Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Hepatic GIST Tumor with Cystic Morphology: A Case Report and Literature Review
*Corresponding author: Bing Ren MD, PhD, Department of Pathology and Laboratory Medicine, Dartmouth-Hitchcock Medical Center, One Medical Center Drive Lebanon, NH, USA.
Received: October 23, 2021; Published: October 27, 2021
DOI: 10.34297/AJBSR.2021.14.002019
Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal-origin tumors of the gastrointestinal tract. They arise in the interstitial cells of Cajal, the cells that generate peristaltic motions. GISTs can demonstrate a variety of genetic mutations including KIT and PDGFRα mutations. They are most frequently located in the stomach (60-70%), small intestine (20-25%), colon and rectum (5%), and esophagus (<5%). Nearly half of all patients diagnosed with GIST will experience recurrence or metastasis-most commonly to the liver or peritoneum. Primary and metastatic GISTs typically present as solid masses. Cases of metastatic and primary liver GIST with cystic morphology are exceedingly rare. Here we present a case of presumed metastatic liver GIST in a patient with a large, symptomatic, cystic liver mass and review other published cases of hepatic GIST with cystic morphology.
Keywords: Gastrointestinal stromal tumor, Extraintestinal gastrointestinal, Stromal tumor, Cystic morphology
Introduction
Gastrointestinal stromal tumors (GISTs) account for roughly 3% of tumors of the gastrointestinal tract [1]. They arise in the interstitial cells of Cajal- the pacemaker cells of the digestive system [2]. GISTs are classified into three categories (spindle, epithelioid, or mixed spindle/epithelioid) based on histological cell type. Immunohistochemical markers including CD34, CD117, DOG1 and genetic analysis for KIT and PDGFRα mutations can assist the diagnosis of GIST [3]. The most common anatomical locations for GIST are the stomach (60-70%) and small intestine (20-25%), followed by colon and rectum (5%), and esophagus (<5%) [4]. A small percentage of GISTs (<5%)5 occur outside of the gastrointestinal tract and are referred to as extraintestinal stromal tumors (EGIST) [5]. Treatment recommendations for GIST and EGIST include surgical resection, which is curative in most cases of localized disease [6]. Risk of recurrence and metastasis must be determined in patients diagnosed with GIST as up to 50% of patients will experience recurrence or metastasis after resection, irrespective of margin status [6]. Risk category is assigned using prognostic criteria such as those provided by the Memorial Sloan Kettering Cancer Center Nomogram, the NIH-Fletcher consensus, or AFIPMiettinen criteria [7]. The scheme includes anatomic sites, tumor size and mitotic activity. In patients with high risk for metastatic disease or recurrence and in those with unresectable masses, treatment with a tyrosine kinase inhibitor (TKI) is indicated [8,9]. Imatinib mesylate is the first-line therapeutic agent in these cases. Treatment with imatinib often induces cystic changes in GISTs [10- 12] Prior to imatinib therapy, GIST typically demonstrates a solid or mixed solid/cystic morphology [4]. Purely cystic morphology is rarely seen in GIST masses prior to treatment with imatinib and few cases of cystic GIST prior to treatment have been reported in the literature [4]. Moreover, reported cases of EGIST of the liver with cystic morphology prior to imatinib therapy are even sparser. Here we present the largest reported case of cystic EGIST of the liver without imatinib therapy and review other such cases that have been reported in the literature.
Case Report
In 2015, a 64-year-old female presented to an outside hospital with a solid 6.0 x 4.0 x 3.8 cm small bowel tumor. The patient underwent partial small bowel resection, and the specimen was submitted to pathology for diagnosis. Histologically, the mass demonstrated a mixed spindled/epithelioid morphology. Immunohistochemical analysis revealed tumor cells with positive CD117, DOG1, and Caldesmon staining. A diagnosis of GIST was rendered. The histologic grade was low with a mitotic rate of <5/5 mm2. The margins of the resection specimen were negative for tumor. Risk of progression was reported as moderate (approximately 24%) based on mitotic count, size and location. The patient received no further treatment at that time.
In 2020, the patient presented to our hospital with a six-month history of abdominal pain and distention. Computed tomography scan revealed a cystic hepatic mass measuring 23 x 20 x 14 cm with an irregular, thickened wall and septations replacing nearly the entire right lobe of the liver. A fine needle aspiration was performed and resulted in nondiagnostic findings with blood, macrophages, and leukocytes., Intraoperatively, 1.5 L of blood-like fluid was drained from the mass and a 3.4 x 2.4 x 0.9 cm portion the cystic wall was excised and submitted for pathologic diagnosis. Histologic exam revealed the cystic wall consisting of a sheet of epithelioid type tumor cells with small round nucleus and moderate eosinophilic cytoplasm, with fibrosis and fibrin deposition along the inner surface. Immunohistochemical staining showed the tumor cells were diffusely positive for CD117 and DOG1, and negative for carcinoma and other tumor type markers including pancytokeratin AE1/3, CKMNF116, CK5D3, HMB45, S100, SOX10, MITF, Arginase 1, PAX 8, Sooth muscle actin, Calponin, Caldesmin, Calretinin, ERG 1/2/3, and CD34. The immunostaining results confirmed the diagnosis of GIST. The histologic grade was high with a mitotic rate of 18/5 mm2. In contrast to the patient’s small intestine mass, this specimen displayed negative staining for Caldesmon. Immunohistochemical analysis of DNA mismatch repair proteins revealed intact nuclear staining for MLH1, MSH2, MSH6, and PMS2. The tumor was submitted for next generation sequencing with a 170 gene panel Significant gene sequence alterations were detected in KIT (p. V559A) and MSH3 (p. T363Nfs*11) (Figure 1).
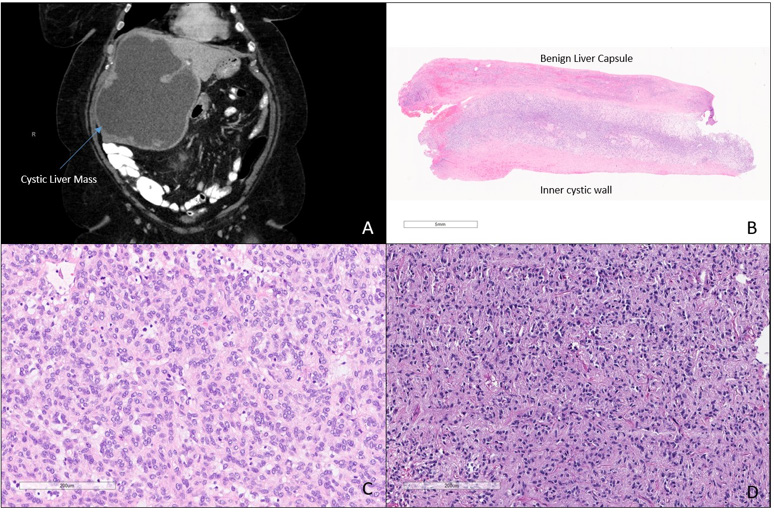
Figure 1. Radiological and Microscopic Characteristics of the Small Bowel and Liver Masses
A. CT of the abdomen and pelvis demonstrates a cystic mass replacing nearly the entire right hepatic lobe B. Microscopic exam of the wall of the cystic liver mass demonstrates a sheet of epithelioid tumor cells underlying benign liver capsule, the inner wall of the mass displays fibrosis and fibrin deposition. Microscopically, the liver tumor demonstrates epithelioid GIST cells. D. Microscopically, the tumor of the small bowel demonstrates mixed epithelioid and spindled GIST cells.
After the excision and drainage of the cystic liver mass, the patient initiated imatinib mesylate treatment. At follow-up seven months later, computed tomography imaging demonstrated a significant decrease in the size of the mass which measured 9.7 x 9.1 x 5.7 cm at that time. This case is unique in that it is the largest reported cystic liver mass demonstrating GIST cells with epithelioid features and a high mitotic rate. Additionally, this cystic liver GIST was presumed to be a metastasis from the patient’s small bowel GIST which had been resected five years prior. This presumption was based on the propensity of GIST to metastasize to the liver. However, the possibility that the liver mass represents a new primary GIST cannot be completely ruled out. Differences in tumor cell type, gross morphological type, and mitotic count, discordant caldesmon staining, as well as the size and unilocularity of the liver mass raise suspicion that this may indeed represent a primary cystic GIST of the liver.
Discussion
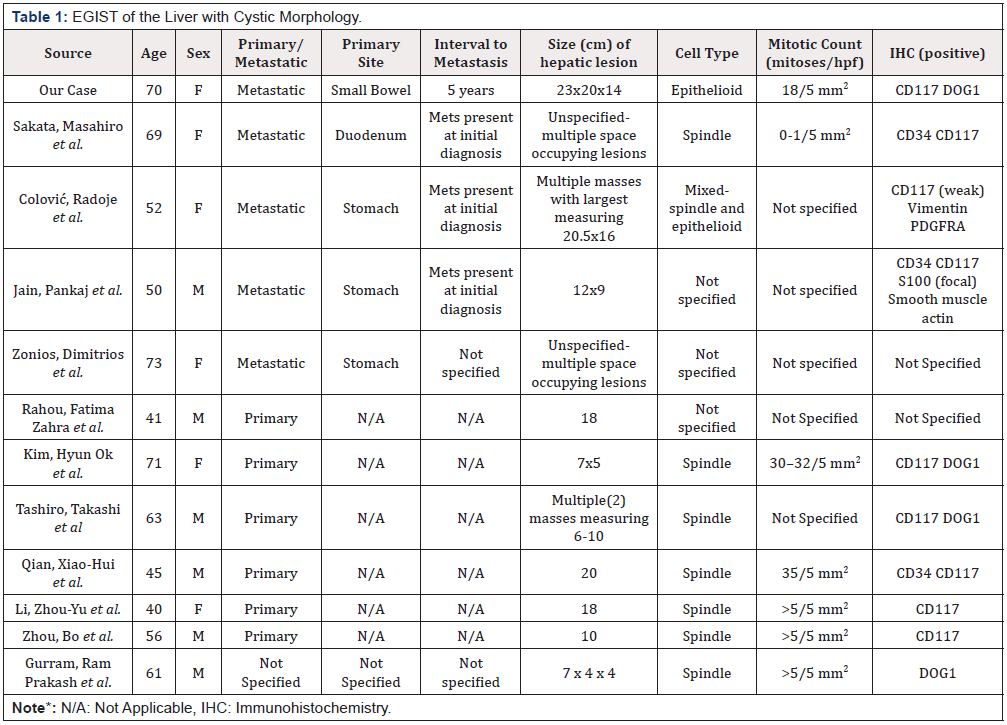
Gross morphology of GIST typically demonstrates a solid or mixed solid/cystic architecture [4]. Although it is common for GIST tumors to transform into cystic masses after imatinib therapy, GIST displaying purely cystic gross morphology prior to receiving treatment is rare [10-12]. Table 1 lists the cases of primary and metastatic EGIST of the liver displaying cystic morphology prior to tyrosine kinase inhibitor (TKI) therapy that have been reported in the English literature (Table 1)[20-30].
Patients with cystic GIST of the liver represent an interesting cohort in terms of risk stratification. At diagnosis, GIST tumors are risk-stratified based on size, mitotic activity, and location [13]. Treatment recommendations, including administration of TKI therapy, vary based on risk category [14,15]. Large size and extraintestinal location are both poor prognostic indicators which affect risk stratification [13]. The average size of a GIST mass at diagnosis in 5 cm. The cases of cystic liver GIST that have been reported in the literature present with larger than average masses at diagnosis. Current criteria for risk stratification, including MSKCC Nomogram, NIH-Fletcher consensus, and AFIP-Miettinen criteria, do not take gross morphology (cystic versus solid versus mixed cystic/solid) into account when assessing risk. A small study conducted by Wang et al. at Zhongshan Hospital in Shanghai, China assessed outcomes for seven patients treated for GIST with cystic morphology. They found that “the biological behavior of cystic GIST is indolent with a low risk of malignancy and favorable prognosis [16]. Analysis of treatment outcomes for a larger cohort of patients with cystic GIST, particularly cystic EGIST of the liver, is necessary to determine if the current risk stratification systems are appropriate in patients presenting with large liver masses with cystic morphology.
Our case represents an additional quandary into the assessment of GIST tumors. Patients with multiple GIST masses in the liver are assumed to have metastatic disease and are placed in an advanced stage category, which affects treatment and screening recommendations [17]. However, the patient we reported had a unifocal large complex cystic GIST replacing the entire right lobe of liver after a resection of small bowel GIST 5 years prior without history of imatinib treatment. However, the patient we reported had a unifocal large complex cystic GIST replacing the entire right lobe of liver after a resection of small bowel GIST 5 years prior without history of imatinib treatment. The gross and histological features of the large cystic liver GIST cause a diagnostic dilemma regarding primary tumor versus metastasis. There is evidence to suggest that a subset of patients with multiple GIST masses presenting at different sites may not have metastatic disease, but rather multiple primary tumors [18,19]. Genetic analysis may be useful in determining metastatic versus primary etiology. Next generation sequencing revealed genetic alteration in KIT (p. V559A) and MSH3 (p. T363Nfs*11) in the cystic liver GIST. Unfortunately, the tumor tissue from the small bowel resection performed at an outside hospital 5 years prior was not available for next generation sequencing analysis and a diagnosis of metastatic GIST was assigned without genetic correlation.
Conclusion
This case highlights the complexity of assessing both risk and stage in cases of cystic liver GIST. Both of these factors can affect the course of treatment patients presenting with cystic liver GIST receive. Genetic analysis of presumed metastatic and primary tumor tissue is of particular utility in determining metastatic versus primary etiology for staging. More research regarding treatment outcomes as well clinical and pathologic behavior is needed to determine if current risk stratification schema are appropriate in this patient population. Presently, these cases should be carefully evaluated and monitored to ensure proper treatment is rendered.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
None.
References
- Miettinen M, Jerzy L (2006) Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130(10): 1466-1478.
- Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, et al. (1999) Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol23(4): 377-389.
- Corless CL, Heinrich MC (2008) Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 3: 557-586.
- Miettinen M, Lasota J (2011) Histopathology of gastrointestinal stromal tumor. J Surg Oncol 104(8): 865-873.
- Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, et al. (2012) Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 13(3): 265-274.
- Miettinen M, Lasota J (2011) Histopathology of gastrointestinal stromal tumor. J Surg Oncol 104(8): 865-873.
- Bamboat ZM, Dematteo RP (2012) Updates on the management of gastrointestinal stromal tumors. Surg Oncol Clin N Am 21(2): 301-16.
- Belfiori G, Sartelli M, Cardinali L, Tranà C, Bracci R, et al. (2015) Risk stratification systems for surgically treated localized primary Gastrointestinal Stromal Tumors (GIST). Review of literature and comparison of the three prognostic criteria: MSKCC Nomogramm, NIH-Fletcher and AFIP-Miettinen. Ann Ital Chir 86(3): 219-227.
- Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK (2016) The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric cancer 19(1): 3-14.
- Kelly CM, Gutierrez Sainz L, Chi P (2021) The management of metastatic GIST: current standard and investigational therapeutics. Journal of hematology & oncology 14(2).
- Bakoyiannis A, Delis S, Triantopoulou C, Dervenis C (2013) Rare cystic liver lesions: a diagnostic and managing challenge. World journal of gastroenterology 19(43): 7603-7619.
- Kim HC, Lee JM, Choi SH, Han H, Kim SS, et al. (2004) Cystic changes in intraabdominal extrahepatic metastases from gastrointestinal stromal tumors treated with imatinib. Korean Journal of Radiology 5(3): 157-163.
- Bechtold RE, Chen MY, Stanton CA, Savage PD, Levine EA (2003) Cystic Changes in Hepatic and Peritoneal Metastases from Gastrointestinal Stromal Tumors Treated with Gleevec. Abdominal Imaging 28(6): 808-814.
- Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, al. (2012) Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. The Lancet. Oncology 13(3): 265-74.
- Duensing A (2012) Closing in on accurate risk prediction and disease management for patients with operable GIST. The Lancet Oncology 13(3): 220-221.
- Zhang H, Liu Q (2020) Prognostic Indicators for Gastrointestinal Stromal Tumors: A Review. Translational oncology 13(10): 100812.
- Wang CZ, Hou YY, Shen KT, Wang HS, Qin J, et al. (2011) Clinicopathological features and prognosis of cystic gastrointestinal stromal tumor. Zhonghua Wei Chang Wai Ke Za Zhi 14(8): 599-602.
- Sakata M, Kaneyoshi T, Fushimi T, Watanabe J (2021) Rare cause of cystic liver lesions: Liver metastasis of gastrointestinal stromal tumors. JGH open: an open access journal of gastroenterology and hepatology 5(3): 408-409.
- Colović R, Micev M, Matić S, Colović N, Grubor N, et al. (2013) Malignant stromal tumor of the stomach with giant cystic liver metastases prior to treatment with imatinib mesylate. Vojnosanitetski pregled 70(2): 225-228.
- Jain P, Jha AK, Rai RR (2009) Cystic hepatic metastasis from gastrointestinal stromal tumor prior to imatinib mimicking a liver abscess. Journal of gastrointestinal and liver diseases 18(1): 121-122.
- Rahou FZ, Miry A, Bennani A, Bouziane M (2020) Neurofibromatosis type 1 associated multiple and cystic gastrointestinal tumors: 02 case reports. International journal of surgery case reports 76: 210-216.
- Kim HO, Kim JE, Bae KS, Choi BH, Jeong CY, et al. (2014) Imaging findings of primary malignant gastrointestinal stromal tumor of the liver. Japanese journal of radiology 32(6): 365-370.
- Tashiro T, Uwamori F, Nakade Y, Inoue T, Kobayashi Y, et al. (2019) Primary Gastrointestinal Stromal Tumor of the Liver with Cystic Changes. Case reports in gastroenterology 13(1): 58-65.
- Zonios D, Soula M, Archimandritis AJ, Revenas K (2003) Cystlike hepatic metastases from gastrointestinal stromal tumors could be seen before any treatment. AJR American journal of roentgenology 81(1): 282.
- Qian XH, Yan YC, Gao BQ, Wang WL (2020) Prevalence, diagnosis, and treatment of primary hepatic gastrointestinal stromal tumors. World journal of gastroenterology 26(40): 6195-6206.
- Li ZY, Liang QL, Chen GQ, Zhou Y, Liu QL (2012) Extra-gastrointestinal stromal tumor of the liver diagnosed by ultrasound-guided fine needle aspiration cytology: A case report and review of the literature. Archives of medical science 8(2): 392-397.
- Zhou B, Zhang M, Yan S, Zheng S (2014) Primary gastrointestinal stromal tumor of the liver: report of a case. Surgery Today 44(6): 1142-1146.
- Gurram RP, Gnanasekaran S, Midha K, Biju P, Kalayarasan R (2020) Atypical Presentation of Gastrointestinal Stromal Tumor as Multiple Intra-Abdominal Cysts: A Case Report. Cureus 12(5): e7999.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.