Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Oral Ingestion of A Novel Supplement Inerbty® Whitens and Brightens Facial Skin, Reduces Facial Melanin and Spots: Results of A Clinical Study
*Corresponding author: Dan Cheng, Ph.D., R&D Center, Shanghai Lithy One Health Group, Shanghai, China.
Received: February 10, 2021; Published: February 18, 2021
DOI: 10.34297/AJBSR.2021.12.001711
Abstract
In the recent years, the oral products for skin beauty and health have been rapid cutting the market value of topical cosmetics, as more and more individuals have realized the importance and value of such oral products. Skin whitening and brightening is one of the first priority needs for individuals at all ages and genders. In our study, a novel formulated pressed candy with worldwide prestigious ingredients was orally supplemented to 30 female individuals for 56 days to test its efficacies and functions for facial skin improvement. After 28 and 56 days, it is demonstrated that this supplement significantly whitened and brightened the facial skin, reduced the melanin and spots compared with those at baseline. No adverse events were reported during the whole study of the oral administration of this supplement. It is reflected by the self-assessment questionnaire that those individuals were highly satisfied with the supplement in terms of functionality and easiness for supplementation and were willing to continue to consume and recommend the product.
Introduction
Skin care has always been one of the first-priority concerns especially for female individuals. In the recent years, orally administered products for skin care have obtained great attention and popularity beyond topical cosmetics [1]. For instance, the market for collagen peptide has grown exponentially and well recognized by female consumers [2]. It is also the trend that more and more oral products have been developed targeting more precise needs to resist aged skin. Except for collagen products, the oral products for skin beauty can be categorized into various aspects as anti-glycation, anti-oxidation, hydration, skin whitening and so on. The market value of oral products for skin whitening has rapidly increased since 2018. It is estimated that 50% of the consumers that purchase oral products for skin care are actually buying oral whitening products [3]. Especially in summer this kind of product is consumed most when outdoor activity is prevalent, and sunlight is strong to cause skin burn.
Skin is the most important barrier between internal and external environment for human beings, as it has the capacity to detect external factors to protect body homeostasis [4]. Among external factors, ultraviolet radiation (UV) generates most adverse effects on skin being exposed to it. For instance, UVA (wavelength between 315 -400 nm) can penetrate deep into the dermis and cause DNA damage by generation of reactive oxygen species (ROS) [5]. As a protective mechanism, the cellular damage actually activates melanin production from melanocytes and melanin deposition in the keratinocytes [6]. Therefore, beyond significant measure of UV fillers and topical cosmetics that protect skin from UV exposure, it is crucial to apply antioxidant that scavenge ROS to reduce the relevant cascades of adverse chain reactions. Extracts from natural plants and fruits can provide promising options as they are rich in anti-oxidative phytochemicals such as polyphenols [7-8]. For instance, it is reported that melon concentrate exhibits photoprotective effects from antioxidant activity in healthy adults [9]. Minimal erythema dose (MED) measurement was used to determine the photoprotective effect of the melon concentrate. Higher MED values correspond to better protection.
In this study, we aim to investigate the effect of a novel formulated food Inerbty® comprising melon concentrate, artichoke extract, yeast extract and grape powder on skin whitening and brightening by a single-blinded, randomized and self-controlled trial including 35 healthy female subjects.
Materials and Methods
Testing Product
The test product in this study was a pressed candy called Inerbty® and provided by Guangzhou Aibaiyi Biotechnolgy Co., Ltd. The ingredients of the pressed candy consist of melon extract powder (SODB® (Bionov, Avignon, France), Holimel M® (Robertet, France)), grape powder, artichoke powder, yeast extract and red rose extract. The melon extract is a unique species that is originally planted in France with high content of endogenous SOD.
Study Design/Intervention
The study was carried out as a single-blinded, randomized and self-controlled trial on the effects of 35 healthy female subjects after 56 days of oral intake. All participants signed consent agreement that manifests their benefits from this test and relevant risks. Before the inception of the study, all testing protocols and consent agreement were scrutinized and approved by Guangdong Cosmetics Ethnicity Committee, China.
Cohort
Thirty-five healthy female subjects in the age group of 32 to 60 years were screened and enrolled in the study. Thirty subjects completed the study and five withdrew due to personal reasons. The participants were supplemented with Inerbty® twice a day at night.
Inclusion Criteria
Healthy female subjects from 18 to 60 years old; they are capable of reading Chinese and comprehending the consent agreement; they are willing to cooperate and complete all the tests during the study and report any adverse events.
Exclusion Criteria
Females that are pregnant or breastfeeding; any obvious facial defects including sunburn, scar, pigmented nevus, which might impair the test characterization; facial microbial infections; chronic skin diseases (such as skin tumors, rosacea, eczema, lupus erythematosus, seborrheic dermatitis, psoriasis, severe epidermal shedding); history of immunosuppressive or immunodeficiency disorders (including HIV or AIDS) or current use of immunosuppressive drugs or radiotherapy; chronic and endocrine diseases such as asthma, epilepsy, diabetes, hypertension, hyperthyroidism or hypothyroidism; participation of other clinical studies during the past 3 months; took any drugs that may affect skin status or response in the past 6 months or currently such as antihistamines, antibiotics, insulin, anti-inflammatory drugs, vitamins A, steroids, aspirin, thyroid drug; treated with facial medical treatment, such as laser treatment, chemical stripping and minimally invasive cosmetic treatment; a history of mental illness or unable to take care of themselves; participant with any of the above were excluded from the study.
Outcome Measures
All tests were conducted at a third-party medical testing center called Landproof in Guangzhou, China. Skin melanin, color and spots were assessed at the facial skin of individuals. All measurements were performed before the first intake of the product, after 28 and 56 days.
Measurement of Facial Images
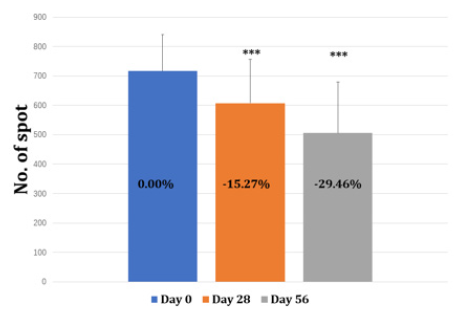
Multi-modality facial images of the subject’s frontal, left-side, and right-side views were captured at baseline, day 28, and day 56 using the VISIA-CR. The number of spots was also analyzed by VISIA-CR with IPP software (Figure 1).
Measurement of Melanin
The Mexameter® MX 18 (Courage+Khazaka electronic GmbH), a very easy, quick and economical tool, was applied to measure melanin index from the intensity of absorbed and reflected light at specific wavelength as recommended [10]. The larger the value, the higher melanin (Figure 2).
Measurement of pigmentation
The overall pigmentation or skin color was determined by a system called ITA° (individual topology angle). By using the method of the L*, a*, b* color space, it is possible to calculate the so-called ITA°. L* stands for lightness (100 corresponds with white and 0 with black), a* denotes red-green coloration and b* denotes blue-yellow coloration. The lighter the skin, the higher the ITA°. The exact ITA was determined by Spectrophotometer, CM-26d as recommended [11] (Figure 3).
Subjective Assessments of Treatment Effects
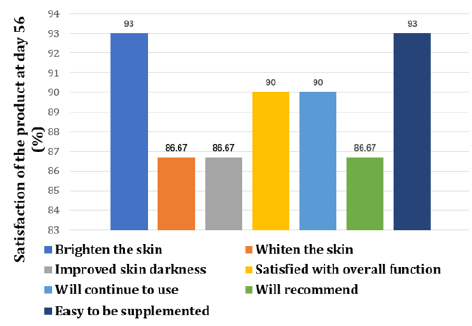
All patients filled in a questionnaire at day 56 consisting of 7 questions in terms of the satisfaction product overall function, supplementation easiness and willingness to use and recommend the product. Each item is rated from score 1 to 7 with higher score indicating better satisfaction. The number of individuals that rate over 5 is reflected in percentage.
Adverse Events
The safety and tolerability of the administration of treatment powder were evaluated at day 28 and day 56. Professional dermatologist asked the subjects whether they had gastrointestinal discomfort, body skin tingling, itching and other symptoms or obvious signs of dry skin, desquamation, flushing and so on. During the whole study, the participants were requested to report instantly to the investigator once they experienced any uncomfortable feelings.
Statistical Analysis
Statistical analyses of this clinical study were completed using IBM Statistical Package for Social Sciences (SPSS 21.0) at an alpha level of 0.05. To evaluate primary and secondary outcome measures, analysis of variance (ANOVA) was used to compare within-group changes and group changes over time.
Results
It can be observed that the melanin decreased by 4.79% at day 28 of orally supplemented product (Figure 4 & Table 1). As individuals continued to orally take the product, the melanin decreased by 7.06% at day 56 compared to that at day 0. The change of melanin at day 0, day 28 and day 56 reach a statistical significance (p<0.001) The facial ITA° value increased from 33.91 to 37.61 during the 56 days of product supplementation indicating the overall facial skin color had been getting lighter (Table 2 & Figure 5). Actually, the ITA° value increased up to 37.05 at day 28 showing a fast effect of whitening and brightening the skin.
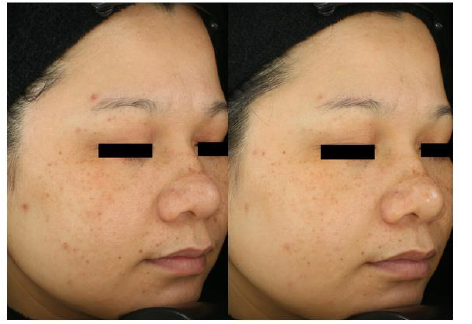
The spot ITA° value increased from 16.58 to 24.62 during the 56 days of product supplementation indicating the spot pigmentation had been getting lighter (Table 3 & Figure 6). Moreover, the number of spots has decreased by 15.27% and 29.46% at day 28 and 56 respectively (Table 4 & Figure 7). The comparison of right-sided facial skin of tester 3 was shown in Figure 8. It is obvious to see that the facial skin is improved regarding spots, color, wrinkle and so on (Table 4 & Figure 7,8). For the self-assessed product satisfaction, at least 26 out of 30 individuals rated score of at least 5 from aspects of the overall function, willingness to continue and use and recommend and easiness for supplementation (Figure 9).
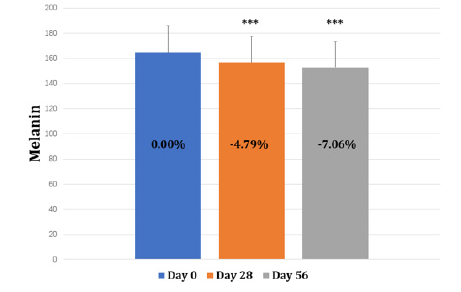
Figure 4: The change of skin melanin at baseline, day 28 and day 56. Note: *** indicates changes reach a statistical significance compared to baseline (p<0.001)
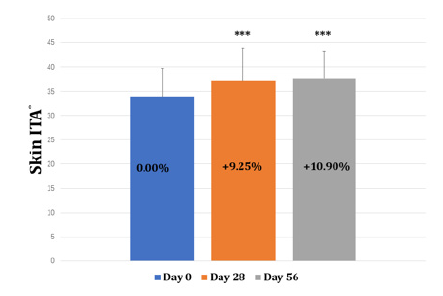
Figure 5: The change of facial skin ITA° at baseline, day 28 and day 56. Note: *** indicates changes reach a statistical significance compared to baseline (p<0.001)
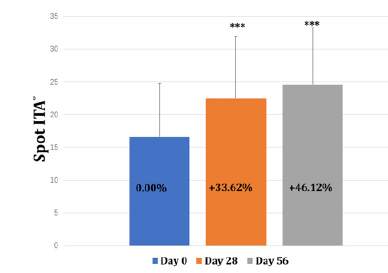
Figure 6: The change of Spot ITA° at baseline, day 28 and day 56. Note: *** indicates changes reach a statistical significance compared to baseline (p<0.001)
Discussion
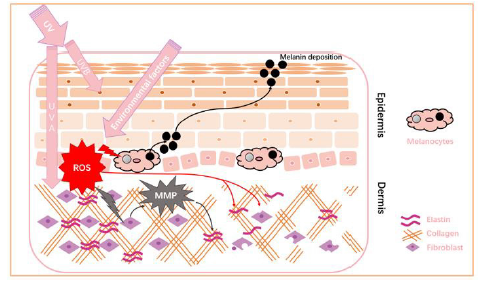
UV is the spectrum of electromagnetic radiation emitted by the sun. There are three varieties of UVs regarding their wavelength: UVC (100–280 nm), UVB (280–315 nm), and UVA (315–400 nm). Ambient sunlight consists of UVA (90–95%) and UVB (5–10%) while most of UVC and UVB are blocked by ozone layer [12]. It can be clearly seen from Figure 9 that the mechanism of UVB and UVA damage different skin layers including epidermis and dermis. While UVB directly causes sunburn clinically characterized as edema and redness, UVA seems more detrimental as a result of ROS generation [13]. ROS can function as a signal to remedy damaged cells by activating melanin production and deposition [14]. Therefore, scavenging ROS is the first and most critical step to prevent the cascades of reactions caused by ROS except topical cosmetical protection. Besides, ROS leads to the production of metalloproteinases that causes the degradation of extracellular matrix including elastin fibers and collagen [15]. That explains the loss of collagen and skin aging associated with photo aging. Therefore, this product that scavenge the ROS should be able to complement and amplify the function of collagen and hydration oral products.
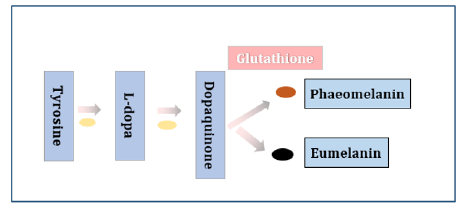
It is found orally supplementing melon concentrate enriched in SOD actually increased individuals’ endogenous antioxidant enzymes including SOD, CAT and GPX and reduced melanin levels and sunburn cells in comparison with placebo on the radiated skin explants [9]. Grape extract can also provide a robust system of antioxidant consisting of anthocyanins, proanthocyanin and resveratrol [16]. In a double-blinded, placebo-controlled study, 60 days oral administration of grape extract significantly increased skin antioxidant power, enhanced skin moisturization and elasticity and reduced skin roughness wrinkles and aged spots [17] (Figure 10). The dominant part for melanin formation involves the transformation of tyrosine to 3,4-dihydroxphenylalanine (L-dopa) and oxidation of L-dopa to dopaquinone (Figure 11). It is tyrosinase that enzymatically catalyzes these reactions [18]. It was found resveratrol could be oxidated by tyrosinase into 2,3 hydroxyresveratrol that inhibited the activity of tyrosinase [19]. Also, it was found HCAs (Hydroxycinnamic Acids) and their derivatives contained in artichoke also exhibited anti-collagenase and anti-tyrosinase activities in addition to UV-protective effect [20].
Glutathione, low molecular weight thiol-tripeptide and essential to the maintenance of intracellular redox balance, is a strong antioxidant with anti-melogenic properties. Glutathione can not only inhibit the activity of tyrosinase but also can skewing of melanogenesis from the darker eumelanin to the lighter phaeomelanin (Figure 11). Two clinical studies have demonstrated orally administered glutathione 4 to 8 weeks significantly reduced melanin index at all tested sites compared with placebo and well-tolerated with no adverse effects [21,22]. On the whole, this comprehensive mechanism of the product lies in free radicals scavenge, inhibition of tyrosinase and favor of production for lighter phaeomelanin. Each of the product ingredients has solid scientific and clinical evidence. This single-blinded, randomized, self-controlled trial demonstrates that the product should be a useful and a daily supplement for individuals who are susceptible to sun exposure.
Conflicts of Interest
The authors declared that they have no conflicts of interest to this work.
References
- Vollmer DL, West VA, Lephart ED (2018) Enhancing Skin Health: By Oral Administration of Natural Compounds and Minerals with Implications to the Dermal Microbiome. Int J Mol Sci 19(10): 3059.
- Bolke L, Schlippe G, Gerß J, Voss W (2019) A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 11(10): 2494.
- The trend for consumption of oral beauty products (2019) CBNdata.
- Marionnet C, Pierrard C, Golebiewski C, Bernerd F (2014) Diversity of biological effects induced by longwave UVA rays (UVA1) in reconstructed skin. PLoS One 9(8): e105263.
- Schuch AP, Moreno NC, Schuch NJ, Menck CFM, Garcia CCM, et al. (2017) Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic Biol Med 107:110-124.
- Al-Jamal MS, Griffith JL, Lim HW (2014) Photoprotection in ethnic skin. Dermatologica Sinica 32(4): 217–224.
- Kao YY, Chuang TF, Chao SH, Yang JH, Lin YC, et al. (2013) Evaluation of the antioxidant and melanogenesis inhibitory properties of pracparatum mungo (lu-do huang). Journal of Traditional and Complementary Medicine 3(3): 163-170.
- Arung ET, Furuta S, Ishikawa H, Tanaka H, Shimizu K, et al. (2011) Melanin biosynthesis inhibitory and antioxidant activities of quercetin-3'-O-beta-D-glucoside isolated from Allium cepa. Z Naturforsch C J Biosci 66(5-6): 209-214.
- Egoumenides L, Gauthier A, Barial S, Marion Saby, Céline Orechenkoff, et al. (2018) A Specific Melon Concentrate Exhibits Photoprotective Effects from Antioxidant Activity in Healthy Adults. Nutrients 10(4): 437.
- Matias AR, Ferreira M, Costa P, Neto P (2015) Skin colour, skin redness and melanin biometric measurements: comparison study between Antera(®) 3D, Mexameter(®) and Colorimeter(®). Skin Res Technol 21(3): 346-362.
- Del Bino S, Bernerd F (2013) Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br J Dermatol. 169 Suppl 3: 33-40.
- Wang SQ, Balagula Y, Osterwalder U (2010) Photoprotection: a review of the current and future technologies. Dermatol Ther 23(1): 31-47.
- Schuch AP, Moreno NC, Schuch NJ, Menck CFM, Garcia CCM, et al. (2017) Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic Biol Med 107: 110-124.
- Young AR, Claveau J, Rossi AB (2017) Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J Am Acad Dermatol 76(3S1): S100-S109.
- Baek B, Lee SH, Kim K, Lim HW, Lim CJ, et al. (2016) Ellagic acid plays a protective role against UV-B-induced oxidative stress by up-regulating antioxidant components in human dermal fibroblasts. Korean J Physiol Pharmacol 20(3): 269-277.
- Auger C, Gérain P, Laurent-Bichon F, Karine Portet, Aurélie Bornet, et al. (2004) Phenolics from commercialized grape extracts prevent early atherosclerotic lesions in hamsters by mechanisms other than antioxidant effect. J Agric Food Chem 52(16): 5297-5302.
- Buonocore D, Lazzeretti A, Tocabens P, Vincenzo Nobile, Enza Cestone, et al. (2012) Resveratrol-procyanidin blend: nutraceutical and antiaging efficacy evaluated in a placebocontrolled, double-blind study. Clin Cosmet Investig Dermatol 5: 159-165.
- Hearing VJ, Tsukamoto K (1991) Enzymatic control of pigmentation in mammals. FASEB J 5(14): 2902-2909.
- Zeng HJ, Li QY, Ma J, Yang R, Qu LB, et al. (2020) A comparative study on the effects of resveratrol and oxyresveratrol against tyrosinase activity and their inhibitory mechanism. Spectrochim Acta A Mol Biomol Spectrosc. 251: 119405.
- Sova M, Saso L (2020) Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 12(8): 2190.
- Arjinpathana N, Asawanonda P (2012) Glutathione as an oral whitening agent: a randomized, double-blind, placebo-controlled study. J Dermatolog Treat 23(2): 97-102.
- Handog EB, Datuin MS, Singzon IA (2016) An open-label, single-arm trial of the safety and efficacy of a novel preparation of glutathi¬one as a skin-lightening agent in Filipino women. Int J Dermatol 55(2): 153-157.









 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.