Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Glutaminate as the Central Transmitter in the Pathology of Diseases
*Corresponding author: Bogdan Feliks Kania, Hugon Kołłątaj Agricultural University, Poland.
Received: October 23, 2021; Published: November 12, 2021
DOI: 10.34297/AJBSR.2021.14.002035
Abstract
This article will refer to the role of glutamic acid (glutamate, Glu) as a transmitter in a pathology of diseases. What kind of receptors are involved in this process, what is the path of transmitting pain? in the further part, there are described such disorders as: neuropathic pain, epilepsy, amyotrophic lateral sclerosis, mood disorders and the role that glutamate plays in them.
Keywords: Glutamate; Metabotropic and Ionotropic Glutamatergic Receptors; Neuropathic Pain; Epilepsy; Amyotrophic Lateral Sclerosis; Mood Disorders
Introduction
Glutamic acid (Glu) five-carbonic, dicarboxylic acid,
endogenous amino acid of an acidic nature. Under physiological
conditions, it occurs mainly in the form of an anion-glutamate,
therefore both terms can be used interchangeably [1]. It is the
major neurotransmitter that has an excitatory effect on the central
nervous system (CNS) and autonomic nervous system (AUN).
Additionally, and importantly, it forms the most extensive nervous
conduction system, as it is used by as much as 35% to 40% of nervous
synapses [2]. When its concentration is balanced with the amount
of pre and postsynaptic inhibitory γ-aminobutyric acid (GABA), it
ensures homeostasis and proper functioning of the nervous system
[3]. The proper action of Glu is related to the following two types of
receptors specific for it, with which it stereospecifically binds [4].
The actions related to the activity of glutamic acid are related to
what are differently mentioned two types of receptors, using the
stereospecific technique [5]:
a) Ionotropic (iGluR): 3 types of glutamatergic (Glu-ergic)
ionotropic receptors (iGluRs) have been classified:
kainate receptor (KAR), α-amino-3-hydroxy-5-methyl-4-
isoxazole propionic acid (AMPAR) and N-methyl-D-aspartic acid
receptor (NMDAR) the most important, which is found in the
highest concentration in the hippocampus. Through AMPAR and
KARs, Glu stimulates the CNS intensively and quickly. In contrast,
NMDARs serve a slow excitatory response that plays an important
role in long-term synaptic potentiation (LTP) and neuroplasticity
[1,5].
b) Metabotropic (mGluR) -After stimulation, mGluRs react
slower than ionotropic receptors (tens of seconds) and are bound
to G proteins. Metabotropic receptors are divided into 3 subgroups:
1. group I (mGluR1 and mGluR5) - Occurs with
triphosphoinositol (IP3) and Ca2+ ions
2. group II (mGluR2 and mGluR3)- Inhibits the activity of
adenylate cyclase (AC), reducing the intracellular concentration of
cAMP
3. group III (mGluR4, mGluR6, mGluR7 and mGluR8)-
Inhibits the activity of adenylate cyclase, but has different
preferences in relation to agonists than group II [4]. Knowing the
basic information on the structure of Glu and its receptors, it is
also necessary to mention its basal actions on animal organisms.
Glu provides homeostasis in the CNS by interacting with the inhibitory neurotransmitter GABA. Glu, by acting on NMDARs in
GABA-containing neurons, increases the synthesis and release of
GABA. This amino acid inhibits the activity of the remaining Gluergic
nerve cells or other neurons [1,5]. Glu, as mentioned above,
is a very important excitatory neurotransmitter in the CNS and
AUN. It forms a permanent connection with mGluRs and iGluRs,
through to which visual, sensory and auditory information reaches
from the periphery to the cortex of the brain via Glu-ergic pathways
[6]. Glutamic acid plays the most important role in learning and
memory. This ability is dependent on the effect of Glu on synaptic
plasticity through Glu binding receptors, which induce LTP.
Recently, more and more studies focus on Glu and its receptors in
various diseases such as neuropathic pain, epilepsy, amyotrophic
lateral sclerosis, affective diseases, depression, intestinal pain,
stress, neurodegenerative diseases, autism, etc. [5, 7, 8].
Glutamate and Neuropatic Pain
Depending on the type of pathomechanism, there are several
types of pain [8-10]:
a) Acute and chronic pain
b) Receptor-nociceptive-somatic, visceral
c) Non-receptor type, also called neuropathic pain, which is
chronic pain.
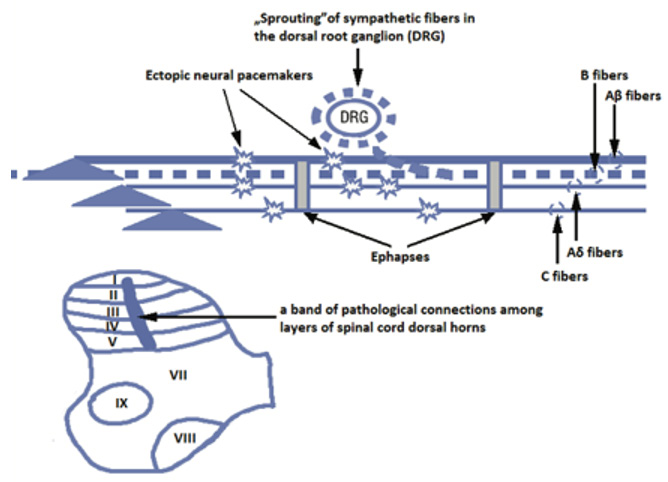
Neuropathic pain is caused by damage to the CNS or AUN, which may be caused by metabolic disorders, infections or mechanical damage [8]. This type of pain is relatively little understood. The exact mechanism, the receptors that are most often involved, is being researched constantly to find the best treatment for patients. Neuropathic pain is paroxysmal, violent. There may be sensory disturbances, such as: paraesthesia, dysaesthesia, hyperalgesia, hyperpathy, allodynia and vegetative disorders [7-10]. It is very important that neuropathic pain causes not only functional but also morphological disorders, thus leading to the so-called neuroplastic changes. At the cellular level, there is a sensitization of neurons in the posterior horns of the spinal cord, which leads to a reduction in the pain threshold, increased response to less intense stimuli and increased spontaneous activity of neurons, which leads to the death of neurons in the posterior horns of the spinal cord [8,11]. Neuropathic pain significantly changes the number of neurons. Microglia and astrocytes proliferate and neurons break down [12].
Due to the relatively poorly understood mechanisms responsible for neuropathic pain, the methods of treatment in this area are still very limited. However, more and more research is emerging on the role of Glu and its receptors (especially the mGluR5) in the pathology of non-receptor pain [7,8]. As mentioned above, Glu-ergic junctions are formed in the spinal cord dorsal horn (SCDH). It is also in this part of the core that mGluR5 metabotropic receptors are intensively expressed. SCDH plays a very important role in the plasticity of the brain, which is mediated indirectly by Glu and pain processes [7]. It is known that in animals, the physiological response to stimulation of mGluR5 receptors in the spinal cord is a pain response. On the other hand, the blockade of the same receptors causes, among others analgesia. Additionally, an increase in Glu concentration is found during pain behavior in animals [7]. Due to the relatively poorly understood mechanisms responsible for neuropathic pain, the methods of treatment in this area are still very limited. However, more and more research is emerging on the role of Glu and its receptors (especially the metabotropic mGluR5 receptor) in the pathology of non-receptor pain. As mentioned above, Glu-ergic junctions are formed in the dorsal horns of the spinal cord (SCDH). It is also in this part of the core that mGluR5 metabotropic receptors are intensively expressed. SCDH plays a very important role in the plasticity of the brain, which is mediated indirectly by Glu and pain processes [13]. It is known that in animals, the physiological response to stimulation of mGluR5 receptors in the spinal cord is a pain response. On the other hand, the blockade of the same receptors causes, among others analgesia. Additionally, an increase in Glu concentration is found during pain behavior in animals [8]. Despite the knowledge of the specific role of mGluR5 in neuropathic pain, the exact course of this process is still unknown, be it via surface receptors or via intracellular pathways. Nevertheless, on the basis of the research performed, the following fact can be stated blocking mGluR5 receptors in the spinal cord inhibits neuropathic pain reactions. In contrast, blockade of surface mGluR5 shows little effect. Diminishing the intracellular Glu concentration by blocking its transporter EAAT-3 mimics the actions of the intracellular antagonist mGluR5. This indicates a direct relationship between the intracellular GPCR and the expression of in vivo behavior [7, 11]. The studies performed in this subject lead to the conclusion that it is intracellular mGluR5 that participate in the pathophysiology of neuropathic pain (due to the significantly increased concentration of nuclear mGluR5 in the neurons of the dorsal spinal horns in animals suffering from neuropathic pain) [8]. This holds the promise for a targeted therapy to block mGluR receptors to relieve neuropathic pain.
Glutamate and Epilepsy
The term epileptogenesis is associated with the development of symptomatic (acquired) epilepsy due to the formation of structural and biochemical changes in the brain. The period of epileptogenesis development begins with the occurrence of an injury causing brain damage and ends with the appearance of the first spontaneous epileptic seizures [14]. Taken together, substantial evidence shows that glutamate plays a pivotal role in normal neuronal signaling. Moreover, excess glutamate release associated with recurrent seizures and observed in chronic epilepsy leads to long-term alterations in normal neuronal signaling and network connectivity [15]. Again, it should be emphasized that the Glu-ergic system is the most important stimulant system, acting in all nervous systems of the body and leading to its plasticity [14]. It is assumed that disorders in its area lead to epilepsy. Moreover, current theories indicate that epilepsy is caused mainly by influencing iGluR receptors. mGluR receptors also play a role, but it is smaller than in the case of NMDA, AMPA or kainate receptors [16].
a) NMDA Receptors: Play a key role in the process of
epileptogenesis. There is an increase in the sensitivity and
density of these receptors in specific areas of the brain,
especially in the hippocampus, where their number increased
by up to 100% in epileptic patients [17].
b) AMPA Receptors: Are composed of four subunits with
different combinations of homologous GluR1-4 (or GluRA-D)
peptides. In animal models of epilepsy, it has been shown that
during the epileptogenesis process, AMPARs with a deficiency
of the GluR2 subunit appear, which leads to an increase in the
permeability of these receptors for Ca2+ ions. This process is
neurotoxic and leads to apoptosis, i.e. programmed cell death
(in this case of nervous cells) [18].
c) mGluR Receptors: It has been shown that changes in the
activity of mGluR1 receptors may play a special role in
epileptogenesis, the activation of which prolongs the duration
of the epilepsy and seizure potentials in the hippocampal
region. The mechanism is very simple here, because
stimulation of mGluR receptors leads to the release of Glu,
which stimulates nervous cells (neurons) [14].
d) It is assumed that, especially in the initial stage of epilepsy, the
synthesis of mGluR group I receptors is intensified and then
decreased during the course of the disease. This can lead to
an imbalance between neurotransmitters and the advantage
of excitatory over inhibitory transmitters [19]. In patients
with drug resistant partial epilepsy, an increase in mGluR5
expression and density has been shown on dendrites and
neuronal bodies in large parts of the hippocampus. This leads
to theories that it is the change in the expression of these
receptors that can lead to persistent hyperactivity in this area
of the brain in people suffering from epilepsy.
Glutamate and Amyotrophic Lateral Sclerosis
Excessive stimulation of glutamate receptors causes excitotoxicity, a phenomenon implicated in both acute and chronic neurodegenerative diseases [e.g., ischemia, Huntington›s disease, and amyotrophic lateral sclerosis (ALS)]. Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease in which motor neurons are damaged. The pathogenesis of ALS is probably multifactorial, complex and not fully understood. It may involve such mechanisms as: oxidative stress, Glu acid toxicity, mitochondrial dysfunction, endoplasmic reticulum stress, protein aggregation, cytoskeleton dysfunction, axonal transport disorders, glial cell involvement, neuroinflammation, lactic acid dyscrasias or genetic factors [16,20- 23]. In ALS, the balance between the processes of producing and removing excess Glu is disturbed [20]. Due to too high a concentration of Glu, an excessive release of Ca2+ occurs, which - as mentioned above - in excess causes the development of apoptosis of neurons. In the course of examinations of patients with ALS, an increased concentration of Glu acid and a reduction in the concentration of the Glu transporter have been demonstrated in an animal experimental model [21-23]. In other cases, a reduction in the concentration of Glu has been observed in many regions of the CNS in the tissues of people who died from ALS. In studies carried out in a transgenic mouse model, altered expression of the Glu AMPAR subunit, decreased expression and activity of the Glu EAAT2 transporter, and disturbed regulation of GluRs in astrocytes were found. It has been suggested that the permeability of AMPARs to Ca2+ ions in the anterior horns of the spinal cord may be impaired due to abnormalities within the GluR2 subunit of the Glu AMPAR [21,23].
Glutamate and Affective Disorders
Affective disorders are a group of endogenous disorders in which mood, emotion and activity disorders occur periodically. These disorders can manifest themselves in the presence of depressive, hypomanic and manic syndromes as well as mixed states. Again, this is a group of diseases whose exact mechanism, cause, is still under careful research. Due to the large influence of the Glu-ergic system on the nervous system, answers are sought in this area [24]. The results of some of the experiments carried out are presented below. Based on biochemical studies on an animal model of depression (learned helplessness), a reduction in the activity of both the excitatory amino acid transporter and the vesicular Glu transporter was demonstrated [25]. The obtained results may indicate that in this model of depression there may be an increased concentration of Glu [26]. In patients with affective disorders, a significant increase in the concentration of Glu in body fluids and tissues has been found. A positive correlation was also found between the increased concentration of Glu in the blood serum and in the cerebrospinal fluid in people showing symptoms of depression. Moreover, postmortem studies have shown increased levels of Glu in the prefrontal cortex in patients with both unipolar and bipolar affective disease [16]. Another type of research carried out was genetic-molecular research. Six genes were found that are associated with affective disorders. This group includes two genes, GRM3 and GRM4, related to the mGluR receptor subunits of the Gluergic system [24].
Conclusion
Research on the role of Glu in the nervous system and its diseases is carried out very intensively. The functions of this transmitter in living organisms are so extensive and diverse that subsequent studies provide more and more recent information that allows a better understanding of the mechanisms and dependencies that occur. It also gives high hopes for more effective therapies for diseases that have been problematic in practice so far.
References
- Kwas L-glutaminowy (Glutaminian). Neuro Expert Encyclopedia Neurofizjologii.
- Treede RD, Jensen TS, Campbell JN, G Cruccu, JO Dostrovsky, etal. (2008) Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 70(18): 1630-1635.
- Kania BF, Bracha U (2020) The Neuropathic Pain in Animals. Arch Vet Animal Sci 2(2).
- Wierońska JM, Cieślik P (2017) Glutamate and its receptors or how the brain can be healed. The Universe 118: 178-189.
- Kania BF (2020) Glutamate as a neural factor for stressoric disorders. Warsaw Univ Life Sci p. 54-48.
- Żylicz Z, Krajnik M (2003) How does pain arise? The mechanism of pain. Pain neurophysiology for beginners. Polish Palliative Medicine 2(1): 49-56.
- Kania BF, Bracha U, Lonc G, Wojnar T (2020) The role of metabotropic glutamate antagonists in experimental neuropathic pain in animals. Medycyna Weter 76(10): 564-571.
- Wordliczek J, Zajączkowska R (2014) Neuropathic pain-pathomechanism. Palliative Medicine in Practice 8: 61-65.
- Vincent K, Cornea VM, Jong YJI, Laferriere A, Kumar N, et al. (2016) Intracellular mGluR5 plays a critical role in neuropathic pain Nat Commun 7: 10604.
- Wordliczek J, Zajączkowska R, Dobrogowski J (2011) Farmakologiczne leczenie bólu neuropatycznego. Polski Przegląd Neurol 7: 39-48.
- Xua Q, Yaksh TL (2011) A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr. Opin. Anaesthesiol 24(4): 400-407.
- Zhou Q, Wang J, Zhang X, Zeng L, Wang L, et al. (2013) Effect of metabotropic glutamate 5 receptor antagonists on morphine efficacy and tolerance in rats with neuropathic pain. Eur. J. Pharmacol 718(1): 17-23.
- Zhuo M, Wu G, Wu LJ (2011) Neuronal and microglial mechanisms of neuropathic pain. Mol Brain 4: 31-34.
- Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, et al. (2005) The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacol 179: 207-217.
- Kazula A, Kazula E (2014) Mechanisms of epileptogenesis and potential new directions of epilepsy therapy. Pharmacy Poland 70 (9): 473-485.
- Idil Cavus, Jullie W Pan, Hoby P Hetherington, Walid Abi-Saab, Hitten P Zaveri, et al. (2008) Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsja 49(8): 1358-1366.
- Chang-Hoon Cho CH (2013) New mechanism for glutamate hypothesis in epilepsy. Front Cel Nejurosci 7: 127.
- Chapman AG (2008) Glutamate and epilepsy. J Nutrition 130: 1043-1045.
- Alcoreza OB, Patel DC, P Tewari BH, Sontheimer H (2021) Dysregulation of ambient glutamate and glutamate receptors in epilepsy: An astrocytic perspective. Front. Neurol 12: 652159
- Iłżecka J (2012) Pathogenetic mechanisms of amyotrophic lateral sclerosis. Pathogenetic mechanisms of amyotrophic lateral sclerosis. Current Neurol 12(4): 222-235.
- Corona JC, Luis B Tovar y Romo LB, Ricardo Tapia R (2007) Glutamate excitotoxicity and therapeutic targets for amyotrophic lateral sclerosis. Expeert Opinion on Therap 11(11): 1415-1428.
- Foran E, Davide Trotti D (2009) Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid. Redox Signal 11(7): 1587-1602.
- Shu S, Zhong-Wei Zhang ZW, Zi Lin Li (2017) Cell death-autophagy loop and glutamate-glutamine cycle in amyotrophic lateral sclerosis Front Mol Neurosci 10: 233.
- Pradhan J, Bellingham MC (2021) Neurophysiological mechanisms underlying cortical hyper-excitability in amyotrophic lateral sclerosis: A review. Brain Sci 11(45): 549-559.
- Permoda Osip A, Rybakowski J (2011) The glutamatergic concept of . Polish Psychiatry, XLV 45(6): 875-888.
- Hashimoto K, Malchow B, Peter Falkai P, Andrea Schmitt A (2013) Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. European Archives of Psychiatry and Clinical Neuroscience 263(5): 367-377.
- Jun C, Choi Y, Lim SM, Baye S, Hong YS, et al. (2014) Disturbance of the Glutamatergic System in Mood Disorders. Exp Neurobiol 23(1): 28-35.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.