Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Inflamyar™ Possesses Anti-Inflammatory Effect and Induces Growth Factor Expression on Human Dermal Fibroblasts In Vitro
*Corresponding author: Dr. rer. nat. Matthias Schulte, Andreas Wentzensen Research Institute BG Trauma Center Ludwigshafen, Rhine, Hand and Plastic Surgery, University Heidelberg, Ludwig-Guttmann-Str.13, D-67071 Ludwigshafen, Germany.
Received: July 22, 2021; Published: August 05, 2021
DOI: 10.34297/AJBSR.2021.13.001919
Abstract
Introduction: Wound healing describes a highly complex process that is essential for protecting the human body from the harmful effects of the outside world after skin structure injury. The search for remedies that support wound healing is one of the oldest challenges in medicine. Since ancient times, herbal extracts have been studied for these purposes. Overall, however, the individual effects of active ingredients from plant extracts are very diverse and have so far been described insufficiently due to the enormous diversity of active substances and their processing, application form and dosage. In this study, the effect of Inflamyar™, a commercially available homeopathic spagyric product, on wound healing was evaluated.
Methods: Primary human dermal fibroblasts were pre-incubated with the test substance with/without a subsequent inflammatory stimulus (LPS). Consecutively, the expression of cytokines was assessed.
Results: In the absence of an inflammatory stimulus, an induced cytokine and growth factor expression was found in human dermal fibroblasts. In the presence of an inflammatory stimulus, most of the cytokines showed either no reaction or a reduction, especially at higher concentration levels of the tested substance. An increase in the anti-inflammatory cytokine IL-1ra and the growth factors bFGF, VEGF and GM-CSF were found at higher concentrations of the tested substance.
Conclusions: The test substance enhances normal immune surveillance of human dermal fibroblasts in absence of an inflammatory stimulus. Under inflammatory conditions, the data suggests a shift from an inflammative environment to a tissue regenerative environment after incubation with the test substance.
Keywords: Inflamyar™, Flamyar™, human dermal fibroblasts, Homeopathy, Spagyric
Abbreviations: FGF: Basic fibroblast growth factor; DMEM: Dulbecco´s Modified Eagle Medium; FBS: Fetal bovine serum; G-CSF: Granulocytecolony stimulating factor; GM-CSF: Granulocyte-macrophage colony stimulating factor; IFN: Interferon; IP-10: Interferon γ -induced protein 10; IL: Interleukin; JAK/STAT: Janus kinase/signal transducer and activator of transcription; LPS: lipopolysaccharide; MIP: Macrophage Inflammatory Protein; MCP-1: Monocyte chemoattractant protein 1; PDGF: Platelet-derived growth factor; RANTES: Regulated on activation, normal T cell expressed and secreted; TNF: Tumor necrosis factor; VEGF: Vascular Endothelial Growth Factor
Introduction
With a surface of 2 m2 and a weight of 10-12 kg, the skin is the largest human organ. In addition to the protection against external mechanical and physical influences, the skin also plays an important role as an active immune organ against the infestation of microorganisms [1]. In the case of a skin structure injury, wound healing and thus the reconstitution of the barrier function is of central importance. Wound healing is a highly complex, dynamic process in which a variety of different cellular and humoral components work together in different phases. The beginning of wound healing is called the inflammatory or exudation phase. If the blood vessels are damaged, blood coagulation begins and a fibrin net, which serves as a matrix for the newly formed granulation tissue, forms. Neutrophil granulocytes migrate through the capillaries into the tissue, secreting cytokines and protein-degrading proteases. Immigrated macrophages eliminate microorganisms through phagocytosis and secrete growth factors and cytokines. These stimulate the proliferation and migration of fibroblasts and vascular endothelial cells into the wound bed. Capillaries sprout into the wound area and precursors of extracellular matrix are synthesized.
The final closure of the wound area is achieved by contraction of the wound area and by immigration of fibroblasts and keratinocytes into the wound bed [2]. The search for remedies that support wound healing is one of the oldest challenges in medicine. Since ancient times, the use of topically applied extracts from medicinal plants has been widespread throughout the world [3]. Many representatives of the plant kingdom show an enormous potential for the treatment of wounds [4,5]. Their natural active ingredients induce healing and regeneration of the injured tissue through different mechanisms [4]. These components include various groups of substances such as terpenoids, polyphenols, alkaloids and essential oils, polypeptides and lectins and other compounds [6].
Active ingredients of plant origin are not only inexpensive, but also have relatively low side effects compared to many synthetic substances [7,8]. This is not least due to the many years of experience in the use of these substances. Important factors for the effect of these substances are their exact composition, dose, processing and administration form. In particular, the dose of these drugs is an important factor in their effectiveness. This was already expressed in the 16th century by the alchemist and physician Paracelsus (“All things are poison and nothing is without poison; just the dose makes sure that a thing is not poison.”) [9]. Thus, different plant compounds show a toxic effect at high concentrations and a healing effect at low concentrations. In fact, the relevant literature does not distinguish between poisonous plants or medicinal plants. Some studies have shown efficacy of plant extracts in different indications even at highly diluted concentrations [10-13]. Overall, the individual effects of active ingredients from plant extracts are very diverse and have so far been described only inadequately [14]. For this reason, further investigations are necessary.
In this study, the effect of Inflamyar™, a commercially available homeopathic spagyric product consisting of plant extracts from Arnica montana, Bryonia cretica, Guajacum, Toxicodendron quercifolium, Bellis perennis, Ledum palustre, Ruta graveolens and Viscum album, on wound healing was investigated in vitro.
Material and Methods
Test Substance
The test substance, Flamyar™, is a homeopathic spagyric natural remedy manufactured by PEKANA Naturheilmittel GmbH (Kißlegg, Germany) and distributed in the USA under the name Inflamyar™. The test substance was developed for the treatment of sports injuries, sprains, joint problems, bruises, and muscle strains. Active ingredients are Arnica montana spag. Peka Dil. D12, Bryonia cretica spag. Peka Dil. D4, Guajacum Dil. D4, Toxicodendron quercifolium Dil. D12, Bellis perennis spag. Peka Dil. D8, Ledum palustre Dil. D4, Ruta graveolens spag. Peka Dil. D6, Viscum album spag. Peka Dil. D4.
Cell Culture
Human dermal fibroblasts obtained from Zenbio (Durham, NC, USA) were cultivated in DMEM (Dulbecco’s Modified Eagle Medium) containing 10% fetal bovine serum (FBS) and antibiotics (100ug/ mL penicillin/streptomycin, Gibco, Life Technologies, Eugene, OR USA) with a density of 32,000 cells per well in 24-well plates. After 16 hours, cell culture medium was replaced by 900ul fresh medium plus 100ul of each treatment and cells cultivated for another 24 hours. After collection, the supernatants were analyzed for cytokine/growth factor quantitation using Luminex-based arrays. Treatments of cells with the test substance were tested in duplicate. Untreated and LPS alone cultures were tested in triplicate.
Cytokine Expression in Human Dermal Fibroblast Cell Cultures
Supernatants were harvested as described above. The expression levels of the following cytokines were tested: Interleukin (IL)-1β, -1ra, -2, -4, -5, -6, -7, -8, -9, -10, -12 (p70), -13, -15, -17, eotaxin, basic fibroblast growth factor (FGF), granulocytemacrophage colony stimulating factor (GM-CSF), granulocytecolony stimulating factor (G-CSF), Interferon γ (IFN-γ), interferon γ -induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein (MIP)-1α, MIP- 1β, platelet-derived growth factor (PDGF)-BB, regulated on activation, normal T cell expressed and secreted (RANTES), tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF). The assay was analyzed using magnetic protein multiplex arrays (BioPlex, Bio-Rad Laboratories Inc.) and xMAP technology (Luminex, Austin, TX, USA).
Statistical Analysis
Calculations and statistical analysis was performed using the two-tailed, independent t-test using Microsoft Excel.
Results
Primary human fibroblasts were used as a model for potential immune modulating activities. The direct effect on the one hand and the effect of the preincubation of fibroblasts with the test substance and the subsequent inflammatory stimulus (LPS) on the other hand were tested. The exposure to the test substance without a subsequent inflammatory stimulus (LPS) showed no effect on proinflammatory cytokine expression of IL-1β, IL-5, IL-13, IL-17A, MIP-1β, RANTES and TNF-α. An induction of proinflammatory cytokine expression was seen in IL-6 (27.03 to 36.37%), IL-8 (4.77 to 34.52%), IL-12p70 (43.87 to 80.88%), IP-10 (38.81 to 503.21%), Eotaxin (up to 85.85%), MCP-1 (208.36 to 346.56%) and MIP-1α (211.54 to 279.33%). Variable results were seen in IFNγ (27.05 to -51.03%). An induction of anti-inflammatory cytokines was seen in IL-1ra (6.47 to 20.86%) and, at higher concentrations of the test substance, in IL-10 expression (up to 25.54%).
Regarding expression of cytokines with pro- and antiinflammatory activity, the treatment of the fibroblasts with the test substance resulted in an increased expression of IL-4 (up to 144.65%), IL-7 (32.52 to 169.92%) and IL-9 (136.11 to 284.32%) and IL-2 (up to 49.66%). No changes were seen in expression of IL- 15. Moreover, an induction of the growth factor VEGF was assessed (15.27 to 31.04%), whereas PDGF-BB showed an induction at higher concentrations (up to 65.2%). Expression of bFGF, G-CSF and GM-CSF showed no changes.
The exposure of fibroblasts to the test substance with a subsequent inflammatory stimulus (LPS) led to consistent decreases of proinflammatory IFNγ (-7.31% to 21.92%), IL-1β (up to -12.95%), IL-13 (-15.20 to -39.61%), Eotaxin (-4.82 to 19.31%) and TNF-α (5.15 to -15.31%). No effects were seen on IP-10 or MCP- 1 levels, whereas IL-17A (0.98 to 10.10%) and RANTES (15.38 to 34.50%) showed an induction of expression. IL-6 (-19.92 to 2.33%), IL-12p70 (-12.08 to 20.21%), MIP-1α (-21.28 to 11.45%) and MIP- 1β (-22.08 to 8.38%) possessed biphasic reactions. Variable effects were measured in the expression of IL-5 (-32.66 to 32.66%) and IL-8 (-21.51 to 8.27%).
A decreased expression of anti-inflammatory cytokine IL- 10 (-38.51 to 0.87%) as well as cytokines with pro- and antiinflammatory activity [IL-2 (up to -35.44%), IL-4 (12.35 to 1.54%), IL-7 (-2.52 to -22.57%), IL-9 (-1.52 to 14.56%), IL-15 (-6.49 to 17.55%)] were also detected. A biphasic expression was seen for IL- 1ra (10.52 to 7.81%). Data from growth factor analysis showed no effect on PDGF-BB and biphasic effects on bFGF (-8.45 to 21.92%) and GM-CSF (-48.31 to 23.48%) expression. G-CSF expression was assessed on a decreasing level (-13.69 to -59.03%) whereas VEGF (-13.73 to 16.27%) possessed an induction of expression.
Discussion
The analysis of cytokine expression after treatment of human dermal fibroblasts without a subsequent inflammatory stimulus possessed, except expression of IFN-γ, either no effect or an increased expression of cytokines and growth factors. Increased cytokines of the chemokine group, e.g. IL-8, MCP-1, MIP-1α, IP-10 and Eotaxin, were described to be involved in important aspects of early stages of wound healing [15,16] and re-epithelialization [17]. An up-regulation of anti- and pro- inflammatory cytokines (IL-6, -12p70, -7, -9, -1ra and -10) by topical application of natural products has been suggested to enhance normal immune surveillance of skin tissue and maintenance of homeostasis [18]. Interestingly, the treatment of skin cells with all four concentrations of the test substance resulted in a 20-30% increase in VEGF production. This regenerative growth factor has a role in tissue repair through its function as an angiogenic factor [19] and topical application of VEGF in a diabetic mouse model has been shown to promote wound healing [20]. Moreover, an induction of PDGF-BB was seen after cell stimulation with higher doses of the test substance. PDGF-BB also plays an important role in the late stage of wound healing [21].
In presence of inflammatory stimuli, most of the cytokines tested showed either no reaction or a reduction, especially at higher test substance concentrations. Interestingly, at higher doses of the test substance an increased expression in the antiinflammatory IL-1ra and the growth factors bFGF, VEGF and GMCSF were found. The anti-inflammatory IL-10 was detected on a moderately reduced level compared to proinflammatory cytokine expression at higher test substance doses. The most important members of the down-regulated proinflammatory cytokines are key molecules in the induction of other proinflammatory proteins via Mitogen-activated Protein Kinase (MAPK) and Nuclear factor κβ (NFκβ) signaling pathways, promote the immune cells proliferation, activate immune cells and promote binding and immigration of immune cells into the tissue [22-25]. In contrast, the moderately reduced anti-inflammatory IL-10 is a major player in inflammatory processes and inhibits the proinflammatory cytokine expression via interaction with the NFκB and the Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) signaling pathway [26]. IL-1ra inhibits the proinflammatory impact of IL1β and adjusts many IL-1 - related inflammatory responses [27].
The growth factors bFGF, VEGF and GM-CSF have an important function and are upregulated in late stages of wound healing like angiogenesis [28], reepithelialization [29], granulation tissue formation [30,31], matrix formation and remodeling [32]. This expression pattern indicates a shift from an inflammative environment to a tissue regenerative environment after incubation with the test substance under inflammatory conditions. Compared to the direct influences of the test substance under normal culture conditions, the reaction under inflammatory conditions is very different and suggests different mechanisms of action under these two different conditions. Overall, the test substance shows an anti-inflammatory effect on human skin cells under inflammatory conditions and the current data suggest that tissue regeneration is promoted.
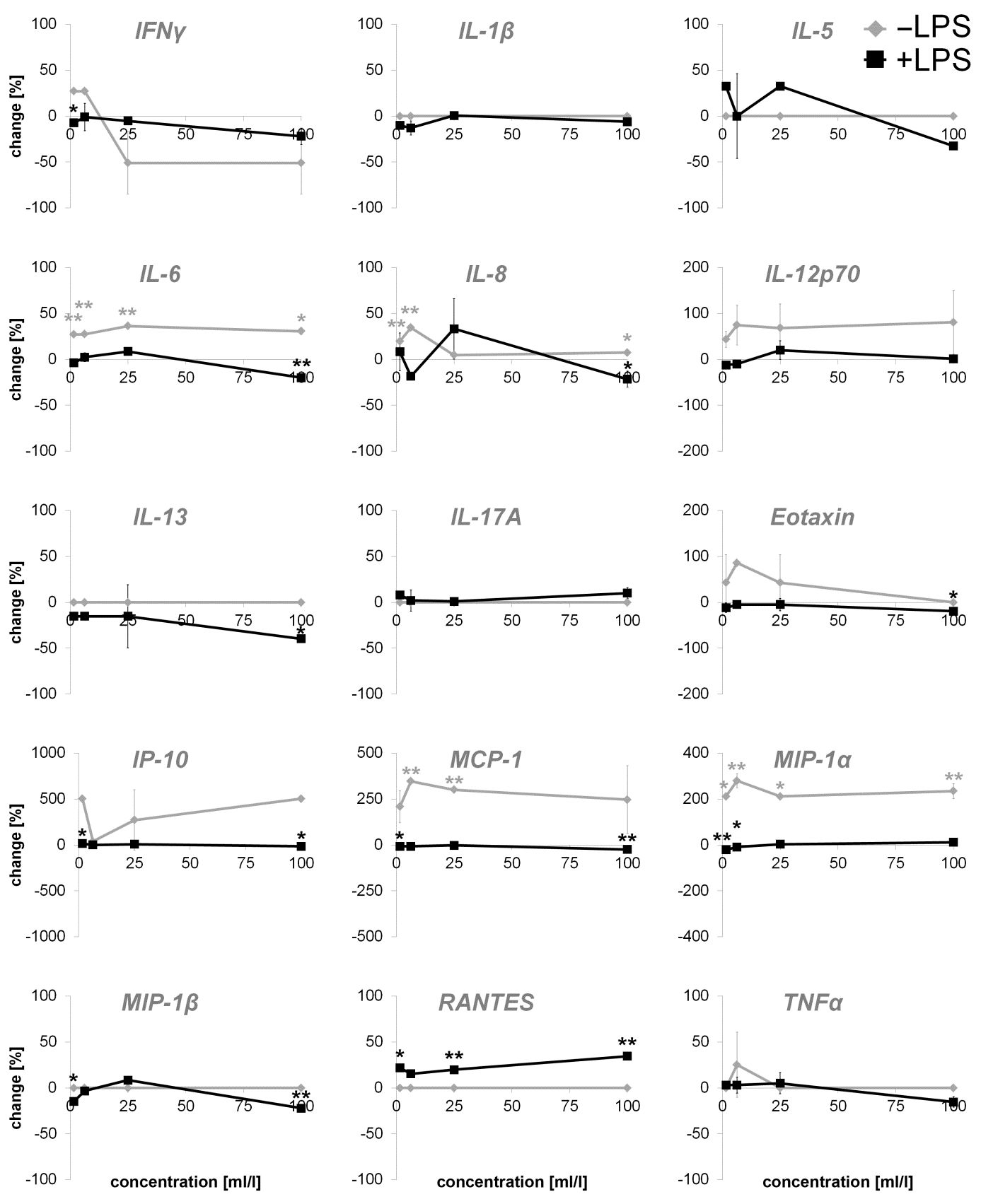
Figure 1: Percent change in proinflammatory cytokine levels in supernatants from dermal fibroblast cultures incubated with serial dilutions of the test product for 24 hours. Effects of the test product alone (-LPS) and effects of a test product pretreatment prior to the addition of the inflammatory compound LPS (+LPS) was observed and displayed in comparison to control cultures without the test substance (* p<0.05; ** p<0.005).
In comparison to previously published work, the data generated in this study is consistent with data from other workgroups. For example, Karow et al. showed reduced wound irritation after treatment of patients with A. montana extracts [33]. Moreover, a stimulation of expression of extracellular matrix genes in a wound-healing phenotype macrophage cell line by A. montana was described by Marzotto et al. [34]. In another study, A. montana showed an inhibition of carragenin-induced rat paw oedema [7]. A. montana extracts have also been reported to promote healing, as some of the modified genes are key factors of tissue remodeling, inflammation, and chemotaxis [35]. In addition, Mahajan et al. show a significant reduction of IL-6, IL-1 and TNFα from human whole blood culture and RAW-264.7 cells after LPS induction and incubation with various homeopathic dilutions of A. montana and Bryonia species in vitro [36]. In a study of dos Santos et al. T. quercifolium extracts have anti-inflammatory effects and appear to act on inflammatory processes with prostaglandins, histamine, and other inflammatory mediators [37].
In the circular excision wound model in rats, a wound healing potential of a topically applied ointment made from B. perennis flowers could also be shown [38]. Tolmacheva et al. described an anti-inflammatory effect of L. palustre extracts [39]. Kuonen et al. described a significant and dose dependent promotion of NIH/3T3 fibroblast migration and an enhanced wound closure by V. album extracts [40]. Although plant extracts have been used as medicinal products for several centuries, unfortunately these are only marginally and insufficiently described in scientific studies. However, previous studies in this area reflect the enormous potential of these naturally occurring drugs. For this reason further studies are urgently needed with regard to the applicability and efficacy of these substances (Figure 1 & 2 & 3).
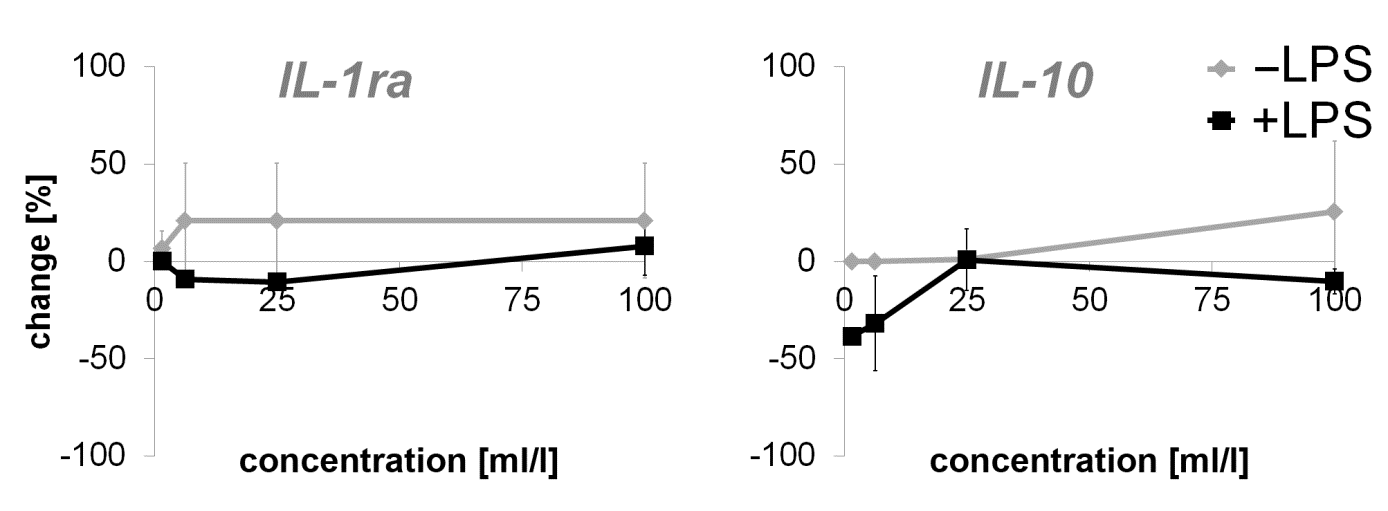
Figure 2: Percent change in anti-inflammatory cytokine levels in supernatants from dermal fibroblast cultures incubated with serial dilutions of the test product for 24 hours. Effects of the test product alone (-LPS) and effects of a test product pretreatment prior to the addition of the inflammatory compound LPS (+LPS) was observed and displayed in comparison to control cultures without the test substance (* p<0.05; ** p<0.005).
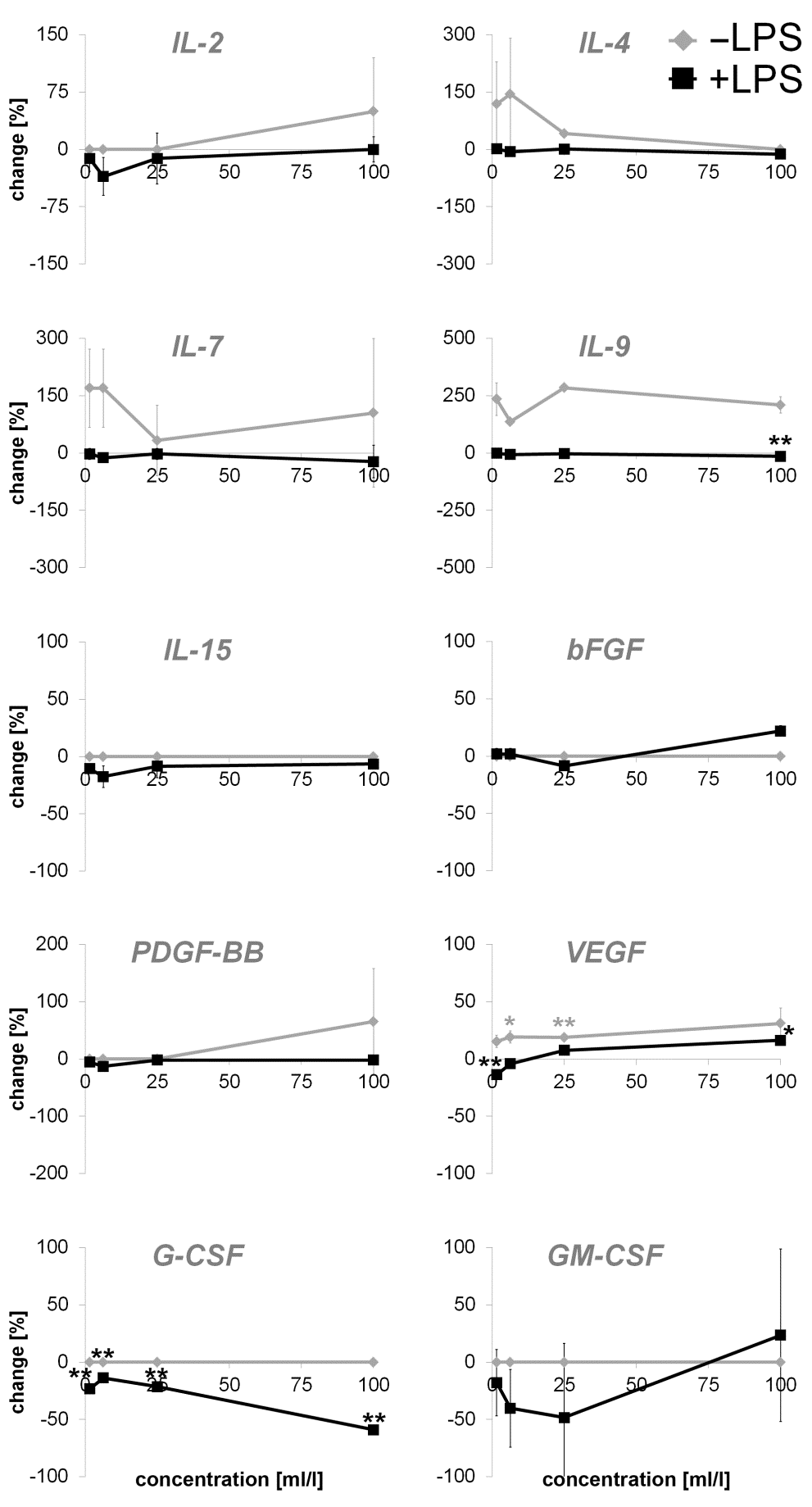
Figure 3: Percent change in levels of cytokines with pro- and anti-inflammatory activity and growth factors in supernatants from dermal fibroblast cultures incubated with serial dilutions of the test product for 24 hours. Effects of the test product alone (-LPS) and effects of a test product pretreatment prior to the addition of the inflammatory compound LPS (+LPS) was observed and displayed in comparison to control cultures without the test substance (* p<0.05; ** p<0.005).
Conclusions
The test substance enhances normal immune surveillance of human dermal fibroblasts in absence of an inflammatory stimulus. Under inflammatory conditions, the data suggests a shift from an inflammative environment to a tissue regenerative environment after incubation with the test substance. In summary, the data of the present study show an anti-inflammatory effect and thus a potential for the test substance for the treatment of acute or chronic inflammatory reactions.
Acknowledgements
All experiments were conducted by NIS Labs, Klamath Falls, USA.
Declarations
Ethics Approval and Consent to Participate
Not applicable
Consent for Publication
Not applicable.
Availability of Data and Materials
All data described in the manuscript are available from the
corresponding author on reasonable request.
Competing interests: The authors declare that they have no
competing interests.
Funding
This work was financially supported by PEKANA Naturheilmittel GmbH (Kißlegg, Germany).
Author’s Contributions
All experiments were conducted by NIS Labs, Klamath Falls, USA. MS, VS, MH and LH have performed data analysis and interpretation, MS prepared the manuscript. VS, MH, UK and LH critically reviewed the manuscript and contributed intellectual content. All authors read and approved the final version of the manuscript.
References
- Thappa DM (2013) Textbook of Dermatology, Leprology & Venereology Elsevier Health Sciences.
- Falabella A, Kirsner R (2005) Wound Healing. Taylor & Francis.
- Bhattacharya S (2012) Wound healing through the ages. Indian J Plast Surg 45(2): 177-179.
- Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P (2007) Ethnopharmacological approaches to wound healing--exploring medicinal plants of India. Journal of ethnopharmacology 114(2): 103-113.
- Petrovska BB (2012) Historical review of medicinal plants' usage. Pharmacogn Rev 6(11): 1-5.
- Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12(4): 564-582.
- Philomena G (2011) Concerns regarding the safety and toxicity of medicinal plants - An overview. J Appl Pharm Sci 1(6): 40-44.
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L, et al. (2011) Extraction, isolation and characterization of bioactive compounds from plants' extracts. Afr J Tradit Complement Altern Med 8(1): 1-10.
- Paracelsus (2014) Die dritte Defension wegen des Schreibens der neuen Rezepte 1538.
- Lussignoli S, Bertani S, Metelmann H, Bellavite P, Conforti A, et al. (1999) Effect of Traumeel S, a homeopathic formulation, on blood-induced inflammation in rats. Complement Ther Med 7(4): 225-230.
- Gebhardt R (2003) Antioxidative, antiproliferative and biochemical effects in HepG2 cells of a homeopathic remedy and its constituent plant tinctures tested separately or in combination. Arzneimittel-Forschung 53(12): 823-830.
- Porozov S, Cahalon L, Weiser M, Branski D, Lider Om, et al. (2004) Inhibition of IL-1beta and TNF-alpha secretion from resting and activated human immunocytes by the homeopathic medication Traumeel S. Clin Dev Immunol 11(2): 143-149.
- Saha SK, Roy S, Khuda-Bukhsh AR (2015) Ultra-highly diluted plant extracts of Hydrastis canadensis and Marsdenia condurango induce epigenetic modifications and alter gene expression profiles in HeLa cells in vitro. J Integr Med 13(6): 400-411.
- Bellavite P, Conforti A, Pontarollo F, Ortolani R (2006) Immunology and homeopathy. 2. Cells of the immune system and inflammation. Evid Based Complement Alternat Med 3(1): 13-24.
- Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, et al. (1998) Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 153(6): 1849-1860.
- Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, et al. (2001) Wound healing in MIP-1alpha (-/-) and MCP-1(-/-) mice. Am J Pathol 159(2): 457-463.
- Gillitzer R, Goebeler M (2001) Chemokines in cutaneous wound healing. J Leukoc Biol 69(4): 513-521.
- Benson KF, Newman RA, Jensen GS (2016) Water-soluble egg membrane enhances the immunoactivating properties of an Aloe vera-based extract of Nerium oleander leaves. Clin Cosmet Investig Dermatol 9: 393-403.
- Johnson KE, Wilgus TA (2014) Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv Wound Care (New Rochelle) 3(10): 647-661.
- Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, et al. (2004) Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 164(6):1935-1947.
- Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79(4): 1283-1316.
- Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104(4): 487-501.
- Schroder K, Hertzog PJ, Ravasi T, Hume DA (2004) Interferon-gamma: an overview of signals, mechanisms and functions. Journal of leukocyte biology 75(2): 163-189.
- Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, et al. (2011) Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. The Journal of allergy and clinical immunology 127(3): 701-721.e1-70.
- Kant S, Swat W, Zhang S, Zhang ZY, Neel BG, et al. (2011) TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway. Genes Dev 25(19): 2069-2078.
- Grutz G (2005) New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol 77(1): 3-15.
- Perrier S, Darakhshan F, Hajduch E (2006) IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett 580(27): 6289-6294.
- Bussolino F, Wang JM, Defilippi P, Turrini F, Sanavio F, et al. (1989) Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 337(6206): 471-473.
- Mann A, Breuhahn K, Schirmacher P, Blessing M (2001) Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing: Stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J Invest Dermatol 117(6): 1382-1390.
- Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, et al. (1996) Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol 270(1 Pt 2): H411-5.
- Yebra M, Parry GC, Stromblad S, Mackman N, Rosenberg S, et al. (1996) Requirement of receptor-bound urokinase-type plasminogen activator for integrin alphavbeta5-directed cell migration. J Biol Chem (46): 29393-29399.
- Ornitz DM, Itoh N (2001) Fibroblast growth factors. Genome Biol 2(3): REVIEWS3005.
- Karow JH, Abt HP, Frohling M, Ackermann H (2008) Efficacy of Arnica montana D4 for healing of wounds after Hallux valgus surgery compared to diclofenac. J Altern Complement Med 14(1): 17-25.
- Marzotto M, Bonafini C, Olioso D, Baruzzi A, Bettinetti L, et al. (2016) Arnica montana Stimulates Extracellular Matrix Gene Expression in a Macrophage Cell Line Differentiated to Wound-Healing Phenotype. PlOS one 11(11): e0166340.
- Olioso D, Marzotto M, Bonafini C, Brizzi M, Bellavite P, et al. (2016) Arnica montana effects on gene expression in a human macrophage cell line. Evaluation by quantitative Real-Time PCR. Homeopathy 105(2): 131-47.
- Mahajan U, Walke A, Kardile M, Goyal S, Siddharth S, et al. (2017) Anti-inflammatory homoeopathic drug dilutions restrain lipopolysaccharide-induced release of pro-inflammatory cytokines: In vitro and in vivo Indian Journal of Research in Homoeopathy 11(3): 158-169.
- Dos Santos AL, Perazzo FF, Cardoso LG, Carvalho JC (2007) In vivo study of the anti-inflammatory effect of Rhus toxicodendron. Homeopathy 96(2): 95-101.
- Karakas FP, Karakas A, Boran C, Turker AU, Yalcin FN, et al. (2012) The evaluation of topical administration of Bellis perennis fraction on circular excision wound healing in Wistar albino rats. Pharmaceutical biology 50(8): 1031-1037.
- Tolmacheva AA, Rogozhin EA, Deryabin DG (2014) Antibacterial and quorum sensing regulatory activities of some traditional Eastern-European medicinal plants. Acta Pharm 64(2): 173-186.
- Kuonen R, Weissenstein U, Urech K, Kunz M, Hostanska K, et al. (2013) Effects of Lipophilic Extract of Viscum album L. and Oleanolic Acid on Migratory Activity of NIH/3T3 Fibroblasts and on HaCat Keratinocytes. Evid Based Complement Alternat Med 718105.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.