Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Intracellular Metabolism in Diabetic Embryopathy
*Corresponding author: Zhiyong Zhao, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Received: July 05, 2021; Published: July 19, 2021
DOI: 10.34297/AJBSR.2021.13.001891
Abstract
Diabetes mellitus in early pregnancy can cause congenital birth defects in infants, a complication known as diabetic embryopathy. Formation of structural abnormalities, commonly seen in the central nervous and cardiovascular systems, is associated with increased programmed cell death (apoptosis) and decreased cell proliferation in the developing organs. Under maternal hyperglycemic conditions, influx of glucose into cells of the embryos disturbs intracellular metabolic homeostasis. Disturbed glycolysis generates factors through side pathways to perturb cell signaling and organelle functions. Perturbed phospholipid metabolism produces signaling metabolites and peroxidation products to suppress cell survival and induce apoptosis. Targeting the key processes and factors in the metabolism of glucose and phospholipids is a potential intervention strategy to prevent birth defects in diabetic pregnancies.
Keywords: Diabetic embryopathy, Birth defects, Hyperglycemia, Glycolysis, Phospholipid, Oxidative stress, Apoptosis
Abbreviations: AA: Arachidonic Acid; AGE: Advanced Glycation End Product; CNS: Central Nervous System; COX-2: Cyclooxygenase-2; cPLA2: Cytosolic Phospholipase A2; CVS: Cardiovascular System; DAG: Diacylglycerol; DHA: Dihydroxyacetone; Fru: Fructose; G6PD: Glucose-6-Phosphate Dehydrogenase; Glc: Glucosamine; Glu: Glucose; GSH: Glutathione; GSSG: Oxidized GSH; NADP+: Nicotinamide Adenine Dinucleotide Phosphate; OGA: O-GlcNAcase; OGT: O-Glcnac Transferase; P: Phosphate; PGE2: Prostaglandin E2; PGF2: Prostaglandin F2; PGP: Phosphatidylglycerolphosphate; PI3K: Phosphatidylinositide 3-kinase; PIP2: Phosphatidylinositol 4,5-bisphosphate; PIP3: Phosphatidylinositol 3,4,5-trisphosphate; PTEN: Phosphatase and Tensin Homolog Deleted on Chromosome 10; ROS: Reactive Oxygen Species; TCA: Tricarboxylic Acid; UDP: Uridine Diphosphate.
Introduction
Diabetes mellitus in early pregnancy imposes a risk of birth defects in infants [1-4]. This type of diabetic complication, known as diabetic embryopathy, account for birth defect rates three times higher than those in the general population [3-7]. Unfortunately, the number of women of childbearing age with type 1 and type 2 diabetes is increasing [8-10], imposing tremendous challenges to perinatal management and health care at large [11,12]. Although aggressive glycemic control and specialized antenatal care, including dietary supplementation of vitamins and folic acid, are widely available in the United States and other developed countries, the birth defect rates in diabetic pregnancies have not been significantly reduced for decades [2,13,14]. Therefore, it is urgent to understand the mechanism of diabetic embryopathy to develop interventions to prevent birth defects in diabetic pregnancies.
Teratogenesis in Diabetic Embryopathy
Birth defects resulting from diabetic embryopathy are commonly seen in the central nervous and cardiovascular systems (CNS and CVS) [1,2,15]. Abnormalities in the CNS include microcephaly, exencephaly, and holoprosencephaly in the brain and spinal bifida in the spinal cord. These are the results of failure in neural tube closure during neurulation, and thus, referred to as neural tube defects (Figure 1) [2,16]. In the CVS, anomalies are commonly seen as hypoplastic ventricular myocardium, ventricular septal defects, and conotruncal abnormalities (Figure 2) [2,17,18].
Work using diabetic animal models, which recapitulate the defects observed in humans [19-22], provides strong evidence that increased cell apoptosis and decreased cell proliferation are associated with structural defects [23-25].
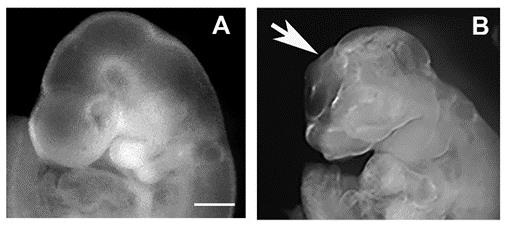
Figure 1: Neural tube defects in mouse embryo. Embryos at 10 days of gestation (equivalent to 4 weeks gestation in humans) from non-diabetic (A) and diabetic (B) mice. Arrow indicates open neural tube in the brain. Scale bar = 2 mm.
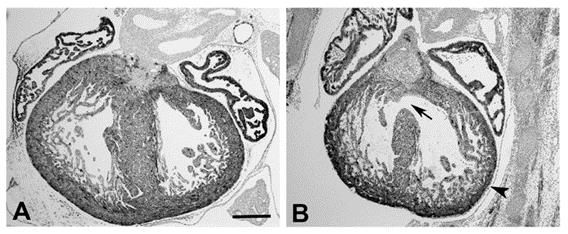
Figure 2: Heart defects in mouse fetus at day 15 of gestation (equivalent to 10 weeks gestation in humans). (A) Non-diabetic. (B) Diabetic. Arrow indicates a ventricular septal defect. Arrowhead indicates hypoplastic ventricular wall. Scale bar = 100 μm.
To investigate the mechanisms underlying the cellular changes seen in diabetic embryopathy, research in this field has characterized a large number of intracellular processes, including metabolism, protein activation, gene expression, epigenetic regulation, and organelle dysfunction [23,25-28].
Dysfunction of mitochondrion generates high levels of reactive oxygen species (ROS) [29,30]. With decreases in endogenous antioxidants, including glutathione (GSH) and thioredoxin, to scavenge the oxygen free radicals [31-34], the imbalance between oxidation and antioxidant buffering leads to oxidative damage of proteins, lipids, and nucleic acids, known as oxidative stress [23,25,28,35].
Hyperglycemia and Teratogenesis
Both clinical and basic research have provided evidence to support the notion that hyperglycemia plays a critical role in embryonic teratogenesis. Clinical studies have found a correlation between the prevalence of fetal anomalies and the status of maternal hyperglycemia, indicated by the levels of glycosylated hemoglobin A (HbA1c) as well as the levels of blood glucose [36-39]. The findings from human studies have been supported by the experiments in animal models which show an association of maternal blood glucose levels and the rates and severity of malformations in the embryos [19,21,25,40]. The notion is further supported by experiments of embryo culture in vitro, in which high concentrations of glucose cause abnormalities in the embryos, similar to those seen in vivo models and observed in human studies [40-43].
It is hypothesized that maternal hyperglycemia disturbs normal glucose metabolism in the cells of the embryo. This leads to perturbation of other metabolic systems, including phospholipids. The aberrant intracellular metabolism causes dysfunction of organelles, generates intracellular stress conditions, and results in increased apoptosis in the developing organs of the embryo and, ultimately, structural malformations (Figure 3).
Glucose Metabolism
Glucose is an important source of energy for cells to survive and function. Cells uptake glucose through glucose transporters (GLUTs) on the cell membrane [44]. Embryos also express GLUTs and take up glucose via a similar mechanism [45-48]. In diabetic pregnancies, it has been shown that Gluts, at least Glut2, are involved in the process of glucose influx into embryonic cells [47].
The metabolism of glucose, known as glycolysis, is a series of chemical reactions to convert glucose into pyruvate to feed the tricarboxylic acid cycle in mitochondria to generate adenosine triphosphate (ATP) molecules (Figure 4) [49].
Along with accelerated glycolysis, associated side pathways are dramatically enhanced. In the polyol pathway, glucose is turned into sorbitol by aldose reductase (Figure 3) [50,51]. It has been shown that sorbitol accumulates in cells of patients with diabetes and exerts adverse effects in diabetic complications [52-54]. Elevated sorbitol levels are also observed in the embryos of diabetic animals or embryos cultured in high concentrations of glucose [55-58]. Treatment with sorbitol in cultured embryos produces adverse effects on embryonic development [59].
In the conversion of glucose to sorbitol, nicotinamide adenine dinucleotide phosphate (NADPH), a co-factor of aldose reductase, is oxidized into NADP+ [50,51]. NADPH is an important antioxidant in the cytosol and mitochondria. Reduction of NADPH leads to a diminishing of antioxidative capacity of the cell and a disturbance of mitochondrial respiratory activity which causes the production of ROS [60,61]. NADPH is also a co-factor of glutathione reductase in the conversion of oxidized GSH (GSSG) into reduced GSH [50].
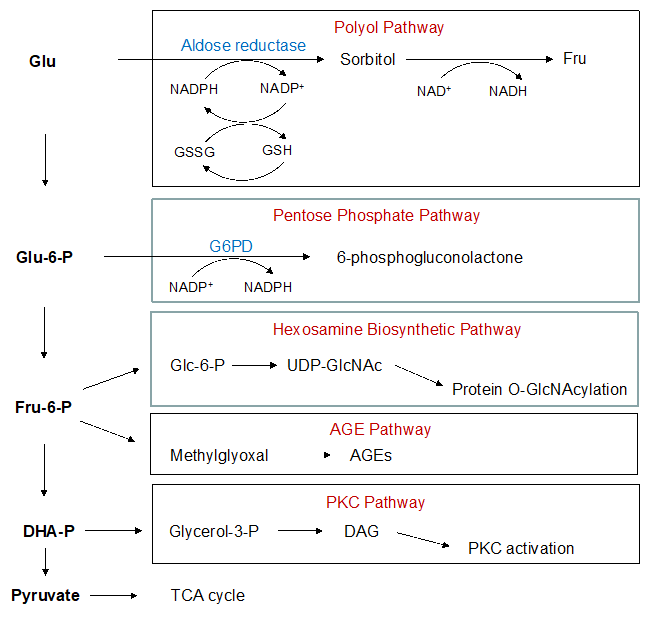
Figure 4: Glycolysis and associated side pathways. AGE: Advanced Glycation End Product; DAG: Diacylglycerol; DHA: Dihydroxyacetone; Fru, Fructose; G6PD: Glucose-6-Phosphate Dehydrogenase; Glu, Glucose; Glc, Glucosamine; GSH: Glutathione; GSSG: Oxidized GSH; NADP+, Nicotinamide Adenine Dinucleotide Phosphate; OGA: O-Glcnacase; OGT: O-Glcnac Transferase; P, Phosphate; ROS: Reactive Oxygen Species; TCA: Tricarboxylic Acid; UDP: Uridine Diphosphate.
In the pentose phosphate pathway, glucose-6-phosphate is converted into 6-phosphogluconolactone by glucose-6-phosphate dehydrogenase [62,63]. In this reaction, NADP+ is reduced to NADPH, which potentially facilitates the conversion of GSSG into GSH to increase antioxidative capacity [62,63]. However, it has been shown that this pathway is inhibited in embryos cultured in high glucose [64].
Overall, the levels of GSH are reduced in the embryos exposed to hyperglycemia [33,65,66]. The depletion of antioxidants further augments oxidative stress, leading to increases in apoptosis in hyperglycemia-induced embryonic malformations.
Hexosamine biosynthetic pathway generates uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc) from fructose- 6-phosphate (Figure 4) [67,68]. GlcNAc can be added to proteins on serine or threonine residues via O-GlcNAcylation by O-GlcNAc transferase (OGT) [69] and removed by β-N-acetylglucosaminidase or O-GlcNAcase (OGA; Figure 4) [70]. Thus, O-GlcNAcylation competes with phosphorylation for the same amino acids to regulate protein functions [71-74].
In the embryos of diabetic mice, global protein O-GlcNAcylation is increased [75]. Administration of glucosamine, a substrate for generating GlcNAc, to normal pregnant mice mimics the effect of high glucose and leads to NTD formation in the embryos [64]. The elevation of protein O-GlcNAcylation is associated with increased activation of OGT. Inhibition of OGT results in reduction of embryonic malformations [75].
Hyperglycemia-accelerated glycolysis also increases production of glyceraldehyde-3-phosphate (Figure 4). This intermediate can be converted into diacylglycerol (DAG), which is a potent activator for members of the protein kinase C (PKC) family [76,77]. Pkc, β2, and α have been shown to play a causative role in diabetic embryopathy [78-80].
Glyceraldehyde-3-phosphate can also be converted into methylglyoxal [81]. Methylglyoxal is a potent radical to react with proteins, lipids, and nucleic acids to generate advanced glycation end-products (AGEs) [82,83]. The cytotoxic and teratogenic effects of AGEs have been demonstrated in diabetic embryopathy [84-86].
Phospholipid Metabolism and Peroxidation
Phospholipids are major components of the plasma membrane [87]. The metabolism of phospholipids gives a rise to a large variety of metabolites, contributing to intracellular signaling [88,89]. Disturbance of lipid metabolism has been characterized in the embryos of diabetic animals or embryos cultured in high glucose [29,90-92].
In the embryos of diabetic animals, phospholipid metabolism increases the levels of ceramide [29]. Ceramide has been shown to perturb intracellular signaling and organelle functions to induce apoptosis [93-95]. In contrast, the levels of phosphatidylglycerolphosphate (PGP) are decreased [29]. Two PGPs form a diphosphatidylglycerol, known as cardiolipin [96]. Deficiency of this molecule is associated with disorders in the CNS and CVS, including neurodegenerations and Barth syndrome with cardiac anomalies [97-99].
Phospholipids play an important role in intracellular signaling. Phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3) are signaling molecules. PIP2 is converted into PIP3 by phosphatidylinositide 3-kinases (PI3Ks) [100,101]. Reversely, PIP3 is dephosphorylated into PIP2 by PTEN (phosphatase and tensin homolog deleted on chromosome 10; Figure 5) [102,103]. PIP3 activates protein kinase Bs (PKBs, also known as AKTs) to regulate cell survival and proliferation [100]. The cell survival regulation also involves mTOR (mechanistic target of rapamycin) [104]. PTEN, which can be activated by PIP2, inhibits PI3Ks and PKBs, to suppress cell survival capability [105,106].
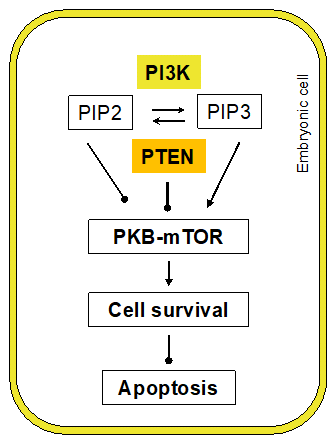
Figure 5: Phospholipid signaling in cell survival. PI3K: Phosphatidylinositide 3-Kinase; PIP2: Phosphatidylinositol 4,5-Bisphosphate; PIP3: Phosphatidylinositol 3,4,5-Trisphosphate; PTEN: Phosphatase and Tensin homolog deleted on chromosome 10.
In the embryos of diabetic mice, levels of PIP2 are increased, along with activation of Pten. In association, the levels of PKB and mTOR activation are decreased [29]. These suggest that maternal hyperglycemia diminishes cell survival via inhibiting the PKB-mTOR system (Figure 4). Inhibition of Pten in embryonic neural stem cells under hyperglycemic conditions, using SF1670, decreases ROS generation, demonstrating its involvement in cell survival regulation [29].
In addition, PIP2 can be cleaved by phospholipase Cs to generate DAG [107,108]. The increases in PIP2 may provide substrates for DAG generation and lead to activation of PKCs. As discussed above, PKCs play an important role in diabetic embryopathy.
Arachidonic acid is another major component of the plasma membrane. Arachidonic acid can be released from the membrane by cytosolic phospholipase A2 (cPLA2) [109]. In the cytoplasm, arachidonic acid can be converted into prostaglandin E2 (PGE2) by cyclooxygenase-2 (COX-2) [110,111], or become PGE2-like isoprostanes, such as 8-iso-prostagladin F2 (8-iso-PGF2) and 8-iso- PGF2, by non-COX-mediated peroxidation, involving ROS (Figure 6) [112,113].
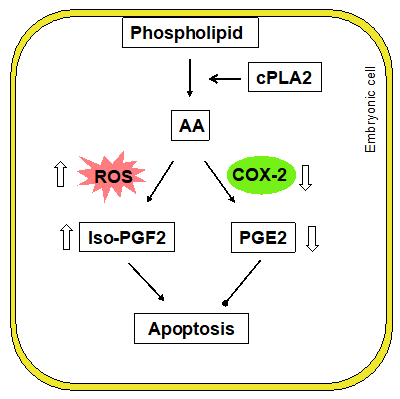
Figure 6: Phospholipid peroxidation. AA: Arachidonic Acid; COX- 2: Cyclooxygenase-2; cPLA2: Cytosolic Phospholipase A2; PGE2: Prostaglandin E2; PGF2: Prostaglandin F2; ROS: Reactive Oxygen Species.
In the cells of embryos from diabetic animals, decreases in arachidonic acid levels have been observed, indicating an active metabolism of the phospholipid [90,114]. Associated with the changes in arachidonic acid, the levels of PGE2 are decreased [115-121]. The reduction in PGE2 in the embryo may be due to decreased COX-2 enzymatic activity and downregulation of COX-2 expression [115,121,122]. Experiments show that blocking Cox-2 in the embryos cultured in normal glucose mimics the effect of high glucose on embryonic development [122]. Administration of PGE2 to embryo cultures in high glucose reduces neural tube defects [123,124]. These suggest that PGE2 may protect embryos from hyperglycemic insult.
In contrast, the levels of 8-iso-PGF2 are dramatically elevated in embryos under hyperglycemic conditions [112,120,125,126]. Isoprostanes have been shown to exert cytotoxic effects in cells [127]. Treatment of embryos in culture with 8-iso-PGF2α causes malformations and growth retardation [125]. These studies indicate a shifted metabolism of arachidonic acid toward producing cytotoxic isoprostanes to exacerbate the perturbed intracellular homeostasis to induce apoptosis.
Potential Interventions Targeting Glucose and Phospholipid Metabolism
Delineation of the molecular mechanisms of diabetic embryopathy provides crucial data for developing interventions to reduce fetal abnormalities. However, limited efforts have been made to target glucose and phospholipid metabolism in diabetic embryopathy.
Delineation of the molecular mechanisms of diabetic embryopathy provides crucial data for developing interventions to reduce fetal abnormalities. However, limited efforts have been made to target glucose and phospholipid metabolism in diabetic embryopathy.
Replenishing arachidonic acid to restore membrane integrity and/or inhibit lipid peroxidation has been explored. In the embryos cultured in high glucose [122], or the embryos in diabetic pregnant animals [131-133], treatment with arachidonic acid decreases malformation rates. These findings indicate that a potential strategy to use certain species of phospholipids, such as fish oil to replenish cardiolipin, to reduce embryonic malformations in diabetic pregnancies.
Concluding Remarks
The mechanisms of diabetic embryopathy is complex, involving various cellular and molecular processes. The influx of glucose and associated glucose and lipid metabolism generate an aberrant intracellular environment, disturb intracellular signaling, and ultimately induce apoptosis in developing embryos and malformations. Interventions to suppress the production of toxic metabolites of glucose and phospholipids or restore normal metabolic balances may be a promising strategy to prevent birth defects in diabetic pregnancies.
Acknowledgement
Authors thank Dr. Julie Rosen for proofreading and editing.
Conflict of interest
Authors declare no conflict of interest.
References
- Mills JL (1982) Malformations in infants of diabetic mothers. Teratology 25(3): 385-394.
- Correa A, Gilboa SM, Besser LM (2008) Diabetes mellitus and birth defects. American Journal of Obstetrics and Gynecology 199(3): 237.
- Reece EA, Homko CJ (1993) Diabetes-related complications of pregnancy. Journal of the National Medical Association 85(7): 537-545.
- Reece EA, Homko CJ (1994) Infant of the diabetic mother. Semin Perinatol 18: 459-469.
- Pedersen LM, Tygstrup I, Pedersen J (1964) Congenital Malformations in Newborn Infants of Diabetic Women. Correlation with Maternal Diabetic Vascular Complications. Lancet 1: 1124-1126.
- Reece EA, Hobbins JC (1986) Diabetic embryopathy: pathogenesis, prenatal diagnosis and prevention. Obstetrical and Gynecological Survey 41(6): 325-335.
- CDC (2008) Update on overall prevalence of major birth defects -- Atlanta, Georgia, 1978--2005. MMWR Morb Mortal Wkly Rep 57(1):1-5.
- CDC (2008) State-specific incidence of diabetes among adults-Participating States, 1995- 1997 and 2005-2007. Morb Mort Wkly Rep 57(43): 1169-1173.
- Mobasseri M, Shirmohammadi M, Amiri T (2020) Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect 10(2): 98-115.
- Dickens LT, Thomas CC (2019) Updates in Gestational Diabetes Prevalence, Treatment, and Health Policy. Current Diabetes Reports 19(6): 33.
- Osterman MJK, Martin JA, Menacker F (2009) Expanded health data from the new birth certificate, 2006. Nat vital Stat Rep 58(5): 1-24.
- Feig DS, Palda VA (2002) Type 2 diabetes in pregnancy: A growing concern. Lancet 359(9318): 1690-1692.
- Farrell T, Neale L, Cundy T (2002) Congenital anomalies in the offspring of women with type 1, type 2 and gestational diabetes. Diabetic Medicine 19(4): 322-326.
- Sheffield JS, Butler KEL, Casey BM, McIntire DD, Leveno KJ (2002) Maternal diabetes mellitus and infant malformations. Obstetrics and Gynecology 100(5 pt 1): 925-930.
- Nasri HZ, Houde NK, Westgate MN, Hunt AT, Holmes LB (2018) Malformations among infants of mothers with insulin-dependent diabetes: Is there a recognizable pattern of abnormalities? Birth Defects Res 110(2): 108-113.
- Zacharias JF, Jenkins JH, Marion JP (1984) The incidence of neural tube defects in the fetus and neonate of the insulin-dependent diabetic woman. American Journal of Obstetrics and Gynecology 150(6): 797-798.
- Loffredo CA, Wilson PD, Ferencz C (2001) Maternal diabetes: An independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology 64(2): 98-106.
- Wren C, Birrell G, Hawthorne G (2003) Cardiovascular malformations in infants of diabetic mothers. Heart 89(10): 1217-1220.
- Zhao Z, Reece EA (2005) Experimental mechanisms of diabetic embryopathy and strategies for developing therapeutic interventions. J Soc Gynecol Investig 12(8): 549-557.
- Loeken MR (2008) Challenges in understanding diabetic embryopathy. Diabetes 57(12): 3187-3188.
- Yang P, Zhao Z, Reece EA (2008) Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol 198(1): 131-137.
- Eriksson UJ, Borg LA, Cederberg J (2000) Pathogenesis of diabetes-induced congenital malformations. Ups J Med Sci 105(2): 53-84.
- Eriksson UJ, Wentzel P (2016) The status of diabetic embryopathy. Ups J Med Sci 121(2): 96-112.
- Loeken MR (2004) Free radicals and birth defects. Journal of Maternal-Fetal & Neonatal Medicine 15(1): 6-14.
- Zhao Z, Reece EA (2013) New concepts in diabetic embryopathy. Clin Lab Med 33(2): 207-233.
- Salbaum JM, Kappen C (2011) Diabetic embryopathy: a role for the epigenome? Birth defects research A Clinical and molecular teratology 91(8): 770-780.
- Loeken MR (2006) Advances in understanding the molecular causes of diabetes-induced birth defects. Journal of the Society for Gynecologic Investigation 13(1): 2-10.
- Yang P, Reece EA, Wang F, Gabbay BR (2015) Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. American Journal of Obstetrics and Gynecology 212(5): 569-579.
- Cao L, Liu P, Gill K, (2016) Identification of novel cell survival regulation in diabetic embryopathy via phospholipidomic profiling. Biochem Biophys Res Commun 470(3): 599-605.
- Yang X, Borg LA, Eriksson UJ (1995) Altered mitochondrial morphology of rat embryos in diabetic pregnancy. Anatomical Record 241(2): 255-267.
- Sakamaki H, Akazawa S, Ishibashi M (1999) Significance of glutathione-dependent antioxidant system in diabetes-induced embryonic malformations. Diabetes 48(5): 1138-1144.
- Menegola E, Broccia ML, Prati M, Ricolfi R, Giavini E (1996) Glutathione status in diabetes-induced embryopathies. Biology of the Neonate 69(5): 293-297.
- Trocino RA, Akazawa S, Ishibashi M (1995) Significance of glutathione depletion and oxidative stress in early embryogenesis in glucose-induced rat embryo culture. Diabetes 44(8): 992-998.
- Kamimoto Y, Sugiyama T, Kihira T (2010) Transgenic mice overproducing human thioredoxin-1, an antioxidative and anti-apoptotic protein, prevents diabetic embryopathy. Diabetologia 53(9): 2046-2055.
- Reece EA (1999) Maternal fuels, diabetic embryopathy: pathomechanisms and prevention. Semin Reprod Endocrinol 17(2): 183-194.
- Greene MF, Hare JW, Cloherty JP, Benacerraf BR, Soeldner JS (1989) First-trimester hemoglobin A1 and risk for major malformation and spontaneous abortion in diabetic pregnancy. Teratology 39(3): 225-231.
- Jovanovic L, Druzin M, Peterson CM (1981) Effect of euglycemia on the outcome of pregnancy in insulin-dependent diabetic women as compared with normal control subjects. American Journal of Medicine 71(6): 921-927.
- Lucas MJ, Leveno K, Williams ML, Raskin P, Whalley PJ (1989) Early pregnancy glycosylated hemoglobin, severity of diabetes, and fetal malformations. American Journal of Obstetrics and Gynecology 161(2): 426-431.
- Miller E, Hare JW, Cloherty JP (1981) Elevated maternal hemoglobin A1c in early pregnancy and major congenital anomalies in infants of diabetic mothers. New England Journal of Medicine 304(2): 1331-1334.
- Gareskog M, Cederberg J, Eriksson UJ, Wentzel P (2007) Maternal diabetes in vivo and high glucose concentration in vitro increases apoptosis in rat embryos. Reprod Toxicol. 23(1): 63-74.
- Wentzel P, Wentzel CR, Gareskog MB, Eriksson UJ (2001) Induction of embryonic dysmorphogenesis by high glucose concentration, disturbed inositol metabolism, and inhibited protein kinase C activity. Teratology 63(5): 193-201.
- Reece EA, Wiznitzer A, Homko CJ, Hagay Z, Wu YK (1996) Synchronization of the factors critical for diabetic teratogenesis: an in vitro American Journal of Obstetrics and Gynecology 174(4): 1284-1288.
- Akazawa S (2005) Diabetic embryopathy: studies using a rat embryo culture system and an animal model. Congenit Anom (Kyoto) 45(3): 73-79.
- Navale AM, Paranjape AN (2016) Glucose transporters: physiological and pathological roles. Biophys Rev 8(1): 5-9.
- Doblado M, Moley KH (2007) Glucose metabolism in pregnancy and embryogenesis. Current Opinion in Endocrinology, Diabetes, and Obesity 14(6): 488-493.
- Hogan A, Heyner S, Charron MJ (1991) Glucose transporter gene expression in early mouse embryos. Development 113(1): 363-372.
- Li R, Thorens B, Loeken MR (2007) Expression of the gene encoding the high-K (m) glucose transporter 2 by the early postimplantation mouse embryo is essential for neural tube defects associated with diabetic embryopathy. Diabetologia 50(3): 682-689.
- Vannucci SJ, Rutherford T, Wilkie MB, Simpson IA, Lauder JM (2000) Prenatal expression of the GLUT4 glucose transporter in the mouse. Developmental Neuroscience 22(4): 274-282.
- Chandel NS (2021) Glycolysis. Cold Spring Harbor Perspectives in Biology 13(7).
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature. 414(6865): 813-820.
- Nishikawa T, Edelstein D, Du XL (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404(6779): 787-790.
- Koh MS, Misch KJ, Yuen CT, Rhodes EL (1986) Accumulation of sorbitol in endothelial cells--a possible cause of diabetic microangiopathy. Diabetes Research 3(4): 217-219.
- Greene DA, Lattimer SA, Sima AA (1987) Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. New England Journal of Medicine 316(10): 599-606.
- Obrosova IG (2005) Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxid Redox Signal 7(11-12): 1543-1552.
- Eriksson UJ, Naeser P, Brolin SE (1986) Increased accumulation of sorbitol in offspring of manifest diabetic rats. Diabetes 35(12): 1356-1363.
- Hashimoto M, Akazawa S, Akazawa M (1990) Effects of hyperglycaemia on sorbitol and myo-inositol contents of cultured embryos: treatment with aldose reductase inhibitor and myo-inositol supplementation. Diabetologia 33(10): 597-602.
- Fu J, Tay SS, Ling EA, Dheen ST (2007) Aldose reductase is implicated in high glucose-induced oxidative stress in mouse embryonic neural stem cells. Journal of Neurochemistry 103(4): 1654-1665.
- Akashi M, Akazawa S, Akazawa M (1991) Effects of insulin and myo-inositol on embryo growth and development during early organogenesis in streptozocin-induced diabetic rats. Diabetes 40(12): 1574-1579.
- Yang P, Zhao Z, Reece EA (2007) Involvement of c-Jun N-terminal kinases activation in diabetic embryopathy. Biochem Biophys Res Commun 357(3): 749-754.
- Raha S, Robinson BH (2000) Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci 25(10): 502-508.
- Wolin MS, Ahmad M, Gao Q, Gupte SA (2007) Cytosolic NAD(P)H regulation of redox signaling and vascular oxygen sensing. Antioxid Redox Signal 9(6): 671-678.
- Ge T, Yang J, Zhou S (2020) The Role of the Pentose Phosphate Pathway in Diabetes and Cancer. Frontiers in Endocrinology 11: 365.
- Patra KC, Hay N (2014) The pentose phosphate pathway and cancer. Trends Biochem Sci 39(8): 347-354.
- Horal M, Zhang Z, Stanton R, Virkamaki A, Loeken MR (2004) Activation of the hexosamine pathway causes oxidative stress and abnormal embryo gene expression: involvement in diabetic teratogenesis. Birth Defects Research A Clinical and Molecular Teratology 70(8): 519-527.
- Wentzel P, Gareskog M, Eriksson UJ (2008) Decreased cardiac glutathione peroxidase levels and enhanced mandibular apoptosis in malformed embryos of diabetic rats. Diabetes 57(12): 3344-3352.
- Yan J, Hales BF (2006) Depletion of glutathione induces 4-hydroxynonenal protein adducts and hydroxyurea teratogenicity in the organogenesis stage mouse embryo. Journal of Pharmacology and Experimental Therapeutics 319(2): 613-621.
- Torres CR, Hart GW (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 259(5): 3308-3317.
- Hart GW, Housley MP, Slawson C (2007) Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446(7139): 1017-1022.
- Hart GW, Greis KD, Dong LY, Blomberg M A, Chou T Y, et al. (1995) O-linked N-acetylglucosamine: the "yin-yang" of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv Exp Med Biol 376L: 115-123.
- Wells L, Gao Y, Mahoney J, Keith V, Chen C, et al. (2002) Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem 277(3): 1755-1761.
- Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291(5512): 2376-2378.
- Wells L, Hart GW (2003) O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett 546(1): 154-158.
- Kamemura K, Hart GW (2003) Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: a new paradigm for metabolic control of signal transduction and transcription. Prog Nucleic Acid Res Mol Biol 73: 107-136.
- O'Donnell N (2002) Intracellular glycosylation and development. Biochim Biophys Acta 1573(3): 336-345.
- Kim G, Cao L, Reece EA, Zhao Z (2017) Impact of protein O-GlcNAcylation on neural tube malformation in diabetic embryopathy. Sci Rep 7(1): 11107.
- Gómez FJC, Torrecillas A, Corbalán GS (2004) Diacylglycerols as activators of protein kinase C. Molecular Membrane Biology 21(6): 339-349.
- Newton AC (1997) Regulation of protein kinase C. Current Opinion in Cell Biology 9(2): 161-167.
- Cao Y, Zhao Z, Eckert RL, Reece EA (2012) The essential role of protein kinase Cdelta in diabetes-induced neural tube defects. J Matern Fetal Neonatal Med 25(10): 2020-2024.
- Cao Y, Zhao Z, Eckert RL, Reece EA (2011) Protein kinase Cbeta2 inhibition reduces hyperglycemia-induced neural tube defects through suppression of a caspase 8-triggered apoptotic pathway. Am J Obstet Gynecol 204(3): 221-225.
- Wang F, Xu C, Reece EA, Xuezheng L, Yanqing W, et al (2017) Protein kinase C-alpha suppresses autophagy and induces neural tube defects via miR-129-2 in diabetic pregnancy. Nat Commun 8: 15182.
- Allaman I, Belanger M, Magistretti PJ (2015) Methylglyoxal, the dark side of glycolysis. Frontiers in Neuroscience 9: 23.
- Ramasamy R, Yan SF, Schmidt AM (2006) Methylglyoxal comes of AGE. Cell 124(2): 258-260.
- Schalkwijk CG, Stehouwer CDA (2020) Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiological Reviews 100(1): 407-461.
- Ejdesjo A, Brings S, Fleming T, et al. (2016) Receptor for advanced glycation end products (RAGE) knockout reduces fetal dysmorphogenesis in murine diabetic pregnancy. Reprod Toxicol 62: 62-70.
- Hao L, Noguchi S, Kamada Y (2008) Adverse effects of advanced glycation end products on embryonal development. Acta Medicinae Okayama 62(2): 93-99.
- Li RL, Zhao W W, Gao BY (2018) Advanced glycation end products induce neural tube defects through elevating oxidative stress in mice. Neural Regen Res 13(8): 1368-1374.
- Van MG, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9(2):112-124.
- Divecha N, Irvine RF (1995) Phospholipid signaling. Cell. 80, 269-278.
- Alb JG, Kearns MA, Bankaitis VA (1996) Phospholipid metabolism and membrane dynamics. Curr Opin Cell Biol 8(4): 534-541.
- Goldman AS, Baker L, Piddington R (1985) Hyperglycemia-induced teratogenesis is mediated by a functional deficiency of arachidonic acid. Proceedings of the National Academy of Sciences of the United States of America 82(23): 8227-8231.
- Pinter E, Reece EA, Ogburn PL (1988) Fatty acid content of yolk sac and embryo in hyperglycemia-induced embryopathy and effect of arachidonic acid supplementation. American Journal of Obstetrics and Gynecology 159(6): 1484-1490.
- Jawerbaum A, Rosello CJ, Gonzalez ET (1994) Glucose metabolism, triglyceride and glycogen levels, as well as eicosanoid production in isolated uterine strips and in embryos in a rat model of non-insulin-dependent diabetes mellitus during pregnancy. Prostaglandins 47(2): 81-96.
- Arboleda G, Morales LC, Benitez B, Arboleda H (2009) Regulation of ceramide-induced neuronal death: cell metabolism meets neurodegeneration. Brain Res Rev 59(2): 333-346.
- Gomez MA (2006) Ceramide 1-phosphate/ceramide, a switch between life and death. Biochim Biophys Acta 1758(12): 2049-2056.
- Hannun YA, Obeid LM (1995) Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci 20(2): 73-77.
- Mejia EM, Nguyen H, Hatch GM (2014) Mammalian cardiolipin biosynthesis. Chem Phys Lipids 179: 11-16.
- Schlame M, Ren M (2006) Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett 580(23): 5450-5455.
- Paradies G, Paradies V, Ruggiero FM, Petrosillo G (2014) Cardiolipin and mitochondrial function in health and disease. Antioxid Redox Signal 20(12): 1925-1953.
- Pointer CB, Klegeris A (2017) Cardiolipin in Central Nervous System Physiology and Pathology. Cell Mol Neurobiol 37(7): 1161-1172.
- Hemmings BA, Restuccia DF (2012) PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol 4(9): a011189.
- Acebes A, Morales M (2012) At a PI3K crossroads: lessons from flies and rodents. Rev Neurosci 23(1): 29-37.
- Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP (2008) Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene 27(41): 464-5476.
- Song MS, Salmena L, Pandolfi PP (2012) The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13(5): 283-296.
- Hemmings BA, Restuccia DF (2015) The PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol 4(9): a011189.
- Worby CA, Dixon JE (2014) Pten. Annu Rev Biochem 83: 641-669.
- Gericke A, Leslie NR, Losche M, Ross AH (2013) PtdIns(4,5)P2-mediated cell signaling: emerging principles and PTEN as a paradigm for regulatory mechanism. Adv Exp Med Biol 991: 85-104.
- Vines CM (2012) Phospholipase C. Adv Exp Med Biol. 740: 235-254.
- Kadamur G, Ross EM (2013) Mammalian phospholipase C. Annu Rev Physiol 75: 127-154.
- Clark JD, Schievella AR, Nalefski EA, Lin LL (1995) Cytosolic phospholipase A2. J Lipid Mediat Cell Signal 12(2-3): 83-117.
- Liang X, Wu L, Wang Q (2007) Function of COX-2 and prostaglandins in neurological disease. Journal of Molecular Neuroscience 33(1): 94-99.
- Rouzer CA, Marnett LJ (2009) Cyclooxygenases: structural and functional insights. Journal of Lipid Research 50 (Suppl): 29-34.
- Morrow JD, Hill KE, Burk RF (1990) A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A 87(23): 9383-9387.
- Morrow JD (2000) The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev 32(3-4): 377-385.
- Jawerbaum A, Gonzalez E (2005) The role of alterations in arachidonic acid metabolism and nitric oxide homeostasis in rat models of diabetes during early pregnancy. Current Pharmaceutical Design 11(10): 1327-1342.
- Wentzel P, Welsh N, Eriksson UJ (1999) Developmental damage, increased lipid peroxidation, diminished cyclooxygenase-2 gene expression, and lowered prostaglandin E2 levels in rat embryos exposed to a diabetic environment. Diabetes 48(4): 813-820.
- Wentzel P, Eriksson UJ (2005) A diabetes-like environment increases malformation rate and diminishes prostaglandin E (2) in rat embryos: Reversal by administration of vitamin E and folic acid. Birth Defects Research A Clinical and Molecular Teratology 73(7): 506-511.
- Piddington R, Joyce J, Dhanasekaran P, Baker L (1996) Diabetes mellitus affects prostaglandin E2 levels in mouse embryos during neurulation. Diabetologia 39(8): 915-920.
- Jawerbaum A, Gonzalez ET, Sinner D (2000) Diminished PGE2 content, enhanced PGE2 release and defects in 3H-PGE2 transport in embryos from overtly diabetic rats. Reprod Fertil Dev 12(3-4): 141-147.
- Jawerbaum A, Sinner D, White V (2001) Modulation of PGE2 generation in the diabetic embryo: effect of nitric oxide and superoxide dismutase. Prostaglandins, Leukotrienes, and Essential Fatty Acids 64(2): 127-133.
- Al Matubsi HY, Salim MD, El Sharaky AS (2010) Activities of cyclooxygenases, and levels of prostaglandins E2 and F2alpha, in fetopathy associated with experimental diabetic gestation. Diabetes and Metabolism 36(1): 43-50.
- El Bassiouni EA, Helmy MH, Abou Rawash N (2005) Embryopathy in experimental diabetic gestation: assessment of PGE2 level, gene expression of cyclooxygenases and apoptosis. British Journal of Biomedical Science 62(4): 161-165.
- Wentzel P, Eriksson UJ (1998) Antioxidants diminish developmental damage induced by high glucose and cyclooxygenase inhibitors in rat embryos in vitro. Diabetes 47(4): 677-684.
- Goto MP, Goldman AS, Uhing MR (1992) PGE2 prevents anomalies induced by hyperglycemia or diabetic serum in mouse embryos. Diabetes 41(12): 1644-1650.
- Baker L, Piddington R, Goldman A, Egler J, Moehring J (1990) Myo-inositol and prostaglandins reverse the glucose inhibition of neural tube fusion in cultured mouse embryos. Diabetologia 33(10) 593-596.
- Wentzel P, Eriksson UJ (2002) 8-Iso-PGF(2alpha) administration generates dysmorphogenesis and increased lipid peroxidation in rat embryos in vitro. Teratology 66(4): 164-168.
- Gerber RT, Holemans K, O'Brien CI (2000) Increase of the isoprostane 8-isoprostaglandin f2alpha in maternal and fetal blood of rats with streptozotocin-induced diabetes: evidence of lipid peroxidation. American Journal of Obstetrics and Gynecology 183(4): 1035-1040.
- Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ (2011) Isoprostane generation and function. Chemical Reviews 111: 5973-5996.
- Zenon GJ, Abobo CV, Carter BL, Ball DW (1990) Potential use of aldose reductase inhibitors to prevent diabetic complications. Clinical Pharmacy 9(6): 446-457.
- Grewal AS, Bhardwaj S, Pandita D, Lather V, Sekhon BS (2016) Updates on Aldose Reductase Inhibitors for Management of Diabetic Complications and Non-diabetic Diseases. Mini Reviews in Medicinal Chemistry 16(2): 120-162.
- Chalk C, Benstead TJ, Moore F (2007) Aldose reductase inhibitors for the treatment of diabetic polyneuropathy. Cochrane Database Syst Rev 17(4): CD004572.
- Reece EA, Wu YK, Wiznitzer A (1996) Dietary polyunsaturated fatty acid prevents malformations in offspring of diabetic rats. American Journal of Obstetrics and Gynecology 175(4 pt 1): 818-823.
- Reece EA, Wu YK (1997) Prevention of diabetic embryopathy in offspring of diabetic rats with use of a cocktail of deficient substrates and an antioxidant. American Journal of Obstetrics and Gynecology 176(4): 790-797.
- Reece EA, Wu YK, Zhao Z, Dhanasekaran D (2006) Dietary vitamin and lipid therapy rescues aberrant signaling and apoptosis and prevents hyperglycemia-induced diabetic embryopathy in rats. Am J Obstet Gynecol 194(2): 580-585.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.