biofilms and implantable prosthetic devices. Int J Impot Res 15(Suppl 5): S150-S154.
- Ivanova K, Fernandes M, Tzanov T (2013) Current advances on bacterial pathogenesis inhibition and treatment strategies. Microbial pathogens and strategies for combating them: science, technology and Education 4.
- Gupta A (2015) Biofilm quantification and comparative analysis of MIC (Minimum Inhibitory Concentration) and MBIC (Minimum Biofilm Inhibitory Concentration) value for different antibiotics against E. coli. International Journal of Current Microbiology and Applied Sciences 4: 198-224.
- Kaplan JB (2010) Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic Uses. J Dent Res 89: 205-218.
- Melo LF, Flemming HC (2010) 18 mechanistic aspects of heat exchanger and membrane biofouling and prevention.
- Puttamreddy S, Cornick NA, Minio FC (2010) Genome-wide transposon mutagenesis reveals a role for pO157 genes in biofilm development in Escherichia coli O157: H7 EDL933. Infection and Immunity 78: 2377-2384.
- Wei Q, Ma Luyan Z (2013) Biofilm matrix and its regulating in Pseudomonas aeruginosa. Int J Mol Sci 14(10): 20983-21005.
- Chiara R, Clemencia CL, Annalisa S, Elisa G, Beniamino Terzo Cenci G, et al. (2016) Influence of incubation conditions on biofilm formation by Pseudomonas fluorescens isolated from dairy products and dairy manufacturing plants. Italian Journal of Food Safety 5(3).
- Agarwal RK, Singh S, Bhilegaonkar KN, Singh VP (2011) Optimization of microtiter plate assay for the testing of biofilm formation ability in different Salmonella serotypes. International Food Research Journal 18(4): 1493-1498.
- Oder M, Fink R, Bohinc K, Torkar KG (2017) The influence of shear stress on the adhesion capacity of Legionella pneumophila. Arh Hig Rada Toksikol 68(2): 109-115.
- Simões M, Simões LC, Vieira M J (2010) A review of current and emergent biofilm control strategies. LWT -Food Science and Technology 43(4): 573-583.
- Prakash B, Veeregowda B, Krishnappa G (2003) Biofilms: a survival strategy of bacteria. Current science 85(9): 1299-1307.
- Nair H, Periasamy S, Yang L, Kjelleberg S, Rice SA, et al. (2017) Real time, spatial, and temporal mapping of the distribution of c-di-GMP during biofilm development. The Journal of Biological Chemistry 292(2): 477-487.
- Cotter PA, Stibitz S (2007) C-di-GMP mediated regulation of virulence and biofilm formation. Current Opinion in Microbiology 10(1): 17-23.
- Valentini M, Filloux A (2016) Biofilms and cyclic-di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J Biol Chem 291(24): 12547-12555.
- Sisti F, Hozbor D, Fernández J, HA DG, Toole GA, et al. (2013) Cyclic-di-GMP signalling regulates motility and biofilm formation in Bordetella bronchiseptica. Microbiology (United Kingdom) 159(Pt 5): 869-879.
- Toyofuku M, Inaba T, Kiyokawa T, Obana N, Yawata Y, et al. (2016) Environmental factors that shape biofilm formation. Biosci Biotechnol Biochem 80(1): 7-12.
- Diana P Cruz, Mónica G Huertas, Marcela Lozano, Lina Zárate, María Mercedes Zambrano, et al. (2012) Comparative analysis of diguanylate cyclase and phosphodiesterase genes in Klebsiella pneumoniae. BMC Microbiology 12: 139.
- Dae-Gon Ha, George A O’ Toole (2015) Cyclic-di-GMP and its effects on biofilm formation and dispersion: A pseudomonas aeruginosa review. Microbiology Spectrum 3(2).
- Mills E, Pultz I, Kulasekara HD, Miller S (2011) The bacterial second messenger c-di-GMP: mechanisms of signalling. Cell Microbiol 13(8): 1122-1129.
- Xiaohui Gao, Sampriti Mukherjee, Paige M Matthews, Loubna A Hammad, Daniel B Kearns, et al. (2013). Functional characterization of core components of the Bacillus subtilis cyclic-di-GMP signaling pathway. Journal of Bacteriology 195(21): 4782-4792.
- Lembre P, Lorentz C, Di Martino P (2012) Exopolysaccharides of the biofilm matrix: a complex biophysical world. The complex world of polysaccharides. In Tech Open.
- Inês B Gomes, Ana Meireles, Ana L Gonçalves, Darla M Goeres, Jelmer Sjollema, et al. (2018) Standardized reactors for the study of medical biofilms: a review of the principles and latest modifications. Critical reviews in biotechnology 38(5): 657-670.
- Purevdorj B, Costerton J, Stoodley P (2002) Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 68(9): 4457-4464.
- Liu Y, Tay JH (2002) The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Research 36(7): 1653-1665.
- Lemos M, Mergulhão F, Melo L, Simões M (2015) The effect of shear stress on the formation and removal of Bacillus cereus biofilms. Food and Bioproducts Processing 93: 242-248.
- Rochex A, Godon JJ, Bernet N, Escudié R (2008) Role of shear stress on composition, diversity and dynamics of biofilm bacterial communities. Water Research 42(20): 4915-4922.
- Simões M, Pereira MO, Sillankorva S, Azeredo J, Vieira M J, et al. (2007) The effect of hydrodynamic conditions on the phenotype of Pseudomonas fluorescens biofilms. Biofouling 23(3-4): 249-258.
- Zhang W, Sileika TS, Chen C, Liu Y, Lee J, et al. (2011) A novel planar flow cell for studies of biofilm heterogeneity and flow–biofilm interactions. Biotechnol Bioeng 108(11): 2571-2582.
- Fu L, Wu C, Zhou Y, Zuo J, Ding Y, et al. (2017) Investigation on evaluation criteria of backwashing effects for a pilot-scale BAF treating petrochemical wastewater. Environmental Technology 38(20): 2523-2533.
- Harald Horn, Helmut Reiff, Eberhard Morgenroth (2003) Simulation of growth and detachment in biofilm systems under defined hydrodynamic conditions. Biotechnol Bioeng 81(5): 607-617.
- Liu Y, Tay JH (2001) Metabolic response of biofilm to shear stress in fixed‐film culture. J Appl Microbiol 90(3): 337-342.
- Qi PS, Wang WB, Qi Z (2008) Effect of shear stress on biofilm morphological characteristics and the secretion of extracellular polymeric substances. Bioinformatics and Biomedical Engineering 3438-3441.
- Karimi A, Karig D, Kumar A, Ardekani AM (2014) Interplay of physical mechanisms and biofilm processes: review of microfluidic methods. Lab on a Chip 15: 23-42.
- Sabater S, Guasch H, Romaní A, Muñoz I (2002) The effect of biological factors on the efficiency of river biofilms in improving water quality. The International Journal of Aquatic Sciences 469: 149-156.
- Besemer K (2015) Biodiversity, community structure and function of biofilms in stream ecosystems. Research in Microbiology 166(10): 774-781.
- Pompilio A, Piccolomini R, Picciani C, D'antonio D, Savini V, et al. (2008) Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: the role of cell surface hydrophobicity and motility. FEMS Microbiol Lett 287(1): 41-47.
- Nostro A, Cellini L, Di Giulio M, Arrigo M, Marino A, et al. (2012) Effect of alkaline pH on staphylococcal biofilm formation. APMIS 120(9): 733-742.
- Chaieb K, Chehab O, Zmantar T, Rouabhia M, Mahdouani K, et al. (2007) In vitro effect of pH and ethanol on biofilm formation by clinical ica-positive Staphylococcus epidermidis strains. Ann Microbiol 57: 431-437.
- Zmantar T, Kouidhi B, Miladi H, Mahdonani K, Bakhrouf A, et al. (2010) A microtiter plate assay for Staphylococcus aureus biofilm quantification at various pH levels and hydrogen peroxide supplementation. New Microbiol 33(2): 137-145.
- Tilahun A, Haddis S, Teshale A, Hadush T (2016) Review on biofilm and microbial adhesion. International Journal of Microbiological Research 7(3): 63-73.
- Nunzia D'Urzo, Manuele Martinelli, Alfredo Pezzicoli, Virginia De Cesare, Vittoria Pinto, et al. (2014) Acidic pH strongly enhances in vitro biofilm formation by a subset of hypervirulent ST-17 Streptococcus agalactiae strains. Appl Environ Microbiol 80(7): 2176-2185.
- A Hostacká, I Ciznár, M Stefkovicová (2010) Temperature and pH affect the production of bacterial biofilm. Folia Microbiol (Praha) 55(1): 75-78.
- Victoria Olusola Adetunji, Ismail Ayoade Odetokun (2012) Biofilm formation in human and tropical foodborne isolates of Listeria strains. American Journal of Food Technology 7(9): 517-531.
- Jama C, Abdallah M, Boukherroub R, Faille C, Chihib NE, et al. (2017) Effect of incubation duration, growth temperature, and abiotic surface type on cell surface properties, adhesion and pathogenicity of biofilm-detached Staphylococcus aureus cells. AMB Express 7: 191.
- Marion-Ferey K, Pasmore M, Stoodley P, Wilson S, Husson GP, et al. (2003) Biofilm removal from silicone tubing: an assessment of the efficacy of dialysis machine decontamination procedures using an in vitro model. Journal of Hospital Infection 53(1): 64-71.
- Chang Y, Fragkopoulos AA, Marque Sm, Kim HD, Angelini T, et al. (2015) Biofilm formation in geometries with different surface curvature and oxygen availability. New Journal of Physics 17: 033017.
- Sang Joon Ahn, Robert A Burne (2007) Effects of oxygen on biofilm formation and the AtIA autolysin of Streptococcus mutans. Journal of Bacteriology 189(17): 6293-6302.
- Keleştemur S, Avci E, Çulha M (2018) Raman and surface-enhanced raman scattering for biofilm characterization. Chemosensors 6: 5.
- Francois Ahimou, Michael J Semmens, Greg Haugstad, Paige J Novak (2007) Effect of protein, polysaccharide, and oxygen concentration profiles on biofilm cohesiveness. Applied and Environmental Microbiology 73(9): 2905-2910.
- Bjergbæk L, Haagensen J, Reisner A, Molin S, Roslev P (2006) Effect of oxygen and growth medium on in vitro biofilm formation by Escherichia coli. Biofilms 3: 1-10.
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, et al. (2002) Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. The Journal of Clinical Investigation 109(3): 317-325.
- T Bühler, S Ballestero, M Desai, M R Brown (1998) Generation of a reproducible nutrient‐depleted biofilm of Escherichia coli and Burkholderia cepacia. J Appl Microbiol 85(3): 457-462.
- Volk C, Le Chevallier M (1999) Impacts of the reduction of nutrient levels on bacterial water quality in distribution systems. Appl Environ Microbiol 65(11): 4957-4957.
- Frias J, Ribas F, Lucena F (2001) Effects of different nutrients on bacterial growth in a pilot distribution system. International Journal of General and Molecular Microbiology 80(2): 129-138.
- Rochex A, Lebeault JM (2007) Effects of nutrients on biofilm formation and detachment of a Pseudomonas putida strain isolated from a paper machine. Water Research 41(13): 2885-2892.
- Jackson DW, Simecka JW, Romeo T (2002) Catabolite repression of Escherichia coli biofilm formation. J Bacteriol 184(12): 3406-3410.
- P J Delaquis, D E Caldwell, J R Lawrence, A R Mc Curdy (1989) Detachment of Pseudomonas fluorescens from biofilms on glass surfaces in response to nutrient stress. Microb Eco 18(3): 199-210.
- Alexis Bazire, Farès Diab, Mohamed Jebbar, Dominique Haras (2007) Influence of high salinity on biofilm formation and benzoate assimilation by Pseudomonas aeruginosa. Official Journal of the Society for Industrial Microbiology 34(1): 5-8.
- Rinaudi L, Fujishige Na, Hirsch AM, Banchio E, Zorreguieta A, et al. (2006) Effects of nutritional and environmental conditions on Sinorhizobium meliloti biofilm formation. Res Microbiol 157(9): 867-875.
- Johnson M, Williams PH, Morrissey JA, Cockayne A (2005) Iron- responsive regulation of biofilm formation in Staphylococcus aureus involves fur-dependent and fur-independent mechanisms. Journal of Bacteriology 187(23): 8211-8215.
- Azeza Falghoush, Haluk Beyenal, Thomas E Besser, Anders Omsland, Douglas R Call, et al. (2017). Osmotic compounds enhance antibiotic efficacy against Acinetobacter baumannii biofilm communities. Applied and Environmental Microbiology 83(19): e01297-e01317.
- Schirmer T, & Jenal U (2009) Structural and mechanistic determinants of c-di-GMP signalling. Nature Reviews Microbiology 7(10): 724-735.
- Adriana Marcia Nicolau Korres, Gloria Maria de Farias V Aquije, David S Buss, Jose Aires Ventura, Patricia Machado Bueno Fernandes, et al. (2013) Comparison of biofilm and attachment mechanisms of a phytopathological and clinical isolate of Klebsiella pneumoniae Subsp. pneumoniae. Scientific World Journal 925375.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.