Case report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Rhabdomyosarcoma after Allogeneic Hematopoietic Stem Cell Transplantation for Adult Acute Lymphoblastic Leukemia: A Case report
*Corresponding author: Guifang Ouyang, Ningbo First Hospital, Zhejiang, People’s Republic of China, China.
Received: February 13, 2021; Published: March 03, 2021
DOI: 10.34297/AJBSR.2021.12.001727
Abstract
Secondary malignancies after allogeneic hematopoietic stem cell transplantation (allo-HSCT) are rare, most of them occur in the long-term survivors after allo HSCT. The probability of a second tumor occurring within two years after transplantation is extremely low. Herein, we report a case of a 40-year-old adult female with Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL) was treated with allo-HSCT after chemotherapy and Tyrosine kinase inhibitor (TKI) treatment. One and a half years after allo-HSCT the patient developed a painless mass on the lateral side of the right thigh root. She was later diagnosed with polymorphic rhabdomyosarcoma (RMS) and underwent surgery subsequently. RMS within two years after allo-HSCT is very rare, which needs to be differentiated from the extramedullary relapse of acute leukemia. Chronic GVHD (cGVHD) and immunosuppressants use increase the difficulty and complexity of second tumor treatment in this stage.
Keywords: Allogeneic hematopoietic stem cell transplantation(allo-HSCT), Adult Rhabdomyosarcoma Secondary Malignancy (RMS), Acute lymphoblastic leukemia (ALL)
Abbreviations: allo-HSCT: Allogeneic Hematopoietic Stem Cell Transplantation; ALL: Acute Lymphoblastic Leukemia; TKI: Tyrosine Kinase Inhibitor; RMS: Rhabdo Myo Sarcoma; cGVHD: Chronic GVHD; PRMS: Pleomorphic RMS; ERMS: Embryonal RMS; ARMS: Alveolar RMS; CR: Complete Remission
Introduction
Advances in transplantation have improved outcomes and led to an increasing number of long-term survivors of allo-HSCT. Previous researches have shown that survivors after allo-HSCT are at risk for developing secondary malignancies [1,2]which are a rare but well-defined late complication. Secondary malignancies occur in up to 15% of patients 15 years after HSCT with myeloablative conditioning, with no plateau in the incidence rates [3,4]. There is a latency period of three to five years before secondary malignancies appearing after allo-HSCT, and cumulative incidence rates of 1–2% at 10 years and 2–6% at 20 years after transplantation [2,3,5,6]. The slope of the curve continues to show a steadily augmented incidence with increased follow-up [3]. The types of secondary malignancies reported mainly include melanoma and other skin cancers, head and neck cancer, brain cancer, liver cancer, uterine cervix cancer, thyroid cancer, breast cancer, lung cancer and possibly gastrointestinal cancers [3,7]. Among these, oropharyngeal cancer is the most frequent secondary malignancy after HSCT [3], but secondary rhabdomyosarcoma is rarely reported. Secondary malignancies are an significant cause of late mortality, and they are responsible for 5-10% of late-stage deaths [1,3].
RMS constitutes a heterogeneous and aggressive malignant soft tissue sarcoma arise from undifferentiated mesodermal tissue [8]. It is the most common soft tissue sarcoma in children and adolescents, with an incidence of 5-10% of all malignancies in childhood tumors [4-9]. However, RMS is uncommon in adults, accounting for less than 4% of all adult soft tissue sarcomas [10,11]. As is well known, RMS is classified into three main histologic subtypes: pleomorphic RMS (PRMS), embryonal RMS (ERMS) and alveolar RMS (ARMS). Among these, ERMS is correlated with a better prognosis than either ARMS or PRMS [12]. It should be noted that the PRMS is most found in adults and little in children [8,13]. RMS is unique among most solid tumors in that it can occur throughout the body [4]. Adult PRMS is mainly involved in limbs and trunk, presenting as painful or painless masses, which is located in muscles with unclear boundaries and characterized by large tumors. Adult RMS is more aggressive and leads to a poor prognosis compared to younger patients, especially in secondary malignancy [12,14]. As far as we know, secondary adult RMS after allo-HSCT has not been reported so far.
In this paper, we report a case of secondary rhabdomyosarcoma in a female adult within 1.5 years after allo-HSCT.
Case Presentation
A 40-year-old adult female was presented in October 2017 with persistent pain in chest and back. Blood routine examination indicated that the white blood cells were 34.7*10^9/L, the hemoglobin was 144g/L and the platelets were 139*10^9/L. Bone marrow aspiration showed hypercellular marrow with 88.5% blasts that were cytochemically negative to myeloperoxidase staining. Flow cytometric immunophenotyping showed that the blasts were positive for CD19, CD79a, CD34, CD10, CD22, and negative for CD33, CD13, CD3. Bone marrow biopsy combined with immunohistochemistry was consistent with acute B lymphoblastic leukemia /B lymphoblastic lymphoma. Chromosome analysis displayed 46, XX, t(9;22) (q34;q11) and PCR showed positive for BCR/ABL. Gene mutation analysis showed INM3a mutation. Since November 2017, the patient has received imatinib+ VDCP (vindesine + idarubicin + cyclophosphamide + dexamethasone) chemotherapy for two cycles and achieved complete remission (CR). The third and fourth schemes were VAP (vindesine + cytarabine + dexamethasone) and MTX+VP (methotrexate + vindesine + dexamethasone) respectively. After each chemotherapy, the patient was given intrathecal injection of cytarabine (Ara-C) + dexamethasone (DXM) to prevent meningeal leukemia.
After four cycles of chemotherapy, the patient underwent HLA fully compatible allogeneic hematopoietic stem cell transplantation in April 2018. The bone marrow morphology of the patients indicated complete remission, while PCR showed negative for BCR/ABL before transplantation. The conditioning regiment was modified BUCY, and the scheme for prevention of graft-versushost disease was cyclosporin, mycophenolate mofetil and shortterm methotrexate (MTX). The patient achieved complete donor engraftment and remained in molecular remission subsequently.
In March 2019, the patient developed cough and shortness of breath, which were diagnosed as chronic pulmonary GVHD, and symptoms were basically controlled after symptomatic treatment including corticosteroid, cyclosporine and ruxolitinib.
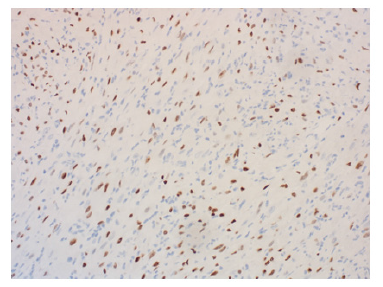
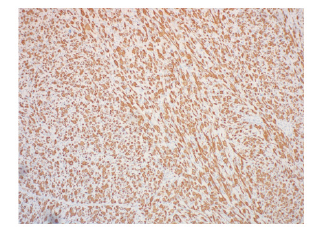
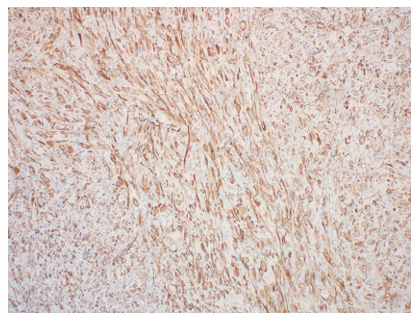
In July 2019, the patient presented with an egg-sized (3.8*1.7cm) painless mass on the lateral side of the right thigh. PETCT showed isolated hypermetabolic mass of right thigh without involvement in other part of the body. The patient underwent a coarse needle biopsy and the histopathological findings suggested neoplastic lesions of soft tissue. Immunohistochemical results showed that the tissue positive for CD31, SMA, Desmin, INI- 1, MyoD1, ALK, Ki-67 was 10% of the cells, and negative for CD34, CD20, EMA, S-100, ER, Melanoma, Calponin, Myogenin. Histopathology combined with immunohistochemistry indicated well differentiated rhabdomyosarcoma (Figure 1). In October 2019, the patient had a mass resection biopsy and further immunohistopathological examination confirmed the diagnose of polymorphous rhabdomyosarcoma (PRMS). After diagnosis, the patient reduced the dose of the corticosteroid, but continued the original dose of rucotinib and imatinib (Figure 2).
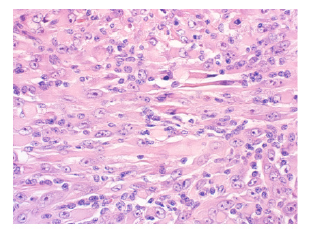
Figure 1: Tumour cells with scanty cytoplasm and round nucleus with clumped chromatin in a fibrous stroma (H & E *400).
In November 2019, the patient underwent an extended resection of mass in the right thigh root under general anesthesia. Because of repeated wound infection, the postoperative wound healing was poor. After the infection of enterococcus faecalis, the patient was treated with chronic ulcer repair of the right thigh plus negative pressure closure and drainage. In the meanwhile, the patient’s bone marrow was in complete remission (Figure 3&4).
Discussion
With larger numbers of long-term survivors, evaluating the late effects of HSCT has been a research priority [2]. To our knowledge, HSCT recipients have been reported to have an increased risk of secondary malignancies [6,15,16], particularly beyond five years after HSCT and without reaching a plateau overtime [7]. Secondary malignancies are uncommon in the survivors of allo-HSCT, comprising approximately 15% of all com plications [2]. Overall, patients develop secondary malignancies at twice the rate of the general population [2]. There is no evidence for any plateau in the incidence rate. On the contrary, the incidence rate continues to show a stably increased incidence with increased follow-up. The histopathological tumor types of secondary malignancies occurring after HSCT were reported peculiarly for colon adenoma [17], invasive breast adenocarcinoma[18], oral squamous cell carcinoma[19-21], Kaposi’s sarcoma [22], Lung adenocarcinoma[21] and glioblastoma multiforme [21]. However, RMS is extremely rare reported in adults after allo-HSCT.
To date, little is known about allo-HSCT secondary malignancies, in relation to both their origin and the initiating mechanisms. Several possible hypotheses for the emergence of secondary malignancies after allo-HSCT have been put forward, including de-novo occurrence, recurrence of malignancy and donor-related malignancy [16,23]. With time going on, there has been reported that an increasing number of cases of secondary malignancies were originated from recipients, donors or unknown sources [23]. In this case, the donor did not demonstrate signs of malignancy at the time of transplant and was free of cancer during the available follow-up period. However, this does not completely exclude a donor source.
After allo-HSCT, when an undifferentiated donor stem cell fuses with a mature differentiated cell, the resultant cell might retain the mature cell phenotype or lead to genetic instability, forming cancer-initiating cells or developing cancer stem cells. By reading the references, the diagnosis of secondary malignancies should not only be based on the histopathological aspect [8]. Some detection techniques have been applied to diagnose secondary malignancies, including chromosome karyotypic analysis [20], DNA short tandem repeat (STR) analysis [18,21], immunostaining [17,20] and fluorescence in situ hybridization assays [19,21]. We sent the pathological section to the company for microsection and STR detection. The results showed that the cell’s DNA was not from a single source, suggesting that the tumor was not simply from the donor or the patient itself. After allogeneic hematopoietic stem cell transplantation, peripheral blood STR results were always in a state of complete chimerism, but the STR results of this tumor cell were in a state of semi-chimerism. We speculated that rhabdomyosarcoma after allogeneic hematopoietic stem cell transplantation might come from both the recipient and the donor. STR analysis was considered to be a good method, but the results were not effective because the samples were contaminated with donor-derived blood cells, which meant that only purified malignant cells could be detected, so we performed microdissection before this to extract individual tumor cells to make the experimental results more reliable.
Various factors have been suggested to be associated with the development of secondary malignancies after allo- HSCT, such as HLA-mismatched allo-HSCT, cytotoxic therapy, irradiation, genetic predisposition, immunodeficiency, chronic GVHD, immunosuppressants, viral infections and advanced age [2,3,5,7,19,23].
First and foremost, chemotherapy exposure can induce breaks in doublestrand DNA that result in gene mutations, deletions and genomic instability conferred by loss of DNA repair[3,24]. Although many cytotoxic agents can add the risk of secondary malignancies, alkylating agents and topoisomerase II inhibitors in particular are known to be the primary cause [25,26]. Platinumbased chemotherapeutic agents also have a weaker association with secondary malignancies. Alkylating agents covalently modify the DNA, causing DNA crosslinking, DNA double-stranded breaks, mutations and cytotoxicity. The cumulative dosage is related to the incidence of secondary malignancies [26]. The most widely used agents in allo-HSCT are cyclophosphamide, total body irradiation and antithymocyte globulin [27]. Cyclophosphamide is an alkylating agent widely used in numerous cytotoxic and immunosuppressive regimens and it is less carcinogenic than other alkylating agents [28-30]. In this case, the main agent is cyclophosphamide, which may increase the risk of RMS development in patient.
In addition, particular immunosuppressants have been correlated with secondary malignancies in previous analyses [2]. An immunosuppressive regimen consisting most commonly of a calcineurin inhibitor (cyclosporine or ruxolitinib) plus a short course of intravenous methotrexate is given after HSCT both to prevent graft rejection and to prevent GVHD [27].
Last but not least, chronic GVHD is also a strong risk factor for secondary malignancies. It may damage target tissues and is connected with prolonged immune-suppression, and both severity and duration of immune-suppression therapy were implicated in the increased risk [3]. In our casethe patient developed chronic GVHD and received corticosteroids and immunosuppressants. Therefore, the step of immune reconstitution can be further disrupted by the chronic GVHD and the prolonged usage of corticosteroids and immunosuppressants [27].
RMS within two years after allo-HSCT is fairly rare, and it requires to be distinguished from the extramedullary relapse (EMR) of acute leukemia. As we all know, acute leukemia relapse usually occurs in the bone marrow, while quite a few reports suggest that EMR also accounts for a significant proportion [31]. According to the reports, EMR occurs in various sites such as the brain, urogenital tract, breast, skin and bone [32]. Due to the particular physiological and anatomical characteristics of the testicular and central nervous systems, these two sites are the most common sites for EMR [32,33]. However, the EMR of acute leukemia may is still a mystery. In this case, the patient was presented with a palpable mass in right thigh, and the diagnosis of thigh infiltration by rhabdomyosarcoma cells was confirmed after immunohistopathological evaluation. Besides, the patient’s bone marrow was in complete remission all the time after HSCT. Therefore, we did not consider the EMR of acute leukemia in this case.
The treatment of RMS involves a multimodal approach [8]. However, there is no standardized treatment described for adults RMS. Studies have found that adults with localized masses were less likely to receive chemotherapy compared to younger patients [34]. Local treatment is one of the key aspects and the main prognostic factor in treatment of RMS [4,35].In this case, the pathologic biopsy of the patient’s thigh root was PRMS, and PET-CT indicated that other sites were not involved. The surgeon believed that they could achieve complete resection of the tumor before starting chemotherapy and recommended the use of Pre-treatment re-excision (PRE). PRE is a wide re-excision of the operative site with adequate margins of normal tissue done prior to the initiation of adjuvant chemotherapy [4]. In this case, the entire mass has been excised by biopsy. However, the patient went to another hospital and underwent an extended radical operation for rhabdomyosarcoma. The development of new treatment techniques and strategies like immunotherapies are still uninitiated for this tumor [34].
The prognosis can be further determined based on the integrity of tumor resection, the degree of postoperative residual lesions, the tumor metastasis to lymph nodes and distant organs after pathological examination of surgical specimens [4]. There are no lymph nodes in the pathological section of this patient. Due to the incompetent immune function, target organ damage by chronic GVHD and co-existing risk of infection use, which increase the difficulty and complexity for the treatment of RMS, the survival and prognosis are not optimistic in this case [14]. Development of new targeted drugs and immunotherapies may improve the prognosis of these patients.
The incidence of secondary malignances after allo-HSCT continued to increase with the extension of follow-up time, and there was no plateau period. Therefore, lifelong follow-up of patients who have received allo-HSCT is quite necessary.
Conclusions
Secondary malignancies are uncommon in the survivors of allo- HSCT, and RMS is an extremely rare secondary malignancy. Chronic GVHD and immunosuppressants use can add the difficulty and complexity of secondary malignancies treatment. Extended followup will be needed to more fully assess the incidence and risk factors for secondary malignancies development. Besides, the development and application of the state-of-the-art detecting techniques and devices would contribute to the diagnosis and treatment of patients with solid malignancies.
Conflict of interest
No potential conflict of interest was reported by the authors.
Acknowledgements
This study was supported by the National Natural Science Foundation of China under grant (number 81401321); Basic Public Welfare Research Project of Zhejiang Province under grant (number LGF19H080002); Medical Health Science and Technology Project of Zhejiang Provincial Health Commission under grant (number 2018PY052); National Science Foundation of Zhejiang Province under grant (number LY17H160005); and Traditional Chinese Medicine Administration of Zhejiang Province under grant (number 2015ZZ018).
References
- Navneet S Majhail, Ruta Brazauskas, J Douglas Rizzo, Ronald M Sobecks, Zhiwei Wang, et al. (2011) Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood 117(1): 316-322.
- J Douglas Rizzo, Rochelle E Curtis, Gerard Socie, Kathleen A Sobocinski, Ethel Gilbert, et al. (2009) Solid cancers after allogeneic hematopoietic cell transplantation. Blood 113(5): 1175-1183.
- Danylesko I, Shimoni A (2018) Second Malignancies after Hematopoietic Stem Cell Transplantation. Curr Treat Options Oncol 19(2): 9.
- Dasgupta R, Fuchs J, Rodeberg D (2016) Rhabdomyosarcoma. Semin Pediatr Surg 25(5): 276-283.
- Olle Ringden, Ruta Brazauskas, Zhiwei Wang, Ibrahim Ahmed, Yoshiko Atsuta, et al. (2014) Second solid cancers after allogeneic hematopoietic cell transplantation using reduced-intensity conditioning. Biol Blood Marrow Transplant 20(11): 1777-1784.
- Adhikari J, Sharma P, Bhatt V R (2015) Risk of secondary solid malignancies after allogeneic hematopoietic stem cell transplantation and preventive strategies. Future oncol 11(23): 3175-3185.
- Y Inamoto, N N Shah, B N Savani, B E Shaw, A A Abraham, et al. (2015) Secondary solid cancer screening following hematopoietic cell transplantation. Bone marrow transplantation 50(8): 1013-1023.
- Askia Alfazaz, Ibrahim Assoumane, Ousseini Adakal, Harissou Adamou, Ibrahim Amadou Magagi, et al. (2019) Oropharyngeal Rhabdomyosarcoma with cranial nerve paralysis in a limited resource setting: a case report and review of literature. Pan Afr med J 34: 51.
- Ireneusz Dziuba, Pawel Kurzawa, Michal Dopierala, Ana B Larque, Danuta Januszkiewicz Lewandowska, et al. (2018) Rhabdomyosarcoma in children – current pathologic and molecular classification. Pol J Pathol 69(1): 20-32.
- Andre Pinto, Ryan M Kahn, Andrew E Rosenberg, Brian Slomovitz, Charles Matthew Quick, et al. (2018) Uterine rhabdomyosarcoma in adults. Hum Pathol 74: 122-128.
- Jorge R Toro, Lois B Travis, Hongyu Julian Wu, Kangmin Zhu, Christopher D M Fletcher, et al. (2006) Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J cancer 119(12): 2922-2930.
- Sarah N Dumont, Dejka M Araujo, Mark F Munsell, Jason A Salganick, Amaury G Dumont, et al. (2013) Management and outcome of 239 adolescent and adult rhabdomyosarcoma patients. Cancer med 2(4): 553-563.
- Attila Kollar, Rupert Langer, Codruta Ionescu, Jennifer L Cullmann, Frank M Klenke (2016) Pleomorphic rhabdomyosarcoma with an impressive response to chemotherapy: case report and review of the literature. Tumori 102(Suppl. 2).
- Paul Youn, Michael T Milano, Louis S Constine, Lois B Travis (2014) Long-term cause-specific mortality in survivors of adolescent and young adult bone and soft tissue sarcoma: A population-based study of 28,844 patients. Cancer 120(15): 2334-2342.
- Roberto Tamma, Luisa Limongelli, Eugenio Maiorano, Domenico Pastore, Eliano Cascardi, et al. (2019) Vascular density and inflammatory infiltrate in primary oral squamous cell carcinoma and after allogeneic hematopoietic stem cell transplantation. Anna hematol 98(4): 979-986.
- Gallagher G, Forrest D L (2007) Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer 109(1): 84-92.
- Christopher R Cogle, Neil D Theise, Dongtao Fu, Deniz Ucar, Sean Lee, et al. (2007) Bone marrow contributes to epithelial cancers in mice and humans as developmental mimicry. Stem cells 25(8): 1881-1887.
- Vassilis Golfinopoulos, George Pentheroudakis, Smaragda Kamakari, Vasiliki Metaxa Mariatou, Nicholas Pavlidis et al. (2009) Donor-derived breast cancer in a bone marrow transplantation recipient. Breast cancer research and treatment 113(2): 211-213.
- Kei Tomihara, Hironari Dehari, Akira Yamaguchi, Masato Abe, Akihiro Miyazaki, et al. (2009) Squamous cell carcinoma of the buccal mucosa in a young adult with history of allogeneic bone marrow transplantation for childhood acute leukemia. Head & neck 31(4): 565-568.
- Y Arai, H Arai, A Aoyagi, T Yamagata, K Mitani, et al. (2006) A solid tumor of donor cell-origin after allogeneic peripheral blood stem cell transplantation. American journal of transplantation. Am J Transplant 6(12): 3042-3043.a
- Itzhak Avital, Andre L Moreira, David S Klimstra, Margaret Leversha, Esperanza B Papadopoulos, et al. (2007) Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem cells 25(11): 2903-2909.
- B C de Medeiros, W N Rezuke, A Ricci Jr, G Tsongalis, P U Shen, et al. (2000) Kaposi’s Sarcoma following Allogeneic Hematopoietic Stem Cell Transplantation for Chronic Myelogenous Leukemia. Acta Haematologica 104(2-3): 115-118.
- Yong-xian Hu, Qu Cui, Bin Liang, He Huang (2010) Donor-derived solid malignancies after hematopoietic stem cell transplantation. Onkologie 33(4): 195-200.
- R E Curtis, P A Rowlings, H J Deeg, D A Shriner, G Socie, et al. (1997) Solid cancers after bone marrow transplantation. The New England journal of medicine 336(13): 897-904.
- Lindsay M Morton, Graça M Dores, Sara J Schonfeld, Martha S Linet, Byron S Sigel, et al. (2019) Association of Chemotherapy for Solid Tumors With Development of Therapy-Related Myelodysplastic Syndrome or Acute Myeloid Leukemia in the Modern Era. JAMA oncology 5(3): 318-325.
- McNerney M E, Godley L A, Le Beau M M (2017) Therapy-related myeloid neoplasms: when genetics and environment collide. Nat rev Cancer 17(9): 513-527.
- Wingard J R, Hsu J, Hiemenz J W (2011) Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Hematol oncol clin North Am 25(1): 101-116.
- Ohad Benjamini, Preetesh Jain, Long Trinh, Wei Qiao, Sara S Strom, et al. (2015) Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk lymphoma 56(6): 1643-1650.
- R E Curtis, J D Boice Jr, M Stovall, L Bernstein, R S Greenberg, et al. (1992) Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med 326(26): 1745-1751.
- L B Travis, R E Curtis, M Stovall, E J Holowaty, F E van Leeuwen et al. (1994) Risk of Leukemia Following Treatment for Non-Hodgkin's Lymphoma. J Natl Cancer Inst 86(19): 1450-1457.
- Alexey Surov, Hayyam Kiratli, Soo Ah Im , Yasuhiro Manabe, Alibhe O Neill, et al. (2015) Intramuscular leukemic relapse: clinical signs and imaging findings. A multicentric analysis. Skeletal radiology 44(4): 491-496.
- Ning Xie, Jian Zhou, Yanli Zhang, Fengkuan Yu, Yongping Song (2019) Extramedullary relapse of leukemia after allogeneic hematopoietic stem cell transplantation: A retrospective study. Medicine 98(19): e15584.
- Jacobs J E, Hastings C (2010) Isolated extramedullary relapse in childhood acute lymphocytic leukemia. Current hematologic malignancy reports 5(4): 185-191.
- Catalina Ruiz Mesa, John M Goldberg, Alvaro J Coronado Munoz, Sarah N Dumont, Jonathan C Trent (2015) Rhabdomyosarcoma in adults: new perspectives on therapy. Curr Treat Options Oncol 16(6): 27.
- Dasgupta R, Rodeberg D A (2012) Update on rhabdomyosarcoma. Semin Pediatr Surg 21(1): 68-78.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.