Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Roles of Autophagy in Regulating ER Stress-Mediated Type 2 Diabetes
*Corresponding author: Md Rafikul Islam, Cardiovascular Research Institute, Yokohama City University Graduate School of Medicine, Japan
Received: June 07, 2021; Published: June 15, 2021
DOI: 10.34297/AJBSR.2021.13.001853
Abstract
Type 2 diabetes (T2D) is a metabolic disorder closely associated with endoplasmic reticulum (ER) stress mediated β-cells loss and/or dysfunction and insulin resistance. On the other hand, ER stress and autophagy are strongly interconnected to maintain cellular homeostasis under metabolic stress and environmental cues. Therefore, co-targeting autophagy and ER stress is a promising strategy for T2D treatment.
Keywords: Diabetes; ER stress; Autophagy
Abbreviations: ER: Endoplasmic Reticulum; T2D: Type 2 diabetes; FFA: Free Fatty Acid; LC3: light chain 3; XBP1: X-Box-Binding Protein 1; TXNIP: Thioredoxin Interacting Protein
Introduction
The endoplasmic reticulum (ER) is the main site in the cell for the post-translational modification and secretion of insulin and other proteins. High blood glucose, starvation, lipid toxicity, free fatty acid (FFA), obesity, oxidative stress, insulin resistance, abnormal inflammatory response and Ca2+ concentration increase the burden of insulin synthesis and/or impair the insulin processing step in the ER and lead to ER stress [1-9]. Many scientific studies reported that ER stress is implicated in β-cell dysfunction, impaired insulin secretion, and insulin resistance that are considered the main reasons for the pathogenesis of T2D [7,10]. Autophagy plays an important role in regulating ER stress-mediated β-cell dysfunction and insulin resistance, although details mechanism of autophagy in diabetes remains to be elucidated. ER stress-mediated autophagy is usually activated through the ER stress-induced transcription factors such as CCAAT enhancer-binding protein (C/ EBP)-homologous protein (CHOP), X-box-binding protein 1 (XBP1), or other signaling pathways such as via JNK or mTOR [11-14]. Microtubule-associated protein 1 light chain 3 (LC3), an autophagic component is reported to be activated by the ER stress-mediated phosphorylation of PERK/eIF2α [15]. Autophagy suppresses ER stress-induced β-cells apoptosis through downregulation of mTORC1 and improves insulin secretion that is hampered by ER stress [16]. Indeed, β-cells Atg7 (autophagy related-7)-knockout mice show degenerated islets, impaired insulin secretion, and glucose intolerance [17,18]. PI3K, p85, and p85β are involved in unfolded protein response (UPR) gene expression through binding to X-box binding protein 1 (XBP 1) in an insulin-dependent manner [19,20,4,21] demonstrated that impaired or reduced expression of functional PI3K, p85, and p85β in the autophagy-deficient β-cells lead to compromised UPR, increase β-cell death, and progression of diabetes in mice. Autophagy inducer (Figure 1) (rapamycin) is found to reduce forced ER stress mediated PERK, CHOP and BiP gene expression, decrease p62 level (an autophagy marker that accumulates in the cells if macroautophagy is suppressed), preserve the ultrastructure of ER and mitochondria, improve β cells function and decrease β-cell death in T2D islets [22]. Rapamycin improves diabetes in diabetic Akita mice model through increasing insulin content and preventing β-cells apoptosis, while inhibition of autophagy exacerbates ER stress and diabetes [23]. It is demonstrated that autophagy-deficient β-cells are more susceptible to forced ER stress-induced cell death and proposed that autophagy plays a critical role in regulating appropriate UPR signaling and lack of autophagy hampers UPR or gene expression and increases prone to diabetes progression [4].
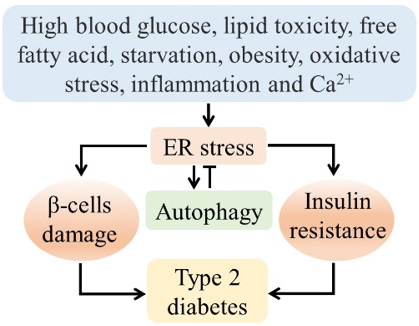
Figure 1: The relationship between ER stress, autophagy, and diabetes. Nutritional stress and environmental cues can develop type 2 diabetes through ER stress-mediated β-cells dysfunction and insulin resistance, where autophagy can play a protective role against diabetes. Although abnormal autophagy exaggerates the pathologic condition.
Several studies demonstrate that autophagy has protective roles by reducing ER stress and inflammatory cytokines (IL1 β) production [5,24,25]. Thioredoxin-interacting protein (TXNIP) is considered a potential therapeutic target for diabetes and other ER stress-mediated diseases as it is an important signaling node that links ER stress, inflammation, and autophagy [5]. TXNIP is activated by ER stress via the PERK and the IRE1 pathways, then stimulates IL1 β production by the NLRP3 inflammasome, and increases β-cell death, whereas autophagy is observed to play important protective roles through reducing ER stress, NLRP3-dependent inflammatory cytokine production and PERK/CHOP mediated apoptosis. In contrast, autophagy is also reported to be associated with β-cells damage in T2D and might contribute to β-cells dysfunction [26]. Forced ER stress is found to trigger autophagy-mediated cell death through downregulation of the Akt/TSC/mTOR pathway [13]. Oxidative stress triggers ER stress-mediated β cell dysfunction through impairing di-sulfide bond formation, and accumulation of misfolded proteins [6,27- 29]. For instance, human diabetic islets lead to accumulating β-amyloid that is correlated with oxidative stress and apoptosis in the lack of ER stress [30,31]. In addition, autophagy is found to control hyperglycemia by reducing the oxidative stress-mediated accumulation of ubiquitinated-proteins aggregate in the β-cells [32]. ER stress under oxidative conditions suppresses insulin production, decreases β-cell mass, or even leads to β- ubiquitin and p62, degenerate proteins, reduce insulin content, increase β-cell death, and suppress β-cell proliferation, while autophagy plays a crucial role to protect β-cell by clearing insoluble or long-lived large protein aggregates [35,4]. Recent studies demonstrate that autophagy is activated in response to lipotoxic ER stress to protect the β-cell failure [36]. Decrease in Ca2+ level in the ER leads to progress T2D through increasing the ER stress, promoting store-operated Ca2+ entry (SOCE), activating calcium-calmodulin kinase II (CaMKII), decreasing lipid removal by autophagy, and increasing insulin resistance [37]. Interestingly, Park HW et al. [38] reported that in obesity and lipotoxicity, an increase in Ca2+ concentration decreases autophagy, while Ca2+ channel blocker restores autophagic flux by enhancing autophagosome-lysosome fusion, prevents large proteins or lipid droplets accumulation, reduces inflammation, and suppresses insulin resistance. Impaired autophagy might entail the development of metabolic disorders through dysregulation of ER stress-mediated insulin resistance. For instance, autophagy is reduced in the liver of obese mice; and Atg7 (autophagy related 7) overexpression restores insulin sensitivity and decreases the expression of ER stress marker [39]. Inversely, insulin resistance inhibits Fox1-dependent expression of key autophagy genes [40]. Activation of X box-binding protein-1 (XBP1) transcription factor through ER stress-mediated phosphorylation of inositol requiring enzyme-1α (IRE1α) plays a vital role in insulin resistance. ER stress by obesity/lipid injury or cytokines is found to develop insulin resistance through serine phosphorylation of insulin receptor system-1/2 (IRS-1/2) by c-Jun N-terminal protein kinase (JNK) [7,41,42].
Autophagy deficiency usually worsens the ER stress-induced inflammatory response. Yoshizaki T et al. [43] found that autophagy is decreased and inflammation is increased in adipose tissue of insulin-resistant mice and hypertrophic 3T3-L1 adipocytes; the activation of autophagy or the inhibition of ER stress (by tauroursodeoxycholic Acid) suppress inflammation through regulating phosphorylation of PERK and e-IF2α, expression of CHOP, and XBP-1 splicing for the expression of autophagy-related genes, such as LAMP1, LAMP2, Atg5 and inflammatory-related genes, such as MCP-1, IL-6, and IL-1β. However, autophagy is also found to contribute to obesity/ER stress-induced insulin resistance by degradation of insulin receptors; and blocking autophagy inhibits ER stress-mediated IR degradation [44]. Autophagy may reduce insulin resistance and promote insulin signaling by reducing the overload of ER stress and facilitating ER-mediated proper IR folding and membrane targeting. In conclusion, autophagy shows a protective effect against diabetes through regulating ER stress, while excess or reduced autophagy would lead to failure of β-cell and hamper glucose homeostasis. Therefore, stimulating autophagy in an appropriate context could be a promising therapeutic strategy for ER stress mediated T2D.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- McNicholl JM, Smith DK, Qari SH, Hodge T (1997) Host genes and HIV: the role of the chemokine receptor gene CCR5 and its allele. Emerg Infect Dis 3(3): 261-271.
- Wu L, LaRosa G, Kassam N, Gordon CJ, Heath H, et al. (1997) Interaction of chemokine receptor CCR5 with its ligands: Multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med 190(8): 1373-1381.
- Sorce S, Myburgh R, Krause K (2011) The chemokine receptor CCR5 in the central nervous system. Prog Neurobiol 93(2): 297-311.
- Giunti D, Borsellino G, Benelli R, Marchese M, Capello E, et al. (2003) Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS Abstract : The recruitment of lymphocytes across the blood brain barrier ( BBB ) is mediated by ad- healthy subject , similarly migrated to. J Leukoc Biol 73(5): 584-590.
- Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT (1997) Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5Δ32. J Biol Chem 272(49): 30603-30606.
- Sullivan AD, Wigginton J, Kirschner D (2001) The coreceptor mutation CCR5∆32 influences the dynamics of HIV epidemics and is selected for by HIV 98(18): 10214 –10219.
- Zajac V (2018) Evolutionary view of the AIDS process. J Int Med Res 46(10): 4032-4038.
- Xu M (2020) CCR5-Δ32 biology, gene editing, and warnings for the future of CRISPR-Cas9 as a human and humane gene editing tool. Cell Biosci 10(1): 1-6.
- Balistreri CR, Grimaldi MP, Vasto S, Listi F, Chiappelli M, et al. (2006) Association between the polymorphism of CCR5 and Alzheimer’s disease: Results of a study performed on male and female patients from northern Italy. Ann N Y Acad Sci. 1089: 454-461.
- Mattson MP (2004) Pathways Towards and Away from Alzheimer’s Disease NIH Public Access. Nature 430(7000): 631-639.
- Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148(6): 1204-1222.
- Xia M, Qin S, Wu L, Mackay CR, Hyman BT (1998) Immunohistochemical study of the βchemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am J Pathol 153(1): 31-37.
- Shafi O (2016) Inverse relationship between Alzheimer’s disease and cancer, and other factors contributing to Alzheimer’s disease: A systematic review. BMC Neurol 16(1): 1-17.
- Nixon DW (2017) The Inverse Relationship Between Cancer and Alzheimer’s Disease: A Possible Mechanism. Curr Alzheimer Res 14(8): 883-893.
- Kandimalla R, Thirumala V, Reddy PH, Biophys B, Author A (2017) Is Alzheimer’s disease a Type 3 Diabetes? A Critical Appraisal Graphical Abstract HHS Public Access Author manuscript. Biochim Biophys Acta 1863(5): 1078-1089.
- Khorram Khorshid HR, Manoochehri M, Nasehi L, Ohadi M, Rahgozar M, et al. (2012) Ccr264i and Ccr5 Δ32 Polymorphisms in Patients with Late-Onset Alzheimer’s disease; A Study from Iran (Ccr2-64i And Ccr5 Δ32 Polymorphisms in Alzheimer’s disease). Iran J Basic Med Sci 15(4): 937-944.
- Combarros O, Infante J, Llorca J, Peña N, Fernández-Viadero C, et al. (2004) The chemokine receptor CCR5-Δ32 gene mutation is not protective against Alzheimer’s disease. Neurosci Lett 366(3): 312-314.
- Passos GF, Figueiredo CP, Prediger RDS, Pandolfo P, Duarte FS, et al. (2009) Role of the Macrophage Inflammatory Protein-1α/CC Chemokine Receptor 5 Signaling Pathway in the Neuroinflammatory Response and Cognitive Deficits Induced by β-Amyloid Peptide. Am J Pathol 175(4): 1586-1597.
- Goldeck D, Larbi A, Pellicanó M, Alam I, Zerr I, et al. (2013) Enhanced Chemokine Receptor Expression on Leukocytes of Patients with Alzheimer’s Disease. PLoS One.
- Schwartz M, Peralta Ramos JM, Ben-Yehuda H (2020) A 20-Year Journey from Axonal Injury to Neurodegenerative Diseases and the Prospect of Immunotherapy for Combating Alzheimer’s Disease. J Immunol 204(2): 243-250.
- Serpente M, Bonsi R, Scarpini E, Galimberti D (2014) Innate Immune System and Inflammation in Alzheimer’s Disease: From Pathogenesis to Treatment. Neuroimmunomodulation 21(2-3): 79-87.
- Nudelman KNH, McDonald BC, Lahiri DK, Saykin AJ (2019) Biological Hallmarks of Cancer in Alzheimer’s Disease. Mol Neurobiol 56(10): 7173-7187.
- Guedes JR, Lao T, Cardoso AL, El Khoury J (2018) Roles of microglial and monocyte chemokines and their receptors in regulating Alzheimer’s disease-associated amyloid-$β$ and tau pathologies. Front Neurol 9: 549.
- Desbois AC, Cacoub P (2017) Diabetes mellitus, insulin resistance and hepatitis C virus infection: A contemporary review. Vol. 23, World Journal of Gastroenterology. Baishideng Publishing Group Co Limited 23(9): 1697-1711.
- Robert R, Wark KL (2012) Engineered antibody approaches for Alzheimer’s disease immunotherapy. Arch Biochem Biophys 526(2): 132-138.
- Atwood CS, Obrenovich ME, Liu T, Chan H, Perry G, et al. (2003) Amyloid-β: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-β 43(1): 1-16.
- López-González I, Schlüter A, Aso E, Garcia-Esparcia P, Ansoleaga B, et al. (2015)Neuroinflammatory signals in alzheimer disease and APP/PS1 transgenic mice: Correlations with plaques, tangles, and oligomeric species. J Neuropathol Exp Neurol. 74(4): 319-344.
- Hwang CJ, Park MH, Hwang JY, Kim JH, Yun NY, et al. (2016) CCR5 Deficiency Accelerates Astrogliosis , Amyloid-Beta Deposit and Impaired Memory Function. Oncotarget 7(11): 11984-11999.
- Li M, Shang DS, Zhao WD, Tian L, Li B, et al. (2009) Amyloid Interaction with Receptor for Advanced Glycation End Products Up-Regulates Brain Endothelial CCR5 Expression and Promotes T Cells Crossing the Blood-Brain Barrier. J Immunol 182(9): 5778–5788.
- Rosi S, Pert CB, Ruff MR (2005) Chemokine Receptor 5 Antagonist D -Ala-Peptide T-Amide Reduces Microglia And Astrocyte Activation Within The Hippocampus In A Neuroinflammatory Rat Model Of Alzheimer ’ S Disease 134: 671-676.
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, et al. (2017) A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169(7): 1276-1290.
- Wojta KJ, Ayer AH, Ramos EM, Nguyen PD, Karydas AM, et al. (2020) Lack of Association between the CCR5 -delta32 Polymorphism and Neurodegenerative Disorders. Alzheimer Dis Assoc Disord.
- Choi DY, Lee MK, Hong JT (2013) Lack of CCR5 modifies glial phenotypes and population of the nigral dopaminergic neurons, but not MPTP-induced dopaminergic neurodegeneration. Neurobiol Dis 49(1): 159-168.
- D’Angelo R, Crisafulli C, Rinaldi C, Ruggeri A, Amato A, et al. (2011) CCR5Δ32 Polymorphism Associated with a Slower Rate Disease Progression in a Cohort of RR-MS Sicilian Patients. Mult Scler Int p. 1-6.
- Louboutin J, Chekmasova A, Marusich E, Agrawal L, Strayer DS (2011) Role of CCR5 and its ligands in the control of vascular inflammation and leukocyte recruitment required for acute excitotoxic seizure induction and neural damage. FASEB J. 25(2): 737-753.
- Behrens M, Lendon C, Roe C (2009) A Common Biological Mechanism in Cancer and Alzheimers Disease? Curr Alzheimer Res. 6(3): 196-204.
- Realmuto S, Cinturino A, Arnao V, Mazzola MA, Cupidi C, et al. (2012) Tumor diagnosis preceding alzheimer’s disease onset: Is there a link between cancer and alzheimer’s disease? J Alzheimer’s Dis 31(1): 177-182.
- Kranjc MK, Novak M, Pestell RG, Lah TT (2019) Cytokine CCL5 and receptor CCR5 axis in glioblastoma multiforme. Radiol Oncol 53(4): 397-406.
- Karin N, Razon H (2018) The role of CCR5 in directing the mobilization and biological function of CD11b+Gr1+Ly6Clow polymorphonuclear myeloid cells in cancer. Cancer Immunol Immunother 67(12): 1949-1953.
- Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315(26): 1650-1659.
- Li J, Peng Y, Liu H, Wu Q (2017) The association between CCR5 Δ32 polymorphism and susceptibility to breast cancer. Oncotarget 8(47): 82796-82802.
- Chen Y, Xu C, Harirforoosh S, Luo X, Wang KS, et al. (2018) Analysis of PTPRK polymorphisms in association with risk and age at onset of Alzheimer’s disease, cancer risk, and cholesterol HHS Public Access. J Psychiatr Res 96: 65-72.
- Harrington C, Song Z, Zhang T, Han Y, Wang J, et al. Comparative Epidemiological Investigation of Alzheimer’s Disease and Colorectal Cancer: The Possible Role of Gastrointestinal Conditions in the Pathogenesis of AD. HYPOTHESIS AND THEORY 10: 176.
- Lim S, Yoo K, Kim H-S, Gilmore HL, Lee Y, et al. (2014) Amyloid-β precursor protein promotes cell proliferation and motility of advanced breast cancer.
- Staropoli JF (2008) Tumorigenesis and neurodegeneration: Two sides of the same coin? BioEssays 30(8): 719-727.
- Sun W, Vanhooke JL, Sondek J, Zhang Q (2011) High-throughput fluorescence polarization assay for the enzymatic activity of gtpase-activating protein of ADP-ribosylation factor (ARFGAP). J Biomol Screen 16(7): 717-723.
- Galvão F, Grokoski KC, da Silva BB, Lamers ML, Siqueira IR (2019) The amyloid precursor protein (APP) processing as a biological link between Alzheimer’s disease and cancer. Ageing Res Rev 49: 83-91.
- Feng Y-CA, Cho K, Lindstrom S, Kraft P, Cormack J, et al. (2017) Investigating the genetic relationship between Alzheimer’s disease and cancer using GWAS summary statistics HHS Public Access. Hum Genet 136(10): 1341-1351.
- Ward ST, Li KK, Hepburn E, Weston CJ, Curbishley SM, et al. (2015) The effects of CCR5 inhibition on regulatory T-cell recruitment to colorectal cancer. Br J Cancer 112(2): 319-328.
- Okereke OI, Meadows ME (2019) More Evidence of an Inverse Association Between Cancer and Alzheimer Disease. JAMA Netw Open 2(6): e196167.
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917): 860-867.
- Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140(6): 883-899.
- Pardoll DM (2002) Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol 2(4): 227-238.
- Gonzalez H, Hagerling C, Werb Z (2018) Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev 32(19–20): 1267-1284.
- Crowther MD, Dolton G, Legut M, Caillaud ME, Lloyd A, et al. (2020) Genome-wide CRISPR–Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat Immunol 21(2): 178-185.
- Olivier IS, Naidoo V (2018) Risk Factors and Pathogenesis of HIV-Associated Neurocognitive Disorder : The Role of Host Genetics. Int J Mol Sci 19(11):3594.
- Basova L, Najera JA, Bortell N, Wang D, Moya R, et al. (2018) Dopamine and its receptors play a role in the modulation of CCR5 expression in innate immune cells following exposure to Methamphetamine: Implications to HIV infection. PLoS One 13(6): 1-23.
- Abdolmohammadi R, Azar SS, Khosravi A, Shahbazi M (2016) CCR5 polymorphism as a protective factor for hepatocellular carcinoma in hepatitis B virus-infected Iranian patients. Asian Pacific J Cancer Prev 17(10): 4643-4646.
- Ganczak M, Skonieczna Zydecka K, Drozd-D, Abrowska M, Zyna Adler G (2017) Possible Impact of 190G > A CCR2 and ∆32 CCR5 Mutations on Decrease of the HBV Vaccine Immunogenicity-A Preliminary Report.
- Blackard JT, Kong L, Rouster SD, Karns R, Horn PS, et al. (2019) CCR5 receptor antagonism inhibits hepatitis C virus (HCV) replication in vitro. PLoS One 14(10): 1-11.
- Marsden V, Donaghy H, Bertram KM, Harman AN, Nasr N, et al. (2015) Herpes Simplex Virus Type 2–Infected Dendritic Cells Produce TNF-α, Which Enhances CCR5 Expression and Stimulates HIV Production from Adjacent Infected Cells. J Immunol 194(9): 4438-4445.
- Almanzar G, Kienle F, Schmalzing M, Maas A, Tony HP, et al. (2019) Tofacitinib modulates the VZV-specific CD4+ T cell immune response in vitro in lymphocytes of patients with rheumatoid arthritis. Rheumatol (United Kingdom) 58(11): 2051-2060.
- Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, et al. (2006) CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med 203(11): 2451-2460.
- Hirako IC, Ataide MA, Faustino L, Assis PA, Sorensen EW, et al. (2016) Splenic differentiation and emergence of CCR5+ CXCL9+ CXCL10+ monocyte-derived dendritic cells in the brain during cerebral malaria. Nat Commun 7: 13277.
- D’Adamo E, Cali AMG, Weiss R, Santoro N, Pierpont B, et al. (2010) Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 33(8): 1817-1822.
- Inayat H, Azim MK, Baloch AA (2019) Analysis of Inflammatory Gene Expression Profile of Peripheral Blood Leukocytes in Type 2 Diabetes. Immunol Invest 48(6): 618-631.
- Słomiński B, Ławrynowicz U, Myśliwska J, Ryba-Stanisławowska M, Skrzypkowska M, et al. (2017) CCR5-Δ32 gene polymorphism is associated with retinopathy in patients with type 1 diabetes. Mol Cell Endocrinol 439: 256-260.
- Carvalho-Pinto C, García MI, Gómez L, Ballesteros A, Zaballos A, et al. (2004) Leukocyte attraction through the CCR5 receptor controls progress from insulitis to diabetes in non-obese diabetic mice. Eur J Immunol 34(2): 548-557.
- Kalev I, Oselin K, Pärlist P, Zilmer M, Rajasalu T, et al. (2003) CC-chemokine receptor CCR5-del32 mutation as a modifying pathogenetic factor in type I diabetes. J Diabetes Complications 17(6): 387-391.
- Buhler MM, Craig M, Donaghue KC, Badhwar P, Willis J, et al. (2002) CCR5 genotyping in an Australian and New Zealand type 1 diabetes cohort. Autoimmunity 35(7): 457-461.
- Vassiliadis S, Balabanidou V, Papadopoulos GK, Athanassakis I (2002) Localization and expression of CCR3 and CCR5 by interleukin-1β in the RIN-5AH insulin-producing model system: A protective mechanism involving down-regulation of chemokine receptors. J Pancreas 3(3): 66-75.
- Muntinghe FLH, Gross S, Bakker SJL, Landman GWD, van der Harst P, et al. (2009) CCR5Δ32 genotype is associated with outcome in type 2 diabetes mellitus. Diabetes Res Clin Pract 86(2): 140-145.
- Hong YS, Chang Y, Ryu S, Cainzos-Achirica M, Kwon MJ, et al. (2017) Hepatitis B and C virus infection and diabetes mellitus: A cohort study. Sci Rep 7(1): 4606.
- Dworzanski J, Drop B, Kliszczewska E, StrycharzDudziak M, Polz-Dacewicz M (2019) Prevalence of Epstein-Barr virus, human papillomavirus, cytomegalovirus and herpes simplex virus type 1 in patients with diabetes mellitus type 2 in south-eastern Poland. PLoS One 14(9): e0222607.
- Heitner J, Dickson DW (1997) Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects: A retrospective postmortem immunocytochemical and histofluorescent study. Neurology 49(5): 1306-1311.
- Chornenkyy Y, Wang W-X, Wei A, Nelson PT (2019) Alzheimer’s disease and Type 2 Diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol 29(1): 3-17.
- Solloch U V, Lang K, Lange V, Böhme I, Schmidt AH (2017) Frequencies of gene variant CCR5- Δ 32 in 87 countries based on next- generation sequencing of 1 . 3 million individuals sampled from 3 national DKMS donor centers. Hum Immunol 78(11–12): 710–717.
- (2019) Global Health Data Exchange.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.