Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Liver and Kidney Function Trends in a Population Under Mass Treatment with Ivermectin in The North West Region of Cameroon (Onchocerciasis Endemic Region)
*Corresponding author: Jules Clement Assob Nguedia, Department of Medical Laboratory Science, Faculty of Health Sciences, University of Buea, Buea, Cameroon.
Received: June 11, 2021; Published: June 30, 2021
DOI: 10.34297/AJBSR.2021.13.001875
Abstract
Background: In Cameroon, despite mass administration for more than 16 years of ivermectin, the transmission of onchocerciasis remains persistant. Therefore community-directed treatment with ivermectin continues. Long-term exposure of ivermectin following repeated annual distribution to treat onchocerciasis on the liver and kidney functions could lead to the discontinuity of community Directed Treatment with Ivermectin (CDTI). Insufficient data exist on the liver and kidney function parameters following yearly ivermectin treatment. This study thus reports the liver and kidney functions of individuals who have been receiving ivermectin compared to those not treated in the Menchum Valley of North- West Region Cameroon.
Methods: A community-based cross-sectional and analytical study was conducted from June 2018 to July 2020. A multistage sampling technique was used to select the participants. Using a semi-structured questionnaire, data on sociodemographic characteristics and ivermectin treatment statues were collected from each participant as well as two skin snips from the iliac region for microscopic detection of O. volovulus. Biochemical markers of liver (Aspartate aminotransferase and alanine aminotransferase) and kidney (creatinine, urea, creatinine clearance) functions were analysed on blood specimens collected from each participant. Urine sample was collected for proteinuria. Statisctical analyses were done to compare the means+/- SEM using SPPS 21.0. p was set at a significant level less than 0.05.
Results: Out of the 1401 Menchum Valley residents aged 15 and above enrolled in this study, 249(17.8%) were males and 1152(82.2%) females. The prevalence of onchocerciasis was 4.02%. A high prevalence of abnormal liver function parameters was observed 782/1401(52.2%) and 668/1401(47.7%) for AST and ALT, respectively). However, in un-infected participants, no statistically significant relationship was detected between those on and not on ivermectin (P=0.088). The prevalence of abnormal kidney function profiles revealed 161/1345(11.1%), 227/1345(16.2%), and 103/1345(9.1%) for creatinine, urea, and creatinine clearance, respectively. Also, a high level of creatinine and urea of un-infected individuals on (P= 0.002 and not on ivermectin P=0.001) indicated a significant association with ivermectin uptake. Mean ± SEM for proteinuria between uninfected individuals on(0.40±0.040) and not on ivermectin (0.44±0.02 ) was statistically significant p= <0.005).
Conclusion: The repeated administration of ivermectin did not affect some liver function such as AST and ALT but induced significant changes in kidney function such as serum creatinine(Scr) and urea levels.
Keywords: Onchocerciasis, Menchum Valley Subdivision, Ivermectin, Aspartate Aminotransferase, Alanine Aminotransferase, Creatinine, Serum Urea
Abbreviations: ALT : Alaninine Aminotransferase; ANOVA : Analysis Of Variance; APOC: African Programme for Onchocerciasis Control; AST: Aspartate Aminotransferase; CDD: Community Drugs Distributors; CDTI: Community Directed Treatment With Ivermectin; CI: Confidence Interval; DALYS: Disability-Adjusted Life Years; GLDH: Glutamate Dehydrogenase; IBM-SPSS: International Business Machine Corporation Statistical Package for Social Science; IRB-FHS: Institutional Review Board Faculty of Health Science; L1: First Stage Larva; L2: Second Stage Larva; L3: Third Stage Larva; MDA: Mass Drug Administration; OCP: Onchocercaisis Control Programme; PPSE: Probability Proportionate to Size Estimation; P-VALUE: Probability Value; REMO: Rapid Epidemiological Mapping; REA: Rapid Epidemiological Assessment; UV: Ultra Violet; X2: Chi-Square
Introduction
Onchocerciasis or river blindness is also known as Robles disease, a parasitic disease caused by one filarial nematode called Onchocerca volvulus. It is one of the leading causes of infectious blindness in Africa [1,2]. Transmission is by the black fly Simuliumdamnosum. l. breed in fast-flowing rivers and streams and is also referred to as river blindness due to the disease condition. The global distribution of onchocerciasis indicates that 37 million individuals are infected, of which 99% of the disease is found in Africa. Thirty-five countries of the world are endemic to river blindness, of which thirty are found in Africa, mainly in rural communities [3]. The parasitic worm forms nodules under the skin. The female worms produce microfilariae (mf) that live in nodules and inflame the skin; microfilariae may enter the eyes, giving rise to inflammatory lesions there as well. These microfilariae are picked up by species of Simulium that serve as vectors, where they develop through three distinct larval stages (L1, L2, and L3). The infective larva (L3) may be passed on to other humans on subsequent bites, completing the life cycle.
The risk of O. volvulus transmission was relatively elevated before the introduction of a coordinated effort to control the black fly vector. Before this , the control of onchocerciasis, the risk of acquiring the disease was relatively high along with riverine breeding sites of the black fly vector. Blindness affected people in most communities; hence they abandoned fertile river valleys for fear of contracting the disease. The result was famine and severe poverty. Economic losses were estimated at U.S. $30 million in West Africa [4]. Control strategies began as far back as 1974 in Africa with the onchocerciasis control program (OCP) in 7 and 11 countries in West Africa [5]. OCP strategy has relied on larvicide spraying on fast-flowing rivers or streams.
The African Program for Onchocerciasis Control (APOC) is a global initiative aimed at reducing the disease burden of human onchocerciasis (river blindness) in Sub-Saharan Africa (SSA) and, where possible, eliminating infection through mass drug treatment (MDA) [1,2]. Since its inception in 1995, APOC and its partnering beneficiary countries expanded their control activities geographically to cover all meso- and hyperendemic areas. Thus, averting 8.9 million disability-adjusted life years (DALYs) by 2010 and aiming to treat over 90 million people annually in 16 African countries by 2015, protecting an estimated population of 118 million people at risk of onchocerciasis [5].
Ivermectin, the medication used to treat onchocerciasis in large groups, is delivered and administered in a single dose of 150-200 g/kg of body weight once a year. Ivermectin is not recommended for chronically sick individuals, pregnant (or lactating) mothers, or children under the age of five [22]. The drug half-life is approximately 12 h, and it is eliminated in about 72 h from the body. The drug must be administered yearly for 12-15 years to break the transmission cycle of human onchocerciasis. Guidelines for stopping mass drug administration and verifying elimination of human onchocerciasis: criteria and procedure [31]. APOC's primary strategy of community-directed treatment with Ivermectin (CDTI) depends on active community participation in ivermectin distribution [6]. Ivermectin is a safe and powerful microfilaricidal drug that has been given by Merck and Co. The drug kills microfilaria( mf) and diminishes the danger of developing eye and skin illness related to the infection. It likewise lessens the fertility of adult worms. It also abbreviates their expectancy, yet treatment should be given for over 15 years [4]. Rapid epidemiological mapping(REMO) distinguishes communities in danger of infection. This was then trailed by rapid epidemiological assessment (REA), a quick and cheap method to identify communities based on their endemicity status.
A collection of observable epidemiologic and entomologic rules is used to check the elimination of morbidity and transmission and to help direct decisions on when to stop semi-annual ivermectin treatment of the eligible population at risk [7]. In other endemic foci in Africa, the entomologic rule of ˂ 0.5 infected flies/1000 flies and an epidemiologic measure of ˂ 5% mf prevalence in all communities were used to evaluate interruption of transmission [7,8]. The right strategy for catch and analysis of grown-up flies for contamination has been the customary procedure of checking the elements of vector infectivity just as the transmission potential [9-11].
The frequency and severity of the effect after annual ivermectin treatment are critical to onchocerciasis control efforts, especially as endemic populations become more empowered to self-treatment. As a result, the adverse response to community-directed treatment with ivermectin (CDTI) must be monitored regularly to avoid treatment discontinuation due to injuries caused on key organs like the liver and kidney. Their function following annual ivermectin treatment should be ascertained regularly in Cameroon because it is one of the world's most affected countries, with 5.1 million people infected [28]. This study thus reports the liver and kidney function parameters of individuals who have received ivermectin compared to those not on treatment in the Menchum valley of North-West region Cameroon in order to ensure its continual mass administration.
Material and Methods
Study Area
The study area was Menchum Valley Subdivision, Menchum Division, Northwest Region of Cameroon. Menchum valley is located between 6º 10’00’’ to 7º 00’00’’ latitude North and from 9º 55’00’’ to 10º 35’00’’ longitude east. The Subdivision is situated at 65 km from Bamenda, the Regional capital, Menchum Valley includes villages like Tingoh, Ibiatem, Agoli, Bangwe, Befang, Modele, Mawong, and Benakuma, with about 40.000 inhabitants.Ivermectin has been administered in this community for more than 15 years since 1998 [29] The bioclimatic area( rainy and dry seasons) is chosen because of good knowledge of the existence of Simulium damnosum complex population, human and animal onchocerciasis, the accessibility of the study site, and ivermectin are distributed annually in this area.
Study Design and Population
A cross-sectional and analytical study was used to determine the long-term effect of treatment with ivermectin on the liver and kidney functions of un-infected individuals on and not on treatment. Participants above 15 years and having stayed in Menchum Valley Subdivision for more than two years were enrolled in the study, which took place from June 20 to July 2020. Onchocerciasis was defined by a positive skin snip detected by parasitological examination. Free-onchocerciasis patients were defined by negative skin snips.
Sample Size Estimation and Sampling Techniques
A multistage randomized sampling technique was used for this study. The minimum sample size was calculated using the Lorentz formula by assuming a 3.5% prevalence of onchocerciasis from the research conducted in Cameroon [12], error at 95% C.I. 5% corresponding to the population which might not be covered by the survey. Four villages were selected randomly (Mawong Bangwi, Befang, and Benakuman), with about 22,000 inhabitants from Menchum Valley Subdivision.A minimum of 51 participants from randomly selected households was included from each village. Globally 1401 participants were recruited based on probability proportionate to size(PPS) approach.
Data Collection
Age, gender, occupation, education, village, compliance with ivermectin treatment, the frequency of ivermectin administration, and the duration of stay in the residency were independent variables collected through a questionnaire structured in English and translated verbally into the local language.
Parasitological Analysis: Two skin snip biopsies from the upper iliac crest and lower extremities were collected using a disposable syringe and a sterilized razor blade by qualified health personnel. Each skin snip biopsy was placed in coded 96 microtiter plate wells containing normal saline and kept at room temperature for 24 hours for complete emergence of mf. Skin snips biopsies were removed from the wells, and each solution from the plate was examined under the microscope for the presence of onchocercal microfilaridermia [21]. The number of mf counted was reported as the number of mf/skin snip. The positive slides were dried and fixed with methanol and stained with Giemsa to differentiate the mf of volvulus from other microfilaria using morphological characteristics [12]. The positive cases were referred to the hospital for treatment through their contact.
Biochemical Analysis: Venous blood of 5 to 10 ml was collected using sterile disposable non - pyrogenic syringes (CATHY YOUGO®, France) into dry vacutainer tubes from each of the respondents by qualified health personnel. A code number and date were written on these tubes. They were centrifuged at 3000rpm for 5 to 10 minutes. Sera were separated from the blood and kept in ice packs, after which they were transported to the university teaching laboratory-Bamenda for liver and kidney function analyses using URIT 810 CHEMISTRY ANALYSER.
Liver Function Analysis: Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) were measured from the serum using kinetic U.V. optimized method international federation of clinical chemistry and laboratory medicine (IFCC) using SGM Italia liquid reagent at 37°c 340 nm wavelength. The test parameters were introduced into the spectrophotometers following the manufacturer’s instruction [19], with normal values as follows: AST women <31 ui/l, men < 38 ui/l ALT women <31 ui/l, men < 41 ui/l. [24].
Kidney Function Analysis: The biochemical parameters quantified consisted of serum urea, and creatinine, creatinine clearance, urine creatinine, and protein. Serum urea was measured by kinetic U.V. method urease-GLDH at 37°c with 340 nm wavelength. The serum and urine creatinine were measured based on Jaffe-Reaction photometric coloration test at 37°c with 510 nm wavelength. Meanwhile, creatinine clearance was calculated according to the equation; Creatinine clearance = urine creatinine volume of urine /serum creatinine x 24 hours. Normal values were serum urea 14 -45 mg/dl, serum creatinine 0.6 - 1.1 and 0.90-1.30 mg/dl for women and men respectively and creatinine clearance 70 - 140 ml/mins/1.73. Also, 24 hours urine was collected from each participant for the analysis of the kidney function. Participants were instructed to discard the first-morning urine and note the time to collect urine for twenty-four hours. The volume of urine collected was noted, and the urine samples were thoroughly sharked for homogeneity, and 3ml of the urine was poured into appropriately labeled test tubes for urine chemistry. This was diluted at 1:100 for urine creatinine, and urine protein was measured using Mission series test strips from ACON company series UR8012130. The strips were dipped into urine up to the test area for about 3seconds, and the color change was read by comparing to a standard provided by the manufacturer, and results were recorded in g/l. Normal values of Proteinuria were less than 0.30 g/l.
Statistical Analysis
Data were entered into and analyzed with IBM-SPSS statistic version 20.0. Descriptive statistics used for the percentages of abnormal participants on and not on ivermectin. Chi-square was used to determine the Relationship between ivermectin treatment and liver/kidney function parameters of individuals who tested negative for onchocerciasis. To compare the mean and standard error mean of participants on ivermectin and those not on treatment, a descriptive one-way ANOVA Fisher test. Bivariate Pearson’s correlation was used for the relationship between the frequency of ivermectin treatment with liver and kidney function profiles of participants negative for onchocerciasis; P-values less than 0.05 were considered significant and a confidence interval of 95%.
Ethical Consideration
Household members were reminded that participating in the study was voluntary and not a requirement for receiving regular medical exams. The ethical committee of the University of Buea in Cameroon gave their approval reference number 2018/0243/UB/ SG/IRB/FHS. The permission of the North West regional delegation of Public Health and community leaders was also obtained. Every qualified person gave informed verbal consent before being included in the study after being told about the study’s goal. Privacy and confidentiality were ensured.
Results
Sociodemographic Characteristics of Study Participants
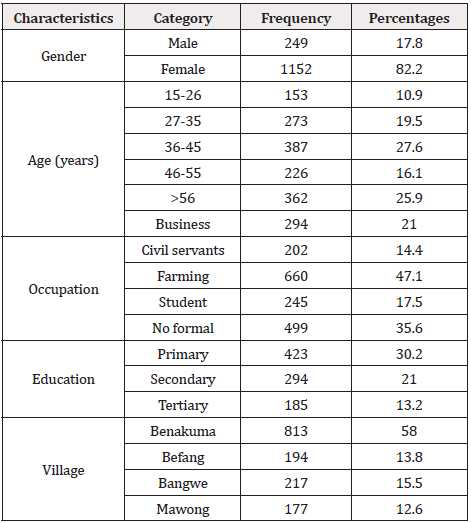
A total of 1401 Menchum Valley residents aged 15 and above enrolled in this study, with 249 males and 1152 females making up the total. According to the data, most participants, 660, were farmers, while 245 were students. Up to 499 of the 1401 participants in this study had no formal education, 423 had primary education, 294 had secondary education, and 185 had tertiary education, with the majority (813) from Benakuma village. Table 1 summarizes the study population's characteristics.
Table 2 shows abnormal liver and kidney function trends among study participants on ivermectin compare to those not on treatment. In our study, 732 of 1401 participants had abnormal Aspartate aminotransferase (AST), with 532 individuals on ivermectin against 200 not on treatment was statistically not significant (P= 0.273). Alanine aminotransferase(ALT) indicated 668 individuals with abnormal values, where 488 were on ivermectin and 180 not on treatment which was statistically not significant(P= 0.503).
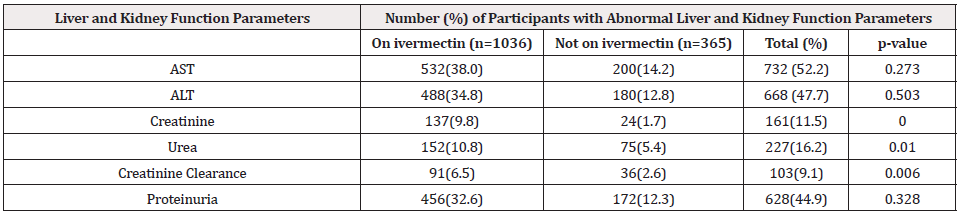
Table 2: Abnormal liver and Kidney function trends among study Participants on ivermectin compare with those not on treatment (n=1401).
In this study, serum creatinine for those on ivermectin revealed 9.8 percent against 1.7 percent for those not on treatment, with a total of 11.5 percent, which was statistically significant(P=0.000). Serum urea indicated 227 participants with abnormal values. One hundred and fifty-two (10.8 percent) on ivermectin against seventy-five(5.4 percent) was statistically significant(P=0.010). Also, 9.1percent of the participants had abnormal creatinine clearance, which was statistically significant (P=0.006), of which 6.5 percent and 2.6 percent were for those on and not on ivermectin, respectively. Proteinuria showed a higher percentage (44.9 percent) among study participants, surprisingly non-significant(0.328).
Table 3 compares the prevalence of abnormal and normal liver function parameters of negative participants on and not on ivermectin. According to our findings, 524 participants on ivermectin had abnormal AST, while those of the control group had 186 individuals. Meanwhile, 488 and 180 participants from those on ivermectin and not on treatment also had abnormal ALT values. From our observation, the comparison of abnormal liver function parameters did not show statistical significance between individuals on ivermectin and the control group ( AST: X2=1.500, P= 0.230, ALT: X2=3.063, P=0.088).
Table 4 shows the mean ± SEM of liver function test among the study population.
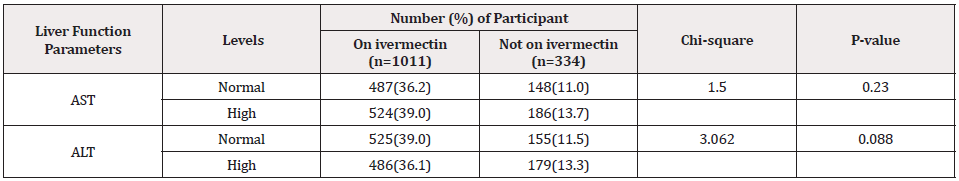
Table 3: Compares the prevalence of abnormal and normal liver function parameters of negative participants on and not on ivermectin (n=1345).
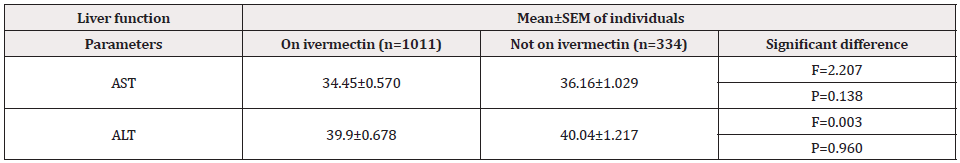
Table 4: Liver function test of O.vovulus non-infected individuals on ivermectin compare with those not on treatment (n=1345).
Individuals who were not taking ivermectin had higher mean serum aspartate aminotransferase levels than those on treatment (36.16.±1.029 and 34.45±.570, respectively). There was no statistically significant difference (F=2.207, P=0.138). Also, a slight elevation of ALT levels was observed with participants not on ivermectin than those on treatment (40.04.±1.217 and 39.9.±0.678, respectively); there was no statistical significance in serum alanine aminotransferase (F=0.003, P=0.960).
Table 5 compares the prevalence of abnormal and normal kidney function parameters of O.volvulus negative participants on and not on ivermectin. This study revealed a significant statistical difference of abnormal creatinine level (10.0 percent) of participants on ivermectin compared with participants not on ivermectin(1.8 percent); X2 =9.161, P= 0.002. Also, serum urea was statistically significant between the number of individuals on ivermectin and those not on treatment 11.0 percent against (5.6 percent) with X2= 10.460, P= 0.001 respectively. Proteinuria indicated a higher abnormal percentage between individuals on ivermectin and those not on treatment but statistically non-significant (X2= 1.780, P= 0.102).
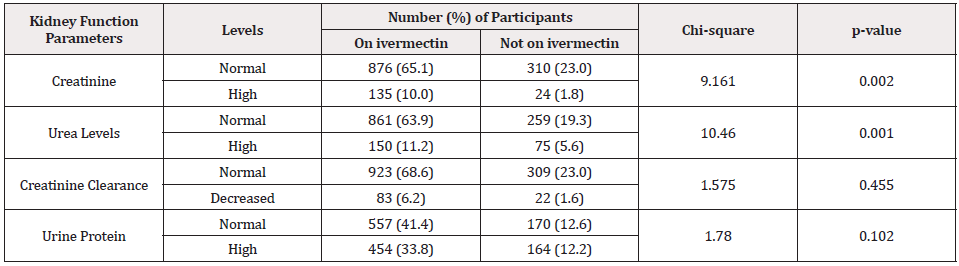
Table 5: Compare the prevalence of abnormal and normal kidney function of participants on and not on ivermectin (n=1345).
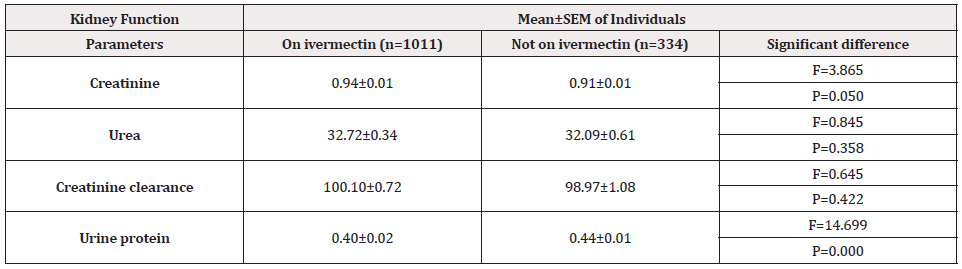
Table 6 shows the mean ± SEM of kidney function test of O. volvulus non-infected individuals compare to those not on treatment. The serum creatinine was higher in participants on ivermectin than those not on treatment, and the difference was statistically significant (F= 3.865, P= 0.050). The mean ± SEM serum urea was higher in individuals on treatment (32.72 ± 0.34) than in the control group (32.09 ± 0.61 ). However, the difference was not significant ( F=0.845, P= 0.58). There was no significant difference between the creatinine clearance of individuals on ivermectin and the control group (F= 0.645, P= 0.422). The mean ± SEM Proteinuria concentration was lower in individuals on treatment (0.40 ± 0.01) than those not on ivermectin(0.44 ± 0.01), and the difference was statistically significant (F= 14.699, P= 0.000).
Discussion
Thanks to dedicated regional programs that have been largely effective in most endemic countries, substantial progress has been made in monitoring and eliminating onchocerciasis in Africa over the last few decades. Onchocerciasis has also been declared a disease in a few African nations, raising expectations that it will be eradicated from the continent entirely by 2025 [28].
However, several obstacles are impeding current efforts to eradicate onchocerciasis in Africa. Uncomplete mapping of all transmission areas, co-endemicity of onchocerciasis and loiasis, the potential emergence of ivermectin resistance. Also, uncoordinated cross-border elimination campaigns, conflict, civil unrest, and a suboptimal program implication are significant challenges. In Cameroon, despite the annual mass administration of ivermectin for more than 16 years, the transmission of onchocerciasis is persisting [12]. As such, uncertainty surrounding the long-term effect of ivermectin following repeated treatment on liver and kidney function could lead to the discontinuity of CDTI, resulting in an increased prevalence of river blindness with the consequences of elevating social, economic, and heavy financial burdens.
Therefore, the present study assessed the effect of ivermectin on the liver and kidney functions of participants on and not on ivermectin not infected with O.volvulus in an onchocerciasis endemic area. A high prevalence of abnormal Aspartate aminotransferase(AST) and Alanine aminotransferase(ALT) was recorded in the study. Our findings also indicated no significant association between participants, irrespective of whether being on ivermectin or not, and abnormal ALT and AST levels. However, an earlier study [25] indicated a significant reduction in the liver and kidney ALT and AST activities in the rat when treated with ivermectin. On the contrary, in an earlier study, high activities of AST and ALT in Vistar rats fed on growers mash and treated with therapeutic doses of ivermectin [26]. Their findings also indicated that an elevated ALT level compared to AST. Elevation of ALT appears to reflect toxic hepatitis, while AST enzyme is useful in early recognition of a toxic hepatocellular origin of drugs [26].
Regarding the diagnosis of chronic kidney disease, serum creatinine levels (sCr) have a substantial prognostic value, and concentrations usually increased in the serum only when approximately 40-50% of renal parenchyma was reversibly or irreversibly damaged [27]. We found a significant difference between participants with abnormal serum creatinine (sCr), Creatinine clearance, urea, and ivermectin uptake. Proteins and intracellular enzymes appear in serum or plasma as a consequence of normal cell turnover. However, an increased level may be a result of cell damage due to drugs or cell proliferation. Currently, the diagnosis of chronic kidney disease is usually made on the levels of blood urea and serum creatinine (sCr). Our findings showed that Creatinine clearance was significantly higher in participants on ivermectin than those not on ivermectin. Urine protein was significantly lower among O. vovulus free individuals on ivermectin than those not. However, an earlier study [30] showed high excretion of total urinary proteins in patients with high microfilariae densities. The discrepancy in both studies might be because the present study did not determine the intensity of infection in the study participants Interestingly, most participants on ivermectin and tested negative for Onchocerca volvulus had normal Creatinine, Creatinine clearance, and Urea levels. We may ascribe that ivermectin seems to cause only minor damage to the glomerulus and disturbances of the reabsorption of low molecular weight proteins in the tubules system.
Conclusion
Transmission of onchocerciasis has not been interrupted in the Menchum Valley. There is still annual mass administration of ivermectin, which might lead to fear of metabolic problems in humans resulting in discontinuity of treatment. From our study, there was mild liver and kidney injury. We may ascribe that ivermectin seems to cause only minor damage to the glomerulus. Therefore, mass drug administration of ivermectin could be continued to ensure onchocerciases elimination. Routine monitoring and evaluation could be regularly done on these organs, not to miss a few cases.
Data Availability
The data used was collected by experienced scientists and health personnel. The data was collected with a questionnaire, case report forms, and observations.
Ethical Approval
Ethical clearances were obtained from the IRB-FHS of the University of Buea.
Acknowledgment
The authors thank the people of Menchum Valley Subdivision for their cooperation and assistance in the data collection and Benakuma District Hospital staff members for their collaboration in laboratory investigation.
Author’s contribution
KSF, and BT conceived and designed the study. KSF and BT collected specimens and performed laboratory and statistical analysis. KSF, KHL NLA and AN critically reviewed the literature and wrote the original draft. KSF and AN supervised the study. All authors contributed to the write-up, reviewed the final draft, read and approved the final manuscript
Competing interest
The authors declare that they have no competing interests.
References
- Zhang L, Ding J, Li HY, Wang ZH, Wu J (2020) Immunotherapy for advanced hepatocellular carcinoma, where are we?. Biochim Biophys Acta Rev Cancer 1874(2): 188441.
- Chiew Woon L, Joycelyn Jie Xin L, Su Pin C (2020) Nivolumab for the treatment of hepatocellular carcinoma. Expert Opin Biol Ther 20(7): 687-693.
- Ghavimi S, Apfel T, Azimi H, Persaud A, Pyrsopoulos NT (2020) Management and Treatment of Hepatocellular Carcinoma with Immunotherapy. J Clin Transl Hepatol 8(2): 168-176.
- Chen Z, Xie H, Hu M, Huang T, Hu Y, et al. (2020) Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res 10(9): 2993-3036.
- Gordan J D, Kennedy E B, Abou Alfa GK, Beg M S, Brower S T, et al. (2020) Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol 38(36): 4317-4345.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.