Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Ratio of Dialysate NGAL to Plasm NGAL (NGAL D/P) Can Reflect Peritoneal Transport in CAPD Patients
*Corresponding author: Huang wen, Department of nephrology, The second affiliated hospital of Wenzhou Medical University, No 109, xueyuan western Road, Wenzhou city, Zhejiang province, 325027, P. R. China.
Received: June 08, 2021; Published: June 21, 2021
DOI: 10.34297/AJBSR.2021.13.001860
Abstract
Objectives: To probe the role of peritoneal NGAL in reflecting peritoneum function.
Methods: 20 CAPD anuria patients without peritonitis in our peritoneal dialysis center were recruited from July 2016 to July 2018. These patients were divided into high-transport peritoneum group and non-high transport peritoneum group according to peritoneal transport type. Patients from both groups received the same dialysis solutions and dialysis modality. Peritoneal transport type, serum C-reactive protein, peritoneal equilibrium test results, dialysate NGAL, plasm NGAL, the ratio of dialysate NGAL to plasm NGAL (D/P NGAL) from both groups were recorded. Spearman’s correlation coefficient was employed to examine the relations between variables. Receiver operating characteristic (ROC) curve analysis was used to calculate the area under the curve (AUC) for ratio of dialysate NGAL to plasm NGAL (D/P NGAL) and peritoneal creatinine to serum creatinine (D/P creatinine).
Results: we had recruited 20 patients (11 male and 9 female, 7 high transport and 13 non-high transport group,). D/P NGAL had a correlation with D/P creatinine in CAPD patients (p < 0.05). The area under the ROC curve for D/P NGAL was 0.81.
Conclusion: The ratio of dialysate NGAL to plasm NGAL (D/P NGAL) can reflect peritoneal transport in CAPD patients.
Keywords: Dialysate NGAL, Plasm NGAL, Peritoneal Transport, CAPD, D/P NGAL
Abbreviations: PD: Peritoneal Dialysis; NGAL: Neutrophil gelatinase-associated lipocalin; CAPD: continuous ambulatory peritoneal dialysis; PDS: Peritoneal dialysis solution; D/P NGAL: Dialysate NGAL to plasm NGAL; D/Pcr: Peritoneal creatinine to serum creatinine; PET: Peritoneal equilibration test; ROC: Receiver operating characteristic; SD: Standard Deviation; AUC: Area under the curve; EMT: Epithelial-to-mesenchymal transition; pKt/V: Peritoneal Kt/V; TGF-ß: Transformation of growth factor; IL-1: interleukin-1; MMP: matrix metalloproteinase; TIMP-1: Tissue inhibitor of metalloproteinase 1; MAPK: Mitogen-Activated Protein Kinase; RAS: Renin-Angiotensin-System
Introduction
Peritoneal dialysis (PD) is a renal replacement therapy which is used by approximately 11% of the total global dialysis population [1]. Continuous exposure to glucose-based dialysis solutions may cause the loss of peritoneal membrane function [2]. Neutrophil gelatinase-associated lipocalin (NGAL) is a secretive glycoprotein which belongs to lipoprotein superfamily. It consists of 178 amino acids with a relative molecular weight of 25 KDa. The human NGAL gene is located in the long arm of the No. 9 chromosome (9q34) and contains 5’ non-transcription regions, 3’ non-transcription regions, seven exons and six introns. Plasm NGAL is known to be high in patients with CKD [3], and high plasm NGAL is positively correlated with peritoneal NGAL (or dialysate NGAL) in PD patients. Furthermore, peritoneal inflammation can also increase dialysate NGAL level in peritonitis patients due to overproduction by mesothelial cells and neutrocyte [4]. It is reported that neutrophil gelatinase-associated lipocalin in peritoneal dialysis could reflects status of peritoneum [5], as evidenced by an inverse correlation between dialysate NGAL and peritoneal ultrafiltration volume [3]. To rule out the impact of inflammation and residual renal function on dialysate NGAL, we recruited continuous ambulatory peritoneal dialysis (CAPD) patients with anuria without peritonitis to explore whether NGAL could reflect peritoneum function in continuous ambulatory peritoneal dialysis (CAPD) patients.
Introduction
Patients Selection
Cross-Sectional study. Recruited CAPD patients in our peritoneal dialysis center from July 2016 to July 2018.The design has been approved by the Ethics Committee of the Second affiliated hospital of Wenzhou Medical University. All patients gave their written informed consent before inclusion in the study. Informed consent was obtained from all participants. This study was performed in accordance with the Declaration of Helsinki. Inclusion criteria of this study: aged than 18 years, peritoneal dialysis time more than 90 days, urine volume less than 100 mL/day. Exclusion criteria: patients who had peritonitis or had other infections in the recent three months before recruitment. A total of 20 CAPD patients were screened, and finally all 20 patients were enrolled.
These patients were divided into two groups based on peritoneal equilibrium test results: high-transport peritoneum group and non-high transport peritoneum group. A 2-L lactate-based PDS (peritoneal dialysis solution) was used, at a concentration of 1.5% glucose in all patients provided by Baxter International Inc. (Wenzhou city, PR china). All patients were treated with 3 exchanges during the day time, with a short dwell (4 hours). The long dwell time was 8-12 hours during the nighttime. (Shown in regular figure). Compare the distributions of dialysate NGAL, plasm NGAL, D/P NGAL in those two groups.
Materials and Methods
Blood samples for plasm NGAL evaluation were collected in chilled vacutainer tubes containing potassium ethylenediaminetetraacetate, and the plasma was promptly analyzed. Plasm NGAL was assayed within 1 hour of sample collection using a Triage NGAL test on a Triage Meter Pro analyzer (Alere, Stockport, UK) on the first day of patients’ visit. Long-dwell effluents were collected for dialysate NGAL, WBC count and effluent ten milliliters of peritoneal liquid was centrifuged at 3,000 rpm for 8 minutes and stored at −80°C until assayed. Dialysate NGAL was evaluated in an overnight effluent collection using the Architect platform (Abbott Diagnostics, Abbott Park, IL, USA). The ratio of dialysate NGAL to plasm NGAL (D/P NGAL) was calculated. Plasm NGAL and dialysate NGAL levels were expressed as ng/mL.
A Standard peritoneal equilibration test (PET) was performed in all recruited patients using the PD Adequest software package according to the manufacturer’s instructions (Baxter Healthcare, McGaw Park, IL, USA) simultaneously. High-transport peritoneum was defined as the ratio of dialysate creatinine at four-hour dwell to plasm creatinine (D/Pcr) more than 0.80. Dialysis adequacy was evaluated in all subjects at the same time and peritoneal Kt/V (pKt/V) was measured. Collect the data of the adequacy of peritoneal dialysis and the peritoneal equilibrium test results. Demographic data including age, gender, body mass index, major comorbidities at the beginning of PD treatment (diabetes, etc.), biochemical data were also collected in both groups.
Statistical Analysis
Statistical analysis was carried out using SPSS software (Version 21.0). Data were presented as means ± SD for normally distributed values (at Kolmogorov-Smirnov test) and median (interquartile range) for non-normally distributed values. Compared the difference between those groups using two independent sample T test for continuous variables. Counting data was expressed in the number of examples or rate. Spearman’s correlation coefficient was searched to examine the relation between variables. Receiver operating characteristic (ROC) curve analysis was employed to calculate the area under the curve (AUC) for ratio of dialysate NGAL to plasm NGAL (D/P NGAL), also to find the best cutoff values capable of identifying the high transport peritoneum. The sensitivity and specificity of D/P NGAL were also calculated. All results were considered significant if p was <0.05.
Results
The clinical patients’ characteristics stratified according to function of peritoneum were reported in Table 1. During the study period, we evaluated 20 patients (7 in the high transport group and 13 in the non-high transport group, 11 male and 9 female). No significant differences were found between two groups in terms of age (p =0.31), gender (p =0.21), comorbidity such as diabetes (p =0.13), dialysis duration (p = 0.31). Significant differences were found between the 2 groups in terms of type of transporter (dialysate to plasma ratio for creatinine, p<0.05), and Kt/V (p<0.05), ultrafiltration volume (p<0.05), also dialysate to plasm ration for NGAL (p <0.05). Analysis of T test showed no significant between two groups in the distribution of peritoneal NGAL and plasm NGAL Showed in Table 1.
No relationship existed between plasm NGAL and D/P creatinine, plasm NGAL and D/D0 glucose (p=0.41, p=0.08 respectively shown in Figure 1a & 2a). D/P NGAL had a correlation with D/P creatinine in CAPD patients with Spearman’s R2 of 0.21 (p < 0.05) (Figure 3a). No relationship existed between dialysate NGAL and D/D0 glucose, dialysate NGAL and D/D0 glucose (p=0.07,p=0.10,shown in Figure 1b & 2b). Similarly, D/P NGAL was negatively correlated with D/D0 glucose in CAPD patients with Spearman’s R2 of 0.34 (p < 0.05, Figure 3b).
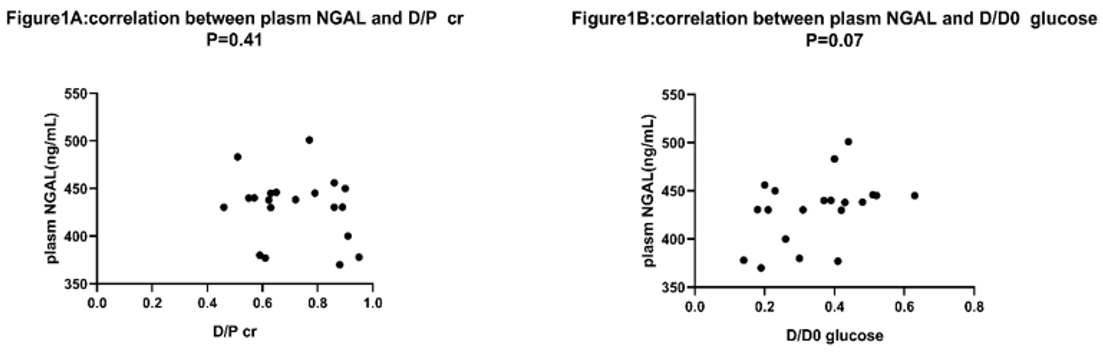
Figure 1: Correlation between plasm NGAL and D/Pcr, D/D0 glucose.
a: correlation between plasm NGAL and D/Pcr, N=20. Spearman’s rank correlation was used. D/Pcr=dialysate to plasma ratio for creatinine. p=0.41.
b: correlation between plasm NGAL and D/D0 glucose, N=20. No correlation found. D/D0 glucose=the ratio of dialysate glucose at 4 hours to
dialysate glucose at 0 hour during the PET test. P=0.10.
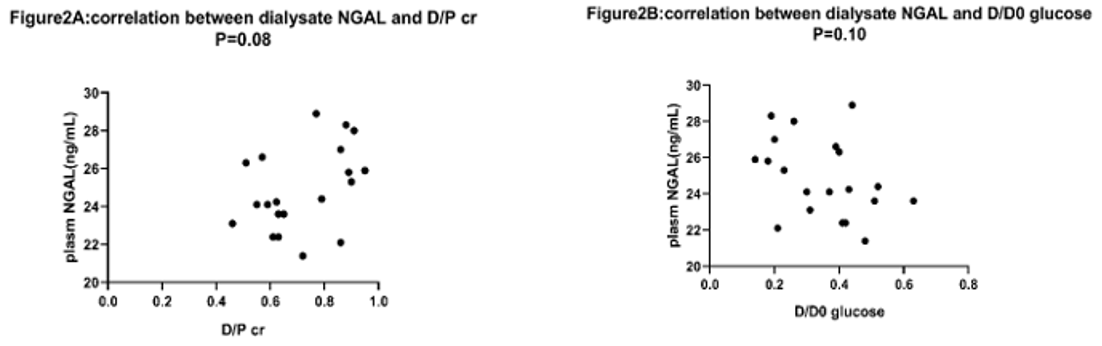
Figure 2: Correlation between dialysate NGAL and D/P cr, D/D0 glucose.
a: correlation between dialysate NGAL and D/P cr, N=20. p=0.08.
b: correlation between dialysate NGAL and D/D0 glucose, no correlation was found by using Spearman’s rank correlation. P=0.10.
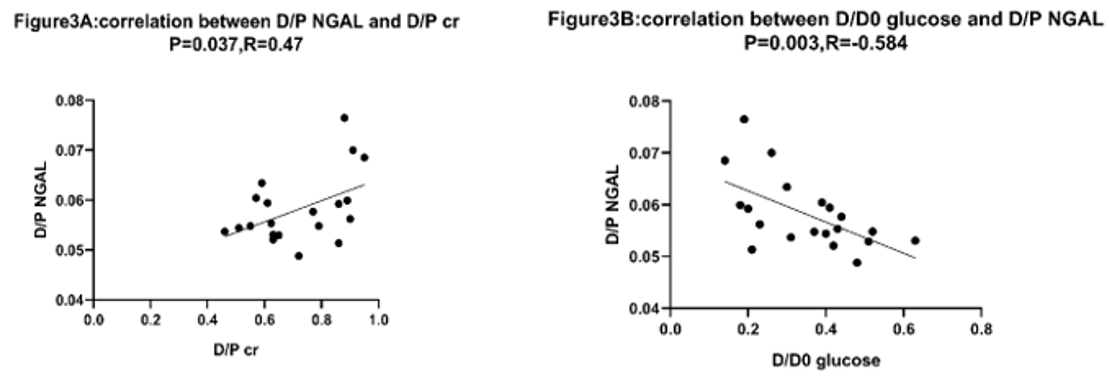
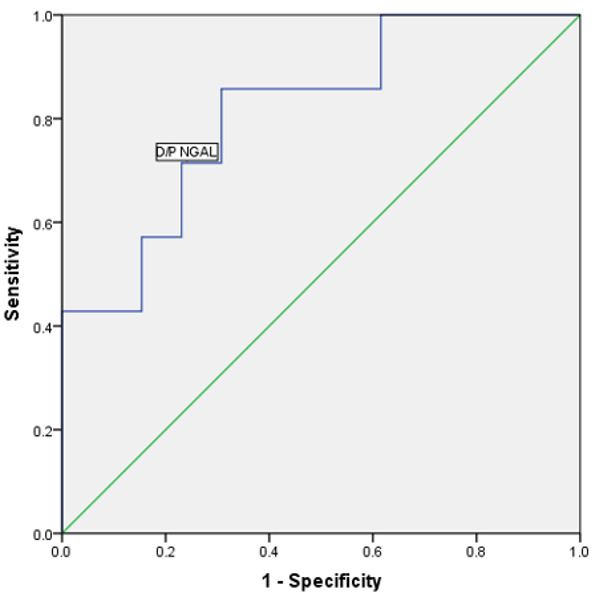
Figure 3: The area under the ROC curve for D/P NGAL was 0.81. When the cutoff value of D/P NGAL was set at 0.056, with sensitivity and
specificity were 85.7% and 70.0%, respectively. P<0.05.
a: Correlation between D/P NGAL and D/P cr was tested by using Spearman’s rank correlation. Positive correlation existed. N=20, p<0.05, R2=0.21.
b: Correlation between D/P NGAL and D/D0 glucose was tested by using Spearman’s rank correlation. Negative correlation existed. N=20, p<0.05,
R2=0.34.
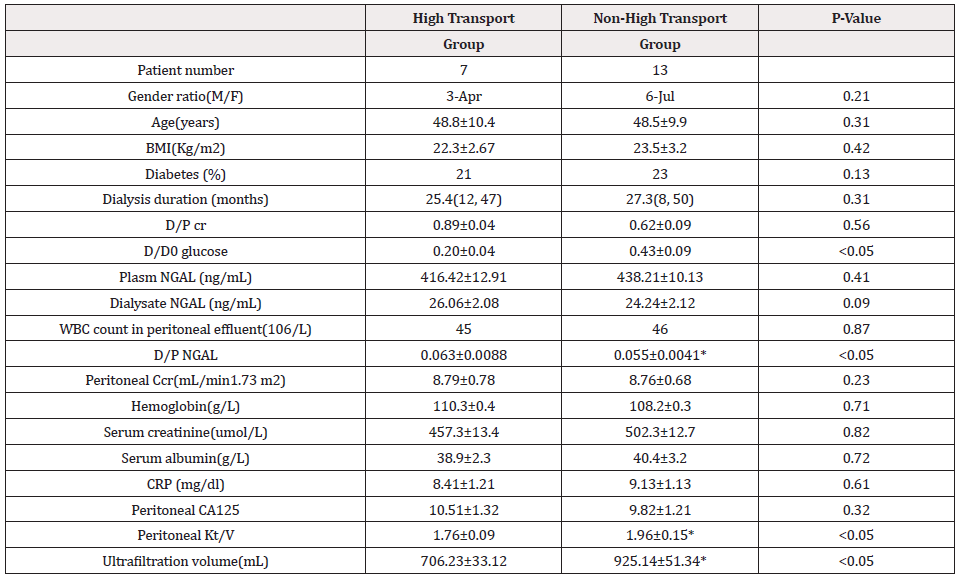
Table 1: Clinical patients’ characteristics in two groups stratified according to function of peritoneum.
BMI = body mass index; CRP = C-reactive protein; NGAL = neutrophil gelatinase-associated lipocalin; WBC = white blood cell. Carbohydrate antigen-125, dialysate to plasma ratio for creatinine, D/D0 glucose =the ratio of dialysate glucose in the end to dialysate glucose at the beginning of PET test, *significant different between two groups.
ROC curve analysis was performed to define the diagnostic profile of D/P NGAL in identifying high transport peritoneum. The area under the ROC curve for D/P NGAL was 0.81(p=0.02, 95% confidence interval [95% CI], 0.61-0.99). When the cutoff value of D/P NGAL was set at 0.56, with sensitivity and specificity were 85.7% and 70.0%, respectively (Figure 4).
Discussion
Chronic exposure to PD solutions is associated with impairment of peritoneal host defense. Alterations of cellular components within the peritoneal cavity led to disturbances in the release of pro-inflammatory and anti-inflammatory mediators [6]. Lowgrade, chronic inflammation and peritoneal injury progressively determine mesothelial cells loss and fibrosis. Expansion of peritoneal vasculature and augmented vessel permeability are important determinants of increased small solute transport across the peritoneal membrane and UF failure [7]. Chronic inflammation can increase the peritoneal NGAL [8]. Many other factors may also affect the peritoneal NGAL level, such as peritoneal mesothelial cell’s function, serum NGAL, residual renal function [3]. Mesothelial cells loss may decrease dialysate NGAL while chronic inflammation and high serum NGAL may increase dialysate NGAL [3]. To further exclude the influence of inflammation and residual renal function on dialysate NGAL, we ruled out CAPD patients with residual renal function and peritonitis.
To exclude the effects of different osmotic pressure on the function of peritoneum, all patients use the same dialysis solutions and dialysis modality, our study demonstrates that, in basal conditions, dialysate NGAL could not reflect peritoneal function. No relationship existed between plasm NGAL and D/P creatinine, dialysate NGAL and D/P creatinine (P=0.41, P=0.08 respectively). However, a positive correlation between D/P NGAL and D/P creatinine in CAPD patients were observed. This discrepancies can be explained by the influence of plasm NGAL on the level of dialysate NGAL. After adjustment of dialysate NGAL in relation to serum NGAL, the positive linear correlation between D/P NGAL and D/P creatinine was revealed. Negative correlation between D/P NGAL and D/D0 glucose in CAPD patients further supported our hypothesis.
NGAL has poor peritoneal diffusive capacity due to large molecular weight (25000 Da) [9]. However, for the first time, we found that the relationship between D/P NGAL and D/P creatinine, which means D/P NGAL can used as a precious marker for peritoneal function. To further explore the role of D/P NGAL in identifying the high transport peritoneum, receiver operating characteristic (ROC) curve analysis was employed. The area under the curve (AUC) for D/P NGAL was 0.81, sensitivity and specificity were 85.7% and 70.0% with the cutoff value of.056 for D/P NGAL, from which we come to the conclusions that D/P NGAL can be used as a reliable marker for high transport peritoneum, as well as D/Pcr and D/D0 glucose.
The relationship between dialysate NGAL and high peritoneal transport peritoneum is very complicated. Peritoneal NGAL may cause peritoneal fibrosis. Chronic exposure to PD solutions is associated with increased small solute transport across the peritoneal membrane and ultrafiltration failure [7]. Particularly, epithelial-to-mesenchymal transition (EMT) of mesothelial cells is often associated with high peritoneal transport [10]. In the state of peritonitis, the transformation of growth factor (TGF-ß), interleukin-1(IL-1) beta can promote reversible EMT [11,12]. Repeated peritonitis is the leading cause of EMT, which may lead to peritoneal fibrosis during CAPD, and finally leading to the termination of PD.
NGAL can impact the process of EMT by regulation of balance between matrix metalloproteinase 9(MMP-9) and tissue inhibitor of metalloproteinase 1 (TIMP-1) [13]. MMP-9, one of the matrix metalloproteinases family, is regulated by NGAL [14]. NGAL can bind to pro-MMP-9 and TIMP-1 to form a ternary complex, blocking the inhibitory efforts of TIMP-1 on MMP-9, thus protect MMP-9 from degradation. NGAL can antagonize the inhibitory effect of TGFbeta on E-cadherin through RAS/Mitogen-Activated Protein Kinase (MAPK) pathway. By inhibiting the RAS/Mitogen-Activated Protein Kinase (MAPK) pathway, NGAL can reverse the phosphorylation and degradation of E-cadherin induced by RASS, which leads to the increased expression of E-cadherin [15]. However, we did not probe the underlying mechanism in our study due to limited time.
This study certainly had a few limitations. Patient number was slightly limited. This was a single-center Cross-Sectional study with poor generalizability of this study as all the patients were from southern China. To resolve these limitations, a prospective, largescale, multicenter randomized trial is necessary. Also, we did not probe the underlying mechanism behind the correlation between D/P NGAL and D/P creatinine in CAPD patients. Further basal research is needed in future.
Acknowledgments
Thanks to Ms. Chen haiyan, Ms. Xu anqi for their assistance in data collection. Informed consent: Written informed consent was obtained from all patients in study. The study was approved by the Ethics Committee of the Second affiliated hospital of Wenzhou Medical University, February 2015.
Author Contributions
The first author, Shi Zhen, contributed data collecting, paper writing to the paper. The second author, Zhao yanling, contributed paper writing and statistical work to the paper. Corresponding author and the third author, Huang wen, designed the research, also collected data, and revised the paper.
Additional Information
Conflict of Interest Statement
We declare that we have no conflicts of interest. All authors made a substantial contribution to the information or material submitted for publication. All read and approved the final manuscript.
Consent For Publication
Written informed consent was obtained from all subjects before the study. See the file of patients’ informed consent form.
Availability of Data and Materials
See the file named date and materials.
Ethical Considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors. Written informed consent was obtained from all patients in study. The study was approved by the Ethics Committee of the Second affiliated hospital of Wenzhou Medical University, February 2015.
Funding Sources
Supported by foundation of Wenzhou science and technology Bureau, Y2020971.
References
- Grassmann A, Gioberge S, Moeller S, Brown G (2005) ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant 12(12): 2587-2593.
- Topley N, Coles GA, Williams JD (1994) Biocompatibility studies on peritoneal cells. Perit Dial Int 14(Suppl 3): S21-S28.
- Francesca Martino, Ilenia Filippi, Davide Giavarina, Gianpaolo Amici, Massimo de Cal, et al. (2013) Neutrophil gelatinase-associated lipocalin in peritoneal effluent: evalution in peritoneal dialysis patients in basal condition. Peritoneal Dialysis International 33(4): 379-381.
- Leung JC, Lam MF, Tang SC, Loretta Y Y Chan, K Y Tam, et al. (2009) Roles of neutrophil gelatinase-associated lipocalin in continuous ambulatory peritoneal dialysis-related peritonitis. J Clin Immunol 29(3): 365-378.
- Lacquaniti, Valeria Chirico, Stefania Mondello, Antoine Buemi, Rosaria Lupica, et al. (2013) Neutrophil gelatinase-associated lipocalin in peritoneal dialysis reflects status of peritoneum. Società Italiana di Nefrologia 26(6): 1151-1159.
- Buemi M, Aloisi C, Cutroneo G, Nostro L, Favaloro A, et al. (2004) Flowing time on the peritoneal membrane. Nephrol Dial Transplant 19(1): 26-29.
- Margetts PJ, Bonniaud P (2003) Basic mechanisms and clinical implications of peritoneal fibrosis. Perit Dial Int 23(6): 530-541.
- Antonio Lacquaniti, Valeria Chirico, Stefania Mondello, Antoine Buemi, Rosaria Lupica, et al. (2013) Neutrophil gelatinase-associated lipocalin in peritoneal dialysis reflects status of peritoneum 26(6): 1151-1159.
- Kabanda A, Goffin E, Bernard A, Lauwerys R, Van Ypersele de Strihou C, et al. (1995) Factors influencing serum levels and peritoneal clearances of low molecular weight proteins in continuous ambulatory peritoneal dialysis. Kidney Int 48(6):1946-1952.
- G del Peso, JA Jiménez-Heffernan, M A Bajo, L S Aroeira, A Aguilera, et al. (2008) Epithelial-to-mesenchymal transition of mesothelial cells is an early event during peritoneal dialysis and is associated with high peritoneal transport. Kidney International (108): S26-S33.
- Zhu Y, Liu Y, Qian Y, Xiaojun Dai, Ling Yang, et al. (1014) Research on the efficacy of Celastrus Orbiculatus in suppressing TGF-β1 induced epithelial-mesenchymal transition by inhibiting HSP27 and TNFαinduced NF-κB/Snail signaling pathway in human gastric adenocarcinoma. BMC Complement Altern Med 14: 433.
- Willis BC, Borok Z (2007) TGF-beta induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 293(3): L525-L534.
- Wereszczynska-Siemiatkowska U, Siemiatkowski A, Swidnicka-Siergiejko A (2015) The imbalance between matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 in acute pancreatitis. Z Gastroenterol 53(3): 199-204.
- Ruiz Morales JM, Dorantes Heredia R, Arrieta O (2015) Neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinase-9 (MMP-9) prognostic value in lung adenocarcinoma. Tumour Biol 36(5): 3601-3610.17.
- Hanai J, Mammoto T, Seth P (2005) Lipocalin diminishes invasiveness and metastasis of Ras-transformed cells. J Biol Chem 280(14): 13641 -13647.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.