Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Bacteriological Profiles and Sensitivity Patterns in Inpatient Urine Culture Isolates
*Corresponding author: Mahvesh Mahmud, Associate Professor, Ziauddin University, Sharah-e-Ghalib, Clifton, Karachi, Pakistan, 75600.
Received: June 04, 2021; Published: June 23, 2021
DOI: 10.34297/AJBSR.2021.13.001864
Abstract
The most common nosocomial infection among hospitalized patients is Urinary Tract Infection (UTI), and this is a major health concern in developing countries. There has been documented increasing resistance to commonly used antibiotics. The aim of this research project was aimed at studying the isolates and their antibiotic resistance patterns in admitted hospital patients.
The study was done in Karachi on inpatient patient urine samples of patients with clinically suspected UTIs, in order to assess the antimicrobial susceptibility pattern of different organisms, which would help in deciding empirical antibiotic treatment and improving patient outcomes. 400 urine sample reports of inpatients clinically suspected to have UTI after 3 days of hospital admission were collected from the bacteriology laboratory results, out of which 283 were culture positive. The positive samples included 183 females and 100 males. The most commonly isolated organism was Escherichia coli (E.coli), followed by Klebsiella species. Escherichia coli was most sensitive to meropenem (90%) and nitrofurantoin (86%) whereas Klebsiella species showed more lower susceptibility rates to commonly used antibiotics. E.coli species was the most common organism causing UTIs. Antimicrobial resistance is seen to be emerging against some antibiotics, and the current susceptibility patterns may be used locally for optimum therapeutic outcomes and for preventing antibiotic misuse.
Keywords: Urinary Tract Infection (UTI); Uropathogen(s); Antibiotic(s)
Introduction
Urinary tract infection (UTI) can be classified as uncomplicated or complicated and involves colonization and infection of the urinary tract by microbes. UTI is categorized by the site of infection as pyelonephritis, cystitis, and urethritis [1]. Urinary tract infections are currently among the most common infectious diseases in the world, and chronic and recurrent infections pose a major health issue [2]. UTI is the most common cause of hospital-acquired infection among hospitalized patients,[3] with most related to the use of an indwelling catheter [4]. The overall prevalence of UTI is higher in females; UTIs without complications in healthy women have an incidence of 50/1000/year. In boys between the ages of 1-5 year, there is a higher frequency of UTIs, and they need to be evaluated in detail [5].
Catheter-related urinary tract infection (UTI) occurs because bacterial adhesion ans colonization occurs on the surface of urethral catheters, through which organisms enter the bladder [6]. The most important risk factor for bacteriuria is the presence of a urinary catheter. The daily incidence of bacteriuria is 3-10% once a catheter is placed. In patients undergoing short-term (i.e. 2-4 days) catheterization, 10-30% develop symptomatic bacteriuria, whereas 90% - 100% of patients who undergo long-term catheterization develop bacteriuria. Approximately 80% of nosocomial UTIs are related to urethral catheterization; and 5-10% are related to genitourinary procedures [7].
The primary etiologic agents are uropathogenic Escherichia coli (UPEC), and they are the most common cause of urinary tract infections (UTIs) worldwide [8,9]. E.coli pathogens reside harmlessly in the human intestines, but in sites outside of the intestine, they become a major cause of disease due to invasive UTI (pyelonephritis, bacteremia, or septicaemia [10]. The virulence factors of E. coli are multiple and unusually complex affecting pathogenicity in combination with one another [11]. Escherichia coli have started expressing multi-drug resistance; the preferable antibiotics for empiric treatment in the treatment of uncomplicated cystitis include nitrofurantoin, trimethoprim/sulphamethoxazole, or ciprofloxacin, with cefuroxime and cefixime being alternate choices. The antibiotics used for complicated and upper UTI cases in hospitalized patients are piperacillin/tazobactam and carbapenems [12].
Among bacteria causing common infections, antibiotic resistance is increasing in all regions of the world [13]. Infections are slowly becoming more difficult to treat and may lead to therapeutic dead-ends [14]. The emergence of resistance to antibiotics points towards the fact that it is very important to us evidence-based strategies for treatment [15]. In cases of UTI, treatment with antibiotics is generally started empirically before the results of urine culture and susceptibility testing are available. The use of antibiotics in an appropriate way in patients with UTI seems to reduce the length of hospital stay, and is thus favorable for patient outcomes and the costs of healthcare [16]. Therefore, it has become important to regularly monitor the antibiotic resistance and susceptibility patterns of uropathogens, so that the can be improvement in the guidelines for empirical antibiotic therapy to include antibiotics with low resistance, and this would help clinicians in the proper management of UTIs with the least number of therapeutic failures [17]. The antibiotic resistance patterns have shown large inter-regional differentiation. The appropriate choice of antibiotic needs to be modified based on the local susceptibility patterns [18]. The factors which should be considered to find out about global data on susceptibility include the type of UTI (complicated or uncomplicated), age, gender and previous history of antibiotic therapy of each UTI patient, which would assist in further appropriate treatments attempts [19]. The data on susceptibility patterns provided by regional microbiology laboratories helps to choose the empirical choice of antimicrobials to treat UTI [20,21]. Generally, antimicrobial treatment is started before the culture susceptibility results, which may lead to the frequent antibiotic misuse [22].
All over the world, resistance against beta-lactam antibiotics is increasing due to Extended Spectrum Beta Lactamases (ESBLs) and Amp-c beta-lactamase production. Carbapenemases are plasmid-encoded and has reduced the activity of all penicillins, monobactams, cephalosporins and carbapenem [23]. Beta lactamases cause resistance to beta-lactam agents and are produced by different aerobic gram-negative bacteria (AGNB) [24] ESBLs were discovered in 1980. The main reservoirs for these resistant organisms are hospital patients [25].
Keeping in view the aforementioned considerations, this study was conducted with the aim of reviewing current antimicrobial sensitivity and resistance pattern in males and females of different age groups, therefore contributing to the prevention of therapeutic failures and antibiotic misuse in patients with UTI.
Materials and Methods
Study area and population
This cross-sectional study was carried out at the Ziauddin Hospital in Karachi, Pakistan. This hospital receives patients from nearby urban and rural areas, as well as from other parts of the province. The study included inpatients’ urine samples collected over a period of one year from Aug 1, 2019 – Jul 30, 2020. The patients had clinical evidence of urinary tract infection as determined by their physician. Culture and susceptibility reports were obtained directly from the micro-biology lab.
Sample size
The urine samples of 400 patients were included in the study. The names of patients were not documented and only laboratory data was used. Out of the 400 samples processed, 183 (46%) depicted bacterial growth, 36 (9%) showed mixed flora, and the rest of the 181 (45%) samples were found to be sterile.
Sample collection and processing
All the patients were catheterized, so samples were collected from the urinary catheters. Samples which were collected after 3 days of catheterization were collected. In the lab, the organisms were isolated and the colony count of each organism was measured. Standard criteria were used to interpret culture results as being significant and insignificant, and significant bacteriuria was considered with the growth of >105 colony forming units/mL.
Anti-microbial susceptibility testing
17 antibiotics were tested as part of the study including, amikacin, amoxicillin-clavulanic acid, ampicillin, aztreonam, cefoperazone/sulbactam, cefixime, cefotaxime, ceftriaxone, colistin, co- trimoxazole, gentamicin, imipenem, meropenem, nitrofurantoin, ofloxacin/ciprofloxacin, polymixin and tazobactam/piperacillin. The microscopic examinations for identification of bacterial strains was done by authorized laboratory technicians, and the results of the antimicrobial discs were validated by appropriate quality control strains. Microsoft Excel was used for the entry and analysis of the data. Mean susceptibility was calculated for the antibiotics for each organism.
Results
Prevalence Rate and Frequency Distribution of UTI among Males and Females in Different Age Groups
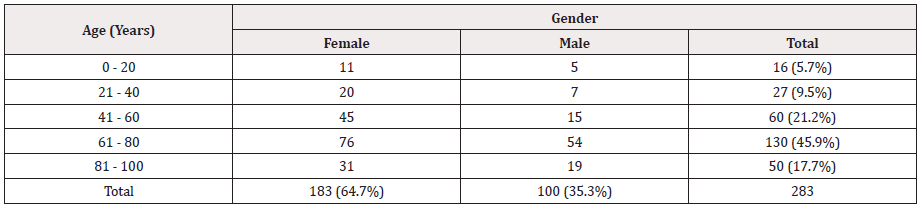
The total prevalence of UTI was found to be 64.7 % in females and 35.3 % in males as seen in Table 1, hence indicating a higher prevalence in female patients. The age group with the highest susceptibility to UTI irrespective of gender, was found to be 61- 80 years (45.9%) followed by 41-60 years (21.2%), and then 81- 100 years (17.7%). The lowest prevalence of UTI was found in age groups of 0-20 years (5.7%), followed by 21-40 years (9.5%) (Table 1).
Distribution frequency of isolated bacterial uropathogens
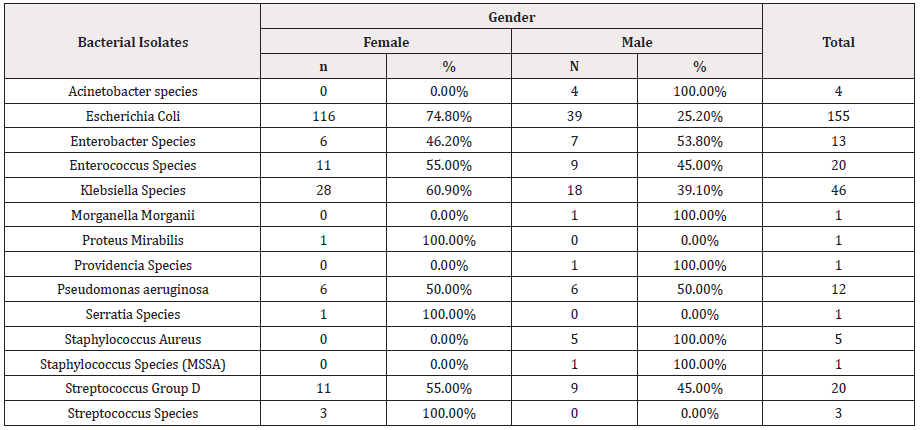
As seen in table 2, among all the isolated bacterial uropathogens from UTI patients, Escherichia coli was found as the dominant bacteria with the highest prevalence (85%), irrespective of gender. The second most prevalent isolate was Klebsiella sp. (31%) followed by Streptococcus Group D and Enterococci sp. The organisms with the lowest prevalence were found to be Morganella Morganii, Proteus sp and Serratia sp followed by Acinetobacter and Streptococcus sp (Table 2).
Gender-wise distribution of uropathogens
The prevalence rate for the occurrence of different uropathogens varied among males and females. E.coli had a higher prevalence in female patients (74.8%) compared to males (2 5.2%). similarly, Klebsiella sp.. had a prevalence rate of 60.9% in females and 39.1% in males. Enterococcus sp and streptococcus group D followed the same pattern. Enterobacter sp had a higher prevalence in males (53.8%) compared to females (46.2%).
Some organisms including Acinetobacter sp, Morganella morganii sp, Providencia sp. and Staphylococcus aureus were isolated only in male patients, whereas Proteus sp. and Serratia sp were among those isolated in only female patients. Pseudomonas sp was equally prevalent in both males and females (Table 3&4).
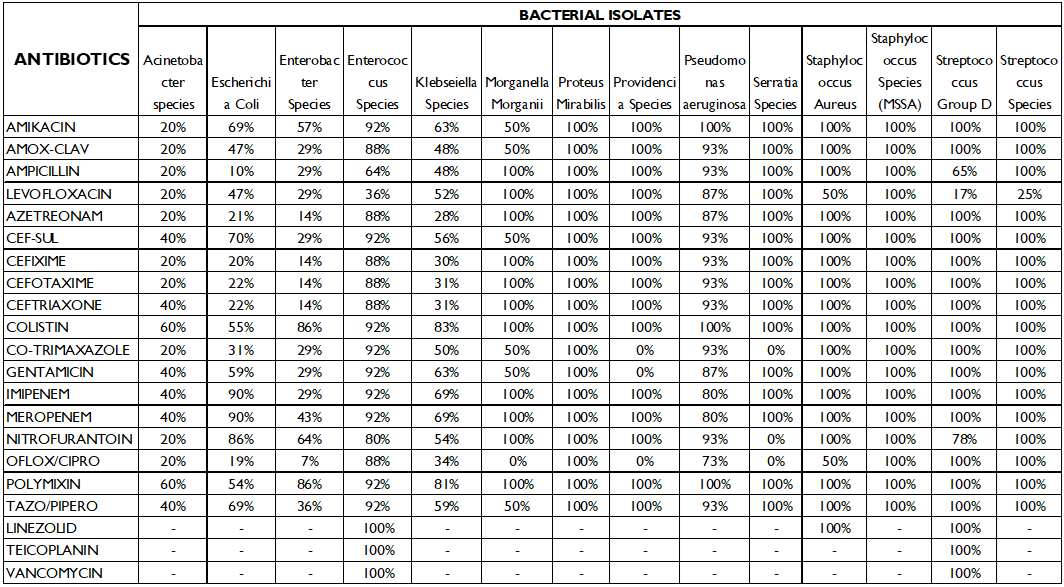
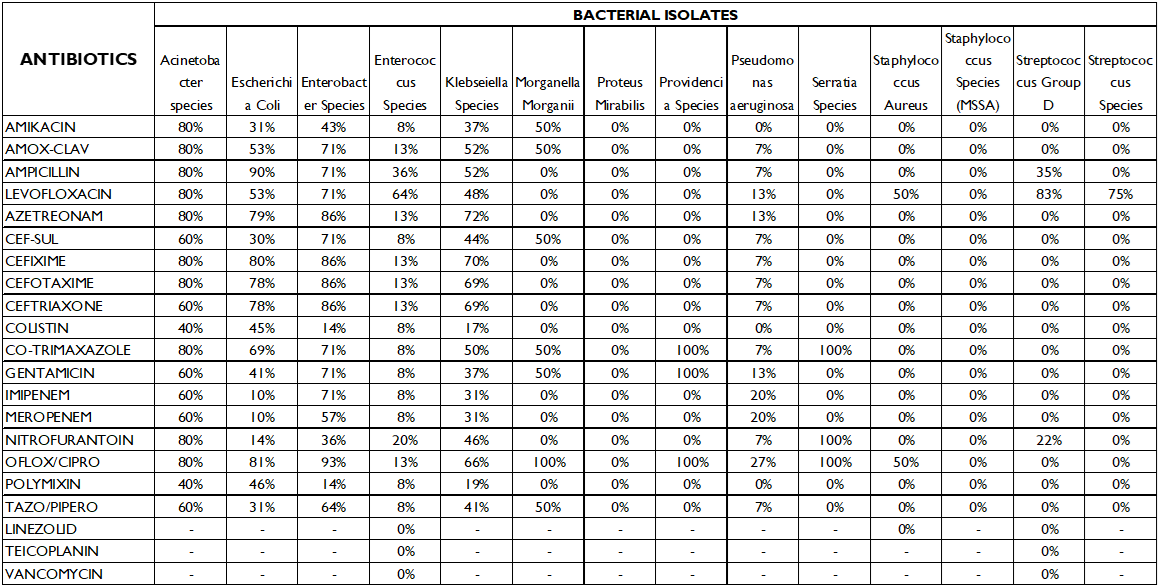
Tables 3 and 4 show the organisms’ susceptibility and resistance pattern to the 17 antibiotics that were part of the study.
Kliebsella species had the highest sensitivity to colistin (83%) and polymixin (81%) followed by imipenem and meropenem (69%). It was most resistant to aztreonam (72%) and cefixime (70%) and quinolones (66%).
Enterobacter sp. also had highest sensitivity to colistin and polymixin (86%).
Enterococcus sp. was 92% susceptible to a number of antibiotics including amikacin, cefoperazone/sulbactam, colistin, polymxin, imipenem and meropenem. It had the highest resistance to levofloxacin (64%).
Morganella morganii was 100% resistant to ofloxacin/ciprofloxacin.
Proteus sp. was 100% sensitive to all antibiotics.
Providencia sp, Pseudomonas, Streptococcus group D and Serratia sp. were susceptible to most antibiotics and had low resistance rates.
Discussion
The age and gender distribution of the patients diagnosed with UTI followed the natural epidemiological pattern of UTI, with older females being the most affected group, which is related to the difference between the male and female genitourinary systems in anatomy and microflora. This study highlights the current scenario of UTI and the anti-microbial susceptibility pattern in the urban and rural settings of other cities in the developing world. E. coli is the most frequently isolated uropathogen in females of all age categories, which correlates with other studies [26-28]. It is followed by Klebsiella sp. which is also the second most commonly isolated organism in various studies [29-31].
Increasing antimicrobial resistance has been documented in this study as well as globally [32-38]. The rapid development of resistance is due to various factors, like self-medication tendencies, noncompliance, financial constraints, and lack of education amongst patients. Other factors include the sale of antibiotic drugs without proper prescription on the part of pharmacists; low surveillance of susceptibility patterns and poor regulatory controls over antibiotics on the part of health care systems. Moreover, prescribing antibiotics before obtaining samples for culture and poor prescribing practices on the part of physicians are among many reasons that lead to the injudicious use of antibiotics, hence causing the quick development of antibiotic resistance [39-42].
The above study shows that E.coli is most susceptible to meropenem and nitrofurantoin, but it also showed a high sensitivity to cefoperazone/sulbactam (70%) and 69% susceptibility to tazobactam-piperacillin and amikacin. On the contrary we see that E.coli is most resistant to ampicillin (90%), and 81% of the isolates were resistant to ofloxacin/ciprofloxacin, therefore these may be excluded in the empiric treatment of E.coli positive patients.
For Klebsiella sp, which is the second most common uropathogen, a high susceptibility to antibiotics colistin (83%) and polymyxin (81%) was noted, however, 70% of the isolates were resistant to cefixime, and66% of the isolates were resistant to ofloxacin/ciprofloxacin, and several of the studies quoted above have shown an increased resistance to fluoroquinolones. This highlights the spread of multidrug resistant catheter associated Klebsiella UTI infections.
The use of indwelling urinary catheters used be used only for proper indications as per the 2009 Centers for Disease Control and Prevention (CDC) guidelines for the prevention of catheter-associated urinary tract infections (UTIs). The use of catheters as well the duration of use should be minimized, especially for those patients who are at higher risk for catheter-associated UTI (eg, women, elderly persons, and patients with low immunity). Catheters should be only for as long as needed. Indwelling catheters placed in patients undergoing surgery should be removed as soon as possible after surgery, and the use of urinary catheters for the treatment of incontinence should be avoided [43].
Conclusion
Resistance to antibiotics is a leading cause of therapeutic failures all over the world. This study was therefore aimed at studying the antimicrobial resistance trends for aiding clinicians in deciding the appropriate empirical treatment, hence improving patient outcome. This may also help in preventing the misuse of antibiotics in UTI patients. As this was a cross-sectional study, further regular monitoring and a continuous review of antibiograms is also necessary to track changes in etiological agents and antimicrobial patterns to help in empirical treatment. A unified antibiotic protocol is necessary to restrict the use of antibiotics injudiciously in order to prevent resistance and reduce the complications of UTI arising from the use of resistant drugs.
Acknowledgment
The authors would like to thank Farzana Aslam, RN, Department of Infectious Diseases, Ziauddin University, Karachi, Pakistan for helping with the provision of the requisite data to the authors for the study.
Funding
No funding sources.
Conflict of interest
The authors declare no conflicts of interest.
References
- Ejrnæs K (2011) Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan Med Bull 58(4): B4187.
- Matthew GB, Matthew AM (2010) Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother 54(5): 1855-1863.
- Ronald AR, Patulo MS (1991) The natural history of urinary infection in adults. Med Clin North Am 75(2): 299-312.
- Munasinghe RL, Yazdani H, Siddique M, Hafeez W (2001) Appropriateness of use of hnindwelling catheters in patients admitted to the medical service. Infect Control Hosp Epidemiol 22(10): 647-649.
- Gupta P, Mandal J, Krishnamurthy S, Barathi D, Pandit N (2015) Profile of urinary tract infections in paediatric patients. Indian Med Res 141: 473-477.
- Vergidis P, Patel R (2012) Novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect Dis Clin North Am 26(1): 173-186.
- John L Brusch, Michael Stuart Bronze (2021) Catheter-Related Urinary Tract Infection (UTI). Drugs & Diseases.
- R N Das, T S Chandrashekhar, H S Joshi, M Gurung, N Shrestha, et al. (2006) Frequency and susceptibility profile of pathogens causing urinary tract infections at a tertiary care hospital in western Nepal, Singapore Med J 47(4): 281-285.
- Ihsan A, Zara R, Safia A, Sajid M, Javid ID (2016) Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan. Asian Pac J Trop Biomed 6(1): 60-66.
- Alteri CJ, Mobley HL (2015) Metabolism and Fitness of Urinary Tract Pthogens. Microbiol Spectr 3(3): 10.
- Hegde A, Bhat GK, Mallya S (2008) Effect of exposure to hydrogen peroxide on the virulence of Escherichia coli. Indian J Med Microbiol 26: 25-28.
- Jharna M, Srinivas AN, Buddhapriya D, Subhash CP (2012) Antibiotic resistance pattern among common bacterial uropathogens with a special reference to ciprofloxacin resistant Escherichia coli. Indian J Med Res 136: 842-849.
- Fair RJ, Tor Y (2014) Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem 6: 25-64.
- Moroh JLA, Fleury Y, Tia H, C Bahi, C Lietard, et al. (2014) Diversity and antibiotic resistance of uropathogenic bacteria from Abidjan. Afr J Urol 20: 18-24.
- Nickel JC (2005) Management of urinary tract infections: Historical perspective and current strategies Part 2-modern management. J Urol 173: 27-32.
- Spoorenberg V, Hulscher ME, Akkermans RP, Prins JM, Geerlings SE (2014) Appropriate antibiotic use for patients with urinary tract infections reduces length of hospital stay. Clin Infect Dis 58: 164-169.
- Sharma N, Gupta A, Walia G, Bakhshi (2016) R Pattern of antimicrobial resistance of Escherichia coli isolates from urinary tract infection patients A three year retrospective study. J Appl Pharm Sci 6: 62-65.
- Shobha Prasada, Archana Bhat, SevithaBhat, Shalini Shenoy Mulki, Sanyuktha Tulasidas (2019) Changing antibiotic susceptibility pattern in uropathogenic Escherichia coli over a period of 5 years in a tertiary care center. Infect drug resist 12: 1439-1443.
- Alos JI (2005) Epidemiology and etiology of urinary tract infections in the community Antimicrobial susceptibility of the main pathogens and clinical significance of resistance. Enfermedades Infecciosas Microbiologia Clinica 4: 3-8.
- McNulty CAM, Richards J, Livermore DM (2006) Clinical relevance of laboratory-reported antibiotic resistance in acute uncomplicated urinary tract infection in primary care. J Antimicrob Chemother 58: 1000-1008.
- Car J (2006) Urinary tract infections in women diagnosis and management in primary care. British Medical Journal 332(7533): 94-97.
- Tambekar DH, Dhanorkar DV, Gulhane SR, Khandelwal VK, Dudhane MN, et al. (2006) Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotechnol 5: 1562-1565.
- Arpin C, Dubois V, Coulange L, Andre C, Fischer I , et al. (2003) Extendspectrum s-lactamase producing Enterobacteriaceae in community and private health care centers. Antimicrob Agents Chemother 47: 3506-3514.
- Aggarwal R, Chaudhary U, Sikka R (2009) Detection of extended spectrum beta-lactamase production among uropathogens. J Lab Physicians 1: 7-10.
- Wiener J, Quinn J, Bradford P, Goering R, Nathan C, et al. (1999) Multiple antibiotic resistant Klebsiella and Escherichia coli in nursing homes. JAMA 281: 517-523.
- Nys S, van Merode T, Bartelds A I M, Stobberingh E E (2006) Urinary tract infections in general practice patients diagnostic tests versus bacteriological culture. J Antimicrob Chemother 57(5): 955-958.
- Hazarika J, Baruah K (2018) Urine culture isolates and their antibiotic sensitivity pattern in a Tertiary Care Hospital of North East India. PARIPEX-INDIAN JOURNAL OF RESEARCH 7(7): 17-18.
- Nys S (2005) Antibiotic resistance and commensal flora. Microbiology, Netherlands pp. 142.
- George CE, Norman G, Ramana GV, Mukherjee D, Rao T (2015) Treatment of uncomplicated symptomatic urinary tract infections Resistance patterns and misuse of antibiotics. J Family Med Prim Care 4: 416-421.
- Prakash D, Saxena RS (2013) Distribution and antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infection in urban community of Meerut city, India. ISRN Microbiology 749629.
- Somashekara SC, Deepalaxmi S, Jagannath N, Ramesh B, Laveesh MR, et al. (2014) Retrospective analysis of antibiotic resistance pattern to urinary pathogens in a tertiary care hospital in South India. J Basic Clin Pharm 5: 105-108.
- Claudia V, Francesca L, Maria PB, Gianfranco D, Pietro EV, et al. (2014) Antibiotic Resistance Related to Biofilm Formation in Klebsiella pneumoniae.Pathogens 3(3): 743-758.
- Kashef N, Djavid GE, Shahbazi S (2010) Antimicrobial susceptibility patterns of community- acquired uropathogens in Tehran Iran. J Infect Dev Ctries 4(4): 202-206.
- Karlowsky JA, Jones ME, Thornsberry C, Critchley I, Kelly LJ, et al. (2001) Prevalence of antimicrobial resistance among urinary tract pathogens isolated from female outpatients across the US in 1999. Int J Antimicrob Agents 18(2): 121-127.
- Rajalakshmi V, Amsaveni V (2011) Antibiotic susceptibility of bacterial pathogens isolated from diabetic patients. International Journal of Microbiological Research 2: 273-275.
- Sharifian M, Karimi A, Tabatabaei SR, Anvaripour N (2006) Microbial sensitivity pattern in urinary tract infections in children: a single center experience of 1,177 urine cultures. Jpn J Infect Dis 59: 380-382.
- Haghi-Ashteiani M, Sadeghifard N, Abedini M, Soroush S, Taheri-Kalani M, et al. (2007) Etiology and antibacterial resistance of bacterial urinary tract infections in children’s medical center, Tehran Iran. Acta Medica Iranica 45: 153-157.
- Rashed marandi FRM, Saremi M (2008) A survey on urinary pathogens and their antimicrobial susceptibility among patients with significant bacteriuria. Iran J Pathol 3(4): 191-196.
- WHO (1996) The World Health Report 1996. Geneva, Switzerland.
- WHO (2017) National Action Plan on Antimicrobial Resistance (NAP-AMR) 2017-2021. New Delhi, India.
- Laxminarayan R, Chaudhury RR (2016) Antibiotic resistance in India: Drivers and opportunities for action. PLoS Med 13: e1001974.
- Porter G, Grills N (2016) Medication misuse in India: A major public health issue in India. J Public Health (Oxf) 38: e150-157.
- Gould CV, Umsheid CA, Agarwal RK, Kuntz G, Pegues DA (2010) Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 31(4): 319-326.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.