Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Biosimilar Drugs in Albania “current situation”
*Corresponding author: Ilda Mallkuçi, National Agency for Medicines and Medical devices, Albania
Received: May 25, 2021; Published: June 14, 2021
DOI: 10.34297/AJBSR.2021.13.001847
Introduction
The use of biologicals is very important in the treatment of mild illnesses ranging from anaemia to life threatening cancers and neurological disorders. These biologics include products such as vaccines, blood and blood components, allergenics, somatic cells, gene therapy, tissues and recombinant therapeutic proteins [1]. However, increasing use of these effective therapies is limited by their costs and accessibility. The expiry of patents of these products created new opportunities for the development of similar biologics (biosimilars) that could offer comparable effective and quality healthcare at an affordable cost [2-4]. To achieve comparable quality, efficacy and safety to their respective originator biologicals, strict compliance with appropriate science-based regulatory standards is essential to approve a biosimilar product [4].
Aim of the Review
To give a present situation of biosimilars and the originators authorized for Marketing in Albania. Issues regarding Law and Regulations, Reimbursement situation.
Legal Basis for Biosimilars in Albania
For the first time the definition Biosimilar was mentioned in
Albanian legislation in 2014, Law No.105/2014, 31.07.2014 “On
drugs and pharmaceutical service”, amended: A biosimilar is a
biological drug that is highly similar to another biological drug
which already has a marketing authorization (reference biological
product) and has the same active ingredient, dosage form and
route of administration as the reference product, for which it
is determined through a quality, safety and efficacy program.
This drug doesn’t fulfill the criteria to be classified as a generic
drug because it differs from the biological drug regarding the
ingredients and manufacturing process and for these reasons is not
substitutable [5]. The total fee, paid by the pharmaceutical company
that has the exclusivity of a certain biosimilar (not an antitumoral
one) for Albania is 800€ (100€ prepayment prior to application)
and 700€ final payment, after the issuance of Order of Minister.
If the biosimilar is designed as an antitumoral drug than the total
fee is 500€ (100€ prepayment prior to application) and 400€ final
payment, the issuance of Order of Minister [5]. This means that the
Albanian Low creates financial incentives only for the antitumoral
drugs, and it is not important if they are originators, generics or
biosimilars. According to the Decision of the Council of Ministers
No. 299, 08.04.2015 “On the regulation for issuing the marketing
authorization for drugs and their classification in the Republic of
Albania” the requirements for issuing the marketing authorization
for a biosimilar in Albania are as follows:
a) Certificate of the Pharmaceutical Product (CPP), according
to WHO (original document).
b) Good Manufacturing Practice Certificate (notarized copy)
or EudraGMP or from the FDA site, valid in the moment of the
application.
c) Marketing authorization certificate/decision from FDA or
EMA, or in two countries of European Community or the last
amendment (translated into English.)
d) Modules 1-5 in CTD format.
Specific Requirements for Special Types of Applications
a) Together with the application for marketing authorization
of a biological drug, the applicant is required to attach the
modules 1, 2, 3, 4 and 5, which contains the information
specified in the regulation. Modules 4 and 5 must contain
additional information on preclinical and/or clinical trials that
are performed, in order to prove similarity of two biological
drugs.
b) Additional data (for example: toxicological and other
non-clinical documentation and appropriate clinical records),
that should be submitted, are determined by the Agency,
in the procedure for individual applications assessment, in
accordance with appropriate scientific findings.
c) Because of the diversity of biological drugs, the applicant
is required for modules 4 and 5 to submit data on performed
evidence taking into account the specific characteristics for
each biological drug.
d) If the reference drug has more than one indication, the
usage safety and effectiveness of biosimilar drug should be
explained/justified or if necessary, must be verified specifically
for each listed indication [6].
Specific Features of Biosimilars According to EMA
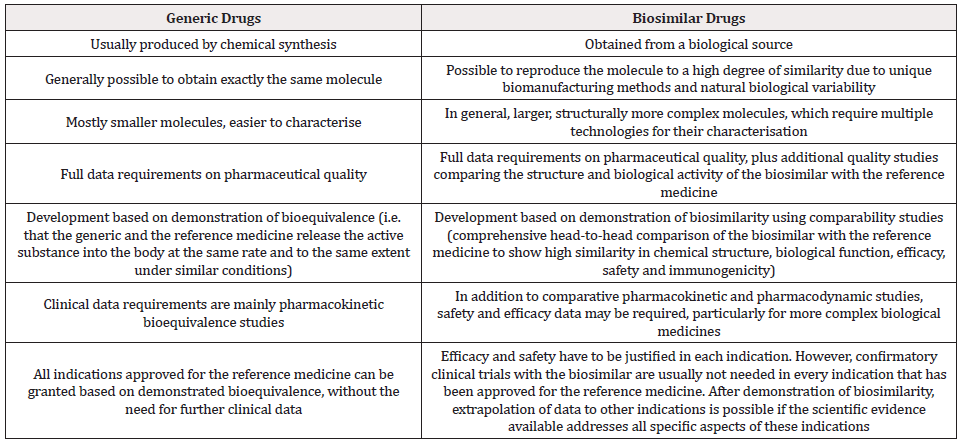
Highly similar to the reference drug: The biosimilar has physical, chemical and biological properties highly similar to the reference drugs. There may be minor differences from the reference medicine which are not clinically meaningful in terms of safety or efficacy. No clinically meaningful differences compared with the reference drug: No differences are expected in clinical performance. Clinical studies that support the approval of a biosimilar confirm that any differences will not have an effect on safety and efficacy. Variability of biosimilar kept within strict limits: Minor variability is only allowed when scientific evidence shows that it does not affect the safety and efficacy of the biosimilar [Table 1]. The range of variability allowed for a biosimilar is the same as that allowed between batches of the reference drug. This is achieved with a robust manufacturing process to ensure that all batches of the drug are of proven quality. Same strict standards of quality, safety and efficacy: Biosimilars are approved according to the same strict standards of quality, safety and efficacy that apply to any other drug [7].
Why Biosimilars are not Considered Generic Drugs
A biosimilar is not considered as a generic of a biological drug. This is mostly because the natural variability and more complex manufacturing of biological drugs do not allow an exact replication of the molecular microheterogeneity. Consequently, more studies are needed for regulatory approval of biosimilars than for generics to ensure that minor differences do not affect safety or efficacy [7]. Generic, or small molecule drugs are produced via a chemical synthesis and are identical copies of their originator drug product. When manufacturers seek the approval for a generic, they must establish bioequivalence tests that deem the two identical. Generic products do not require the additional testing requirement of clinical studies since they do not derive from living organisms, unlike biologics. With generics, the responsibility to prescribe lies with the physician, whereas accountability to dispense lies with the delivering pharmacist. Given the widespread use of generics, physicians are being encouraged to prescribe medicines using their international non-proprietary names (INN), as opposed to their commercial ones. With biosimilars, due to the complexity of recreating a product which is made by living organisms, strict quality, safety and efficacy criteria need to be followed. These criteria include the submission of data from preclinical and clinical studies, among other requirements, to test the degree of similarity to the originator and the consequent safety and efficacy of the final product. Importantly, due to the complexity of the process, different batches of a particular moAb could even be considered biosimilar versions of the moAb given they do not follow a purely chemical pathway but are made from living cells [8].
Methods & Results
Authorized Biosimilars in Albania
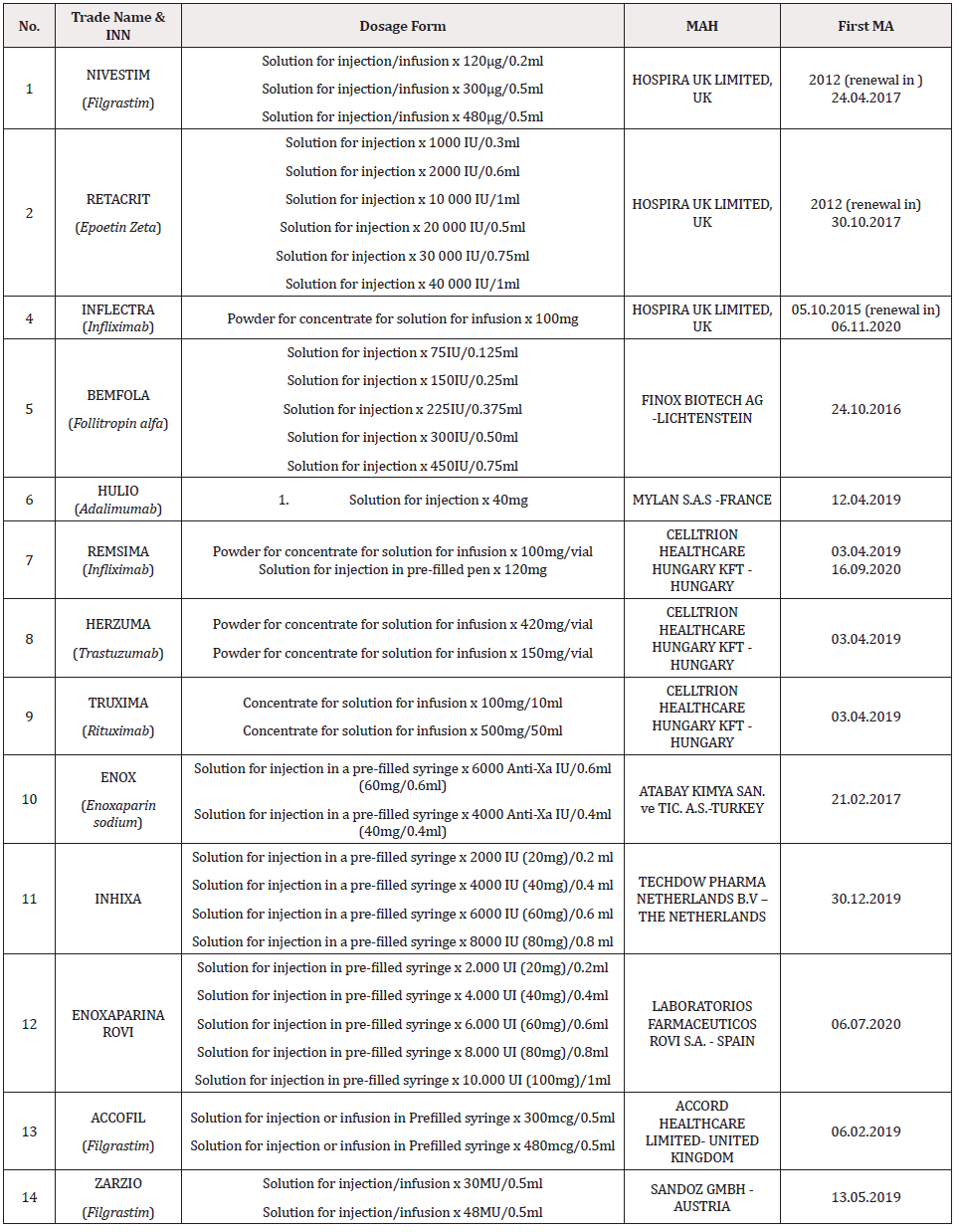
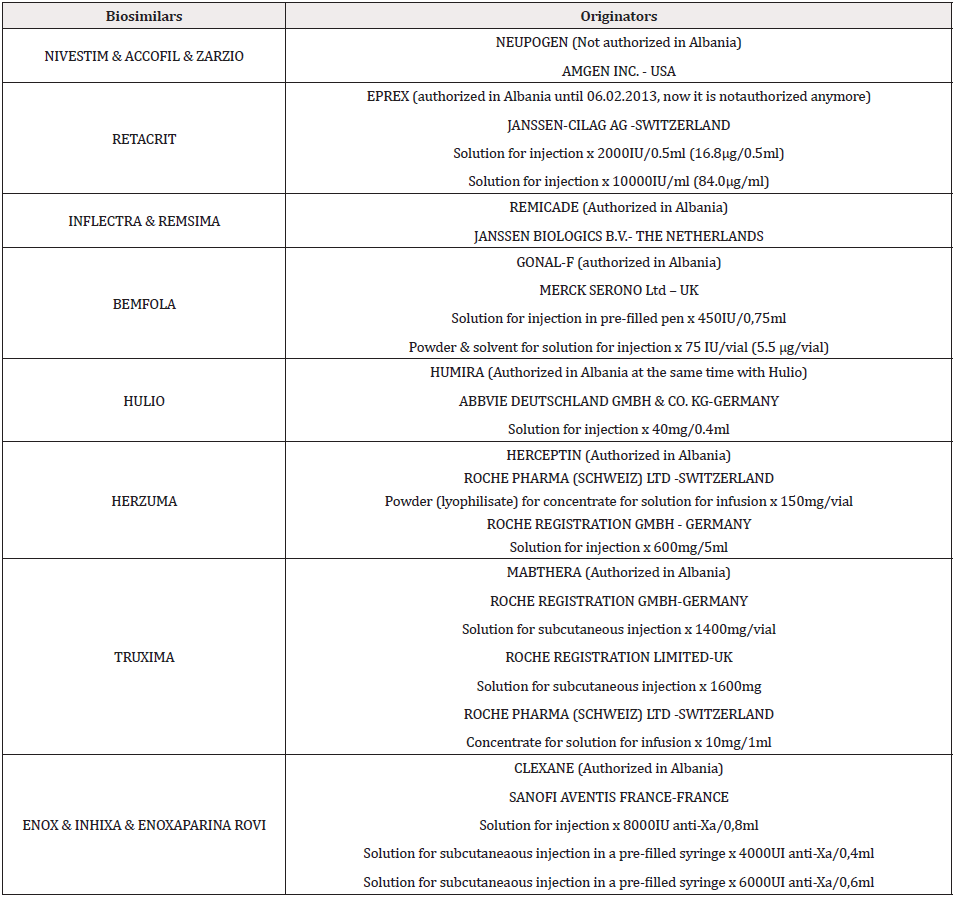
There are 14 biosimilars (each of them with different dosage forms), (see table 2) authorized for marketing in Albania designed for different diseases, from different marketing authorization holders. As it is demonstrated in Table 2, Nivestim is authorized for marketing in three dosage forms since 2012, Retacrit in six dosage forms since 2012, Binocrit in two dosage forms since 2015, Inflectra in one dosage forms since 2015, Bemfola in six dosage forms in 2016, Enox in two dosage forms in 2017, Inhixa in 4 dosage forms in 2019 and last ones are Remsima, Herzuma, Truxima, Hulio, Accofil and Zarzio in 2019. Remsima 120mg was authorized for marketing in 2020. Enoxaparina Rovi was authorized for marketing in 2020 in 5 dosage forms. As it is seen in Table 3, in Albania are authorized for marketing both the originator and the biosimilar for Bemfola, Hulio, Truxima Herzuma, Inflectra, Remsima Enox, Inhixa and Enoxaparina Rovi. In their cases we can talk about interchangeability between the biosimilar and his originator, but it is very important to know that in Albania there are no Legal Basis about the interchangeability, indication extrapolation or traceability of biosimilars and this is a big issue, because for the moment it is not controlled from the Authorities and it is only in the hands of the healthcare professionals. Without any Legal basis it is very difficult to control the situation among patients and it is always the risk to switch back and forth between the biosimilar and the originator without any criteria and control. This uncontrolled interchangeability may cause risks for the patients and is mandatory in our country to create Legal Basis for these issues. Nivestim, Accofil, Zarzio and Retacrit are the only drugs that we have in Albania, their originators are not authorized, so they are the only alternative in the market for patients, this means that the healthcare professionals don’t need to discuss about interchangeability, and this is another important issue in our country, because the Authorities don’t possess the dossiers for the originators of this biosimilars in order to compare it with the one that are submitted for the biosimilars.
Important Parameters which Define Biosimilars
Indication Extrapolation: For prescribers, extrapolation is an extremely important component to the concept of bio similarity. The EMA defines extrapolation as ‘extending information and conclusions available from studies in one or more subgroups of the patient population (source population) to make inferences for another subgroup of the population (target population), or condition or product, thus reducing the need to generate additional information… to reach conclusions for the target population [9]. For a biosimilar product to be approved, it is not always necessary to conduct clinical efficacy studies in every indication for which the reference drug was approved. The biosimilar must demonstrate that there are no clinically meaningful differences relative to the reference drug in a sensitive patient population i.e. a group of patients where any differences between the two drugs are most likely to be revealed. The scientific justification for extrapolation to other indications not studied in the biosimilar clinical programme is evaluated as part of the assessment process on a case-by-case basis, based on the totality of data (quality, non-clinical and clinical data). In line with this the biosimilar may be approved in all indications for which the reference product is approved. This is referred to as indication extrapolation. It is an important concept for healthcare professionals to be familiar with, given that efficacy trials for the medicine may not have been carried out in all proposed treatment groups. However, extrapolation is based on scientific principles requiring specific structural, physiochemical and biological comparability data justifying its acceptance [9,10].
Interchangeability: Interchangeability between biosimilars
and reference products is an ongoing area of debate. While
prescribing practices are at the discretion of healthcare
professionals, it is not recommended that patients are switched
back and forth between a biosimilar and the reference drug product.
It is recommended to have consultations between prescribers,
pharmacists and procurement staff in relation to deciding on
treatment preferences for using a reference or a biosimilar drug [9,
10].
Traceability: There are specific issues surrounding the traceability and pharmacovigilance of all biological drugs including biosimilars. All biologicals, including biosimilars, should be prescribed, dispensed and sold in a way where the product supplied to the patient is clearly identifiable. Similarly all appropriate measures should be taken to clearly identify any biological drug product which is the subject of a suspected adverse reaction report, with due regard to its brand name and batch number. Requirements and recommendations for recording relevant information when dispensing biosimilars, and ensuring the maintenance of product traceability are outlined in the guide [9,10].
Pharmacovigilance and Adverse Drug Reaction Reporting: Pharmacovigilance and monitoring of adverse events are usual components of the authorization process and use of any new drug, including biosimilars in Albania. As is the case for all biological drugs, the clinical safety of biosimilars must be monitored closely on an ongoing basis during the post-approval phase, including continued benefit-risk assessment. Any specific safety monitoring imposed on the reference drug or drug class will also apply to the biosimilar. Reporting of adverse reactions to drug authorities is mandated for marketing authorization holders in Albania. Adverse reactions are reported through a database maintained by the EMA known as Eudravigiilance. Healthcare professionals should also report any adverse reactions they are aware, near the Albanian Agency [9,11-13].
Reimbursement of biosimilars in Albania, “present
situation” [10]:
In the reimbursement list (the last one that was released by the
Authorities in Albania) the only biosimilars reimbursed in Albania
are Retacrit 2000IU, 30000IU, Binocrit 2000IU, 30000IU & Inflectra
100mg.
Biological & Biosimilar Drugs in Reimbursement list of 2016:
B Blood and hematopoiesis organs
B03 Drugs against anemia
a) 544/335 B03XA01 Epoetin Zeta 2000 Nj.N - Retacrit
b) 633/335 B03XA01 Epoetin Zeta 30 000 Nj.N - Retacrit
c) 55/237 B03XA01 Epoetin Alfa 2000 Nj. N – Binocrit
L04 Imunosuppresive
a) 631/307 L04AB02 Infliximab 100 mg - Inflectra
631/307 L04AB02 Infliximab 100 mg - Inflectra
Conclusion
As noted above, a biosimilar is not identical to the reference product chosen. While science and scientific and therapeutic principles play a role in both interchangeability/substitutability and extrapolation of indications, each country has a unique approach, based on national and local laws and practice issues and perceptions. In Albania we have a urgent need for regulations that govern interchangeability/substitutability of biosimilars and the extrapolation of their indications, are also needed administrative processes regarding the substitution of a prescribed product with another ‘equivalent product’. It is also recognized that local medical practice and standards of practice contribute to the use of a drug which, in the case of a new drug such as a biosimilar, carries several unknowns. That is why in many, if not most, countries automatic substitution of biosimilar with the reference product is not recommended and why Albania has to take a cautious approach to extrapolation of indications and uses. Increased awareness amongst the stakeholders regulators, prescribing physicians, medical societies, pharmacists and patients – to the barriers and plausible solutions could help improve further uptake of biosimilars. It is hoped that with increased experience, some of these uncertainties and misgivings will be overcome.
Recommendations
Approaching the Albanian legislation with EU legislation and with the legislation of region countries regarding biosimilar drugs. Creating a data base on the extension of use of biosimilars in Albania. Creating new cooperation bridges with the relevant Agencies of other countries to share the experiences in the field of orphan medicines.
References
- Rader RA (2008) (Re) defining biopharmaceutical. Nat Biotechnol 26(7): 743-751.
- Dranitsaris G, Amir E, Dorward K (2011) Biosimilars of biological drug therapies: regulatory, clinical and commercial considerations. Drugs 71(12): 1527-1536.
- Roger SD (2010) Biosimilars: current status and future directions. Expert Opin Biol Ther 10(7): 1011-1018.
- McCamish M, Woollett G (2012) The state of the art in the development of biosimilars. Clin Pharmacol Ther 91(3): 405-417.
- (2015) National Agency for Medicines and Medical devices, Tirana, Albania. (Law No.105/2014, 31.07.2014 “On drugs and pharmaceutical service” amended and by the Decision of the Council of Ministers.
- (2015) National Agency for Medicines and Medical devices, Tirana, Albania. Decision of the Council of Ministers.
- Biosimilars in the EU, Information guide for healthcare professionals.
- Josep Tabernero, Malvika Vyas, Rosa Giuliani, Dirk Arnold, Fatima Cardoso, et al. (2016) Biosimilars: a position paper of the European Society for Medical Oncology, with particular reference to oncology prescribers, ESMO Open 1(6): e000142.
- (2013) European Medicines Agency. Concept paper on extrapolation of efficacy and safety in medicine development. London: European Medicines Agency.
- Albania reimbursement list 2016 database.
- Joan O'Callaghan, Margaret Bermingham, Maurice Leonard, Frank Hallinan, Michael Morris, et al. (2017) Assessing awareness and attitudes of healthcare professionals on the use of biosimilar medicines: A survey of physicians and pharmacists in Ireland. Regulatory Toxicology and Pharmacology 88: 252-261.
- National Agency for Medicines and Medical devices Database.
- Agnes V Klein, Jian Wang, Patrick Bedford (2014) Subsequent entry biologics (biosimilars) in Canada: approaches to interchangeability and the extrapolation of indications and uses. Generics and Biosimilars Initiative Journal 3(3): 150-154.
- Covid-19 Pandemic. European Medicines Agency.
- (2017) Pharmacovigilance of biologics and biosimilars.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.