Opinion Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Vaccine Potential of Mycobacterium Tuberculosis ‘PE Only’ Subfamily Antigens
*Corresponding author: Md Kaisar Ali, College of Animal Science and Technology, Chongqing, China.
Received: February 08, 2021; Published: March 09, 2021
DOI: 10.34297/AJBSR.2021.12.001730
Introduction
Mycobacterium tuberculosis (M. tuberculosis) is responsible pathogen for causing Tuberculosis (TB) infection. TB is a serious infectious disease globally and major cause of death, then other diseases. Recent WHO (World Health Organization) report estimated that approx. 10 million people became infected with TB [1]. This situation has become complicated and deadly by the arising of HIV-TB co-infection and MDR (multi-drug resistant) and XDR (extensively drug-resistant) M. tuberculosis strains, which has worsened for the prognosis and treatment of TB [2,3]. Many years of concentrated research, the Bacille Calmette-Guerin (BCG) remains only licensed vaccine against TB with variable efficacy in the adult. This vaccine provides the protection against severe meningeal TB and miliary TB in the children and infants, but ineffective against pulmonary TB in adult [4,3]. As a result of continuous research in comprehensing the TB vaccinology, there are several vaccine candidates have been proposed. In which, some of these are under pre-clinical trials and some under clinical development, which either boost or replace the BCG vaccine, and might overcome the limitations that BCG vaccine faced [3]. The M. tuberculosis transmitted through the aerosol droplets form one individual to the other individuals. Upon infection of M. tuberculosis to the individual lungs, they reach to the alveoli, where its bacilli engulfed by the alveolar macrophages.
Keywords: Bacilli Engulfed, Bacille Calmette-Guerin, XDR, Immunopathogenesis, Investigation, Children, Comprehensing, Pre-Clinical, Pulmonary, WHO, Macrophages, Antigen
Opinion
The M. tuberculosis activates the alveolar macrophages, which leads to activate and recruiter the other immune cells including T cells and B cells, as well as induce the immune response against the pathogens [3]. The continuous research and investigation of immunopathogenesis of TB suggested that the “PE only” subfamily” antigens of M. tuberculosis are capable to induce the protective immune response in the host to reduce the replication or eliminate of bacilli form the host. The “PE only” subfamily” antigens named because of contains a conserved Pro-Glu (PE) motif at the N-terminal of 90-110 amino acids length [5]. This antigen family are exclusively present in the pathogenic strain of mycobacteria. PE antigens exhibit several repetitive sequences and abundance of immunogenic regions, which represent a source of T cell and B cell epitopes [6]. The highly immunogenic nature of PE subfamily antigens has been demonstrated by epitope mapping to show the considerable degree of cross-reactivity of these antigens in the elicited T cells [7, 8], and generation of IFN-γ induced T cell responses during infection. The PE18 and PE19 proteins are rich of CD4+-specific epitopes, which significantly induce the cell-mediated immune response [9]. The mice infected with PE4 antigen, detected the higher secretion of pro-inflammatory cytokines, including IL-2, TNF, and IL-6, which capable to induce the protective immunity in mice against M. tuberculosis challenge [10]. PE13 increase the expression of IL-6 and IL-1β and decreased SOCS3 expression in macrophages [11].
PE27 functionally and phenotypically induces the maturation of dendritic cells, by increasing the expression of MHC class I, MHC class II, CD80 and CD86 on the surface of dendritic cells to induce the production of IL-1β, IL-6, IL-12p70 and TNF-α, via NF- κB and MAPK signaling pathways. The PE27-induced dendritic cells regulate the naïve CD4+T cells to increase the secretion of IFN-γ. In M. tuberculosis-infected mice, PE27 activates the memory T cell to induce the production of IFN-γ, indicating the contribution of this antigen in Th1-polarization [12]. These finding suggested that PE27 induced DC maturation and Th1-polarizing could be helpful to design the vaccine against TB [12]. The mice immunize with PE3 protein, can significantly induce the secretion of pro-inflammatory cytokines such as IL-2, IL-6 and TNF and induce the strong protective immune response against mice challenged with live mycobacteria, could be a prospective subunit vaccine candidate against TB [13]. The PE35 and PPE68 recombinant, single or combined, stimulated THP-1 macrophages induce the dose-dependent secretion of anti-inflammatory cytokine IL-10 and chemokine monocyte chemoattractant protein-1, as well as reduce the secretion of pro-inflammatory cytokine IL-12 [14].
Conclusion
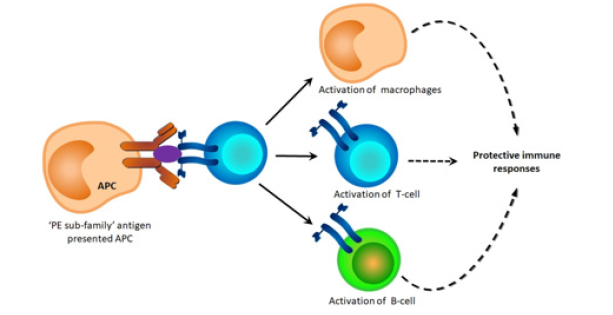
The PE32/PPE65 protein complex reduces the secretion of pro-inflammatory cytokines IL-6 and TNF-α, while induce the secretion of anti-inflammatory cytokine IL-10 in the macrophages. Co-transcription and co-translation of PE32 and PPE65 antigens modulates the protective host immune response against mycobacteria, by impeding the Th1 cells response [15]. The PE9- PE10 protein complex interact with TLR4 in the macrophages, leads to increase the phospho-IRF-3 level, which associate with the inducing transcription level of its target gene IL-1β. The PE9-PE10 protein complex stimulated macrophages induce the transcription level of IL-10, while reduce the transcription level of IL-1β (Tiwari et al. 2015). The Δppe25-pe19 mutant strain is capable to secrete the ESX-1 substrates, which leads to evoke the CD4+ T-cell responses against these protective immunogens [16]. Research so far revealed that PE only” subfamily antigens are capable to influence the host cellular immunity by modulating the macrophages, T lymphocytes, B lymphocytes, and cytokines profile, which contribute to the protective immune responses against M. tuberculosis-induced disease (Figure 1). In my opinion, “PE only” subfamily antigens could be considerable candidates having good potential to develop the powerful peptide vaccine against TB.
References
- Ali Md K, Nzungize L, Abbas K, Eckzechel N, Abo kadoum, et al. (2021) Mycobacterium tuberculosis Rv0580c Impedes the Intracellular Survival of Recombinant Mycobacteria, Manipulates the Cytokines, and Induces ER Stress and Apoptosis in Host Macrophages via NF-κB and p38/JNK Signaling. Pathogens 10(2): 143.
- Furin J, Cox H, Pai M (2019) Tuberculosis. Lancet 393: 1642-1656.
- Fatima S, Kumari A, Das G, Dwivedi VP (2020) Tuberculosis vaccine: A journey from BCG to present. Life Sci 252: 117594.
- Zhu B, Dockrell HM, Ottenhoff TM, Evans TG, Zhang Y, et al. (2018) Tuberculosis vaccines: Opportunities and challenges. Respirology 23(4): 359-368.
- Ali MK, Zhen G, Nzungize L, Stojkoska A, Duan X, et al. (2020) Mycobacterium tuberculosis PE31 (Rv3477) Attenuates Host Cell Apoptosis and Promotes Recombinant M. smegmatis Intracellular Survival via Up-regulating GTPase Guanylate Binding Protein-1. Front Cell Infect Microbiol 10: 40.
- Copin R, Coscolla M, Seiffert S N, Bothamley G, Sutherland J, et al. (2014) Sequence diversity in the pe_pgrs genes of Mycobacterium tuberculosis is independent of human T cell recognition. mBio. 5(1): e00960-00913.
- Vordermeier HM, Hewinson RG, Wilkinson RJ, Wilkinson KA, Gideon HP, et al. (2012) Conserved immune recognition hierarchy of mycobacterial PE/PPE proteins during infection in natural hosts. PLoS One 7(8): e40890.
- Fishbein S, Van Wyk N, Warren RM, Sampson SL (2015) Phylogeny to function: PE/PPE protein evolution and impact on Mycobacterium tuberculosis pathogenicity. Mol Microbiol 96(5): 901-916.
- Qian J, Chen R, Wang H, Zhang X (2020) Role of the PE/PPE Family in Host-Pathogen Interactions and Prospects for Anti-Tuberculosis Vaccine and Diagnostic Tool Design. Front Cell Infect Microbiol 10: 594288.
- Singh S K, Kumari R, Singh D K, Tiwari S, Singh P K, et al. (2013) Putative roles of a proline-glutamic acid-rich protein (PE3) in intracellular survival and as a candidate for subunit vaccine against Mycobacterium tuberculosis. Med Microbiol Immunol 202(5): 365-377.
- Singh P, Rao RN, Reddy JR, Prasad RB, Kotturu SK, et al. (2016) PE11, a PE/PPE family protein of Mycobacterium tuberculosis is involved in cell wall remodeling and virulence. Sci Rep 6: 21624.
- Kim WS, Kim JS, Cha SB, Kim SJ, Kim H, et al. (2016) Mycobacterium tuberculosis PE27 activates dendritic cells and contributes to Th1-polarized memory immune responses during in vivo infection. Immunobiology 221(3): 440-453.
- Singh SK, Tripathi DK, Singh PK, Sharma S, Srivastava KK, et al. (2013) Protective and survival efficacies of Rv0160c protein in murine model of Mycobacterium tuberculosis. Appl Microbiol Biotechnol. 97(13): 5825-5837.
- Tiwari B, Soory A, Raghunand TR (2014) An immunomodulatory role for the Mycobacterium tuberculosis region of difference 1 locus proteins PE35 (Rv3872) and PPE68 (Rv3873). Febs J 281(6): 1556-1570.
- Khubaib M, Sheikh JA, Pandey S, Srikanth B, Bhuwan M, et al. (2016) Mycobacterium tuberculosis co-operonic PE32/PPE65 proteins alter host immune responses by hampering Th1 response. Front microbiol 7: 719.
- Sayes F, Pawlik A, Frigui W, Gröschel MI, Crommelynck S, et al, (2016) CD4+ T Cells Recognizing PE/PPE Antigens Directly or via Cross Reactivity Are Protective against Pulmonary Mycobacterium tuberculosis Infection. PLoS Pathog 12(7): e1005770.
- Tiwari B, Ramakrishnan UM, Raghunand TR (2015) The Mycobacterium tuberculosis protein pair PE9 (Rv1088)-PE10 (Rv1089) forms heterodimers and induces macrophage apoptosis through Toll-like receptor 4. Cell Microbiol 17(11): 1653-1669.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.