Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Material Sustainability and Patient’s Experiences of Silver Tracheostomy Tubes-An In-vitro and In-vivo Case Study
*Corresponding author: Gunilla Björling, Department of Neurobiology, Care and Society, Karolinska Institute, Stockholm, Sweden
Received: September 25, 2021; Published: October 26, 2021
DOI: 10.34297/AJBSR.2021.14.002016
Abstract
Introduction: Tracheostomy tubes are used for enabling a free airway for patients with respiratory failures. In trachea, tracheostomy tubes are exposed to the alkaline tracheal secretion and lining fluid containing proteins, enzymes, organic acids, inorganic salts, and bacteria. Research shows severe material degradation of tracheostomy tubes made of polymeric materials after patient use. Silver tracheostomy tubes are made for long-term exposure up to several years.
The objectives were
a) To evaluate and describe the performance and durability of sterling silver tracheostomy tubes,b) To preliminarily describe and discuss the resulting morphological changes of the material surface after 6 months of clinical use
c) To describe the patient’s own experiences of having a silver tracheostomy tube for a prolonged period of time and
d) To study the release rate of metal ions into synthetic biological fluids in-vitro.
Methods: Two different brands of silver tracheostomy tubes were studied in-vitro in synthetic body fluids. Six patients with silver tracheostomy tubes were included for long-term study of 6 months. Scanning Electron Microscopy was used to evaluate surface changes on the tube material. Patient’ s experiences were measured with a study specific questionnaire with open ended questions.
Results: There were initial irregularities and defects on the reference silver tubes. A significant difference in material degradation between the tube brands were found after patient use. The release rate of metal ions into synthetic biological fluids revealed higher concentrations of copper ions than of silver ions. Patient´s own experiences revealed the importance of having a properly fitted tube to avoid complications.
Conclusion: There was severe degradation on all tubes and significant differences between the tube brands. Patients found it important with a properly fitted tube which could make it possible for them to live a good life despite the chronic tracheostomy.
Keywords: Respiratory Care; Tracheostomy Tube; Silver; Material Degradation; Patient Experience; Chronic Tracheostomy
Abbreviations: PVC: polyvinylchloride; PU: polyurethane; NRC: National Respiratory Centre; SEM: Scanning electron microscopy; EDX: Energy- Dispersive X-Ray Spectroscopy; SBF: Simulated Biological Fluids; ALF: Artificial Lysosomal Fluid; ALF: Artificial Lysosomal Fluid; FAAS: Flame Atomic Absorption Spectroscopy; OSHA: Occupational Safety and Health Administration
Introduction
Tracheostomy tubes are used for enabling a free airway for patients with respiratory failures [1-6]. The environment of the human body can facilitate a complex form of degradation in which biological, chemical, and physical phenomena interact [7]. In trachea, tracheostomy tubes are exposed to the alkaline tracheal secretion and lining fluid containing proteins, enzymes, organic acids, inorganic salts, and bacteria [8-10]. Adsorption of proteins onto the surface of the device material is the first event that occurs after insertion into trachea [9,11]. Alteration of PH, because of protein adsorption onto a metal surface, is thought to accelerate the corrosion of the metal. Moreover, the biological environments are not stable: factors such as the overall health status of the patient can cause variations in the oxygen level, as well as the pH level, and thereby change the corrosive nature of the environment of the patient´s body [10].
The material surface of a tracheostomy tube or an endotracheal tube is prone to microbial colonization [12,13] because the tube violates the natural defense system of the body [12]. In most cases, the tube is accessible for environmental bacteria that can use the device as a direct pathway into the body. An opening in the airway, caused by intubation, can also stimulate mucosal secretions, and thus give rise to bacterial adherence [11,14]. In this case, the microorganisms that have attached to the material’s surface can either initiate or accelerate the deterioration of the device material by inducing a concentration cell and/or by releasing metabolic products [15]. The material’s deterioration can facilitate further colonization by bacteria since pits and cracks can provide a shelter and thereby optimum conditions for the adhesion of microorganisms [2,16-20]. The National Respiratory Centre (NRC) in Stockholm, Sweden is an outpatient clinic for patients with respiratory failure in which one third of patients have long-term tracheostomy [1]. The majority of the patients currently use disposable tracheostomy tubes made of silicone rubber (PDMS), polyvinylchloride (PVC), or polyurethane (PU) [2]. Apart from their lower costs, the popularity of polymeric tubes is also due to their greater lightness and flexibility than metallic tubes. Their thermo-plasticity makes them rigid enough during insertion and causes them to soften at body temperature, which in turn allows them to conform to the contour of the trachea when inserted [10]. Thus, polymeric tubes can be considered to be more comfortable in use by patients [2]. All polymeric tubes on the market today are disposable devices and should not be reused for longer periods of time, and they should be taken out regularly and cleansed [16]. However, metallic tubes (i.e., tubes made of sterling silver, medical grade stainless steel, or copper) are considered to be more suitable for prolonged clinical use, although they are prone to corrosion by alkaline tracheal secretion. Hence, at the NRC there is only a small group of outpatients with long-term tracheostomy who have metallic tubes made of sterling silver. Silver is historically known to be sustainable, have excellent corrosion resistance and antimicrobial properties. The level of bacterial colonization is lower among patients using silver tracheostomy tubes [17], as shown in a clinical study. Sterling silver has lower corrosion resistance than pure silver due to the presence of ~7.5wt% other metals, mostly copper. Regarding corrosion, the weak points of a tracheostomy tube are the junctions between the tube and the neck plate (Area 1), the fenestration site-mid tube (Area 2), and the distal end of the tube (Area 3) [21-27] (Figure 1).

Figure 1. The tracheostomy tube, placed in the trachea below the patients’ vocal folds, can for evaluation purposes be divided into three different areas, i.e., the junctions between the tube and the neck plate (Area 1), the fenestration site (Area 2) and the distal end of the tube (Area 3). (Pictures are taken at the NRC (Unpublished reference)).
Further, are of the weak points of a silver tracheostomy tube illustrated in Figure 2, which shows a silver tracheostomy tube immediately after extubating revealing these areas, where the alkaline bronchial secretion haBBBBB s attached to the material surface. The picture of the tube was taken at the National Respiratory Centre in Sweden. As indicated by Figure 2, the highest concentration of attached secretion was found at the distal end of the tube, resulting in severe oxidation of the metallic surface. Prolonged wear and aging of the device material (polymeric or metal), as well as repeated sterilization, have been proposed as possible risk factors for tracheostomy tube fracture [16-18, 21- 23, 27-33]. Alkaline bronchial secretion, tissue reactivity, internal material stresses, and manufacturing defects have also been reported as possible reasons for tube failure [23,29,30]. In this context, the performance of tracheostomy tubes has only been studied randomly, and the optimum (real) service time of such a tube is not well known. The present authors studied the rates of degradation of polymeric tracheostomy tubes exposed to the environment of the trachea for periods of 1, 3 and 6 months [16,17]. The deterioration was clearly more severe after 3 and 6 months of clinical use than after 1 month. But, even after one-month, clear surface changes were observed. The optimum service time recommended by the authors for the polymeric tubes is thus under 3 months to promote the safety of the patient [16].
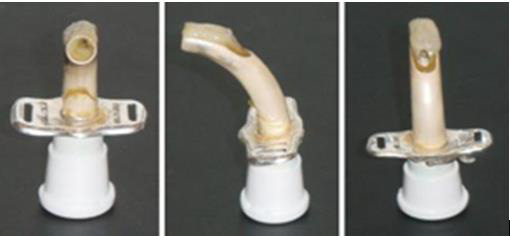
Figure 2. A silver tracheostomy tube immediately after extubation. The picture highlighting the different area where the alkaline bronchial secretion has attached to the material surface. (Brand: Bauer Häselbarth®) (Pictures are taken at the NRC (Unpublished reference)).
As silver tracheostomy tubes are more sustainable than
polymeric tubes and therefore used for chronic tracheostomy in
stable patients as well as the need for more research in the field,
the aim of the present study were
a) To evaluate and describe the performance and durability
of sterling silver tracheostomy tubes,
b) To preliminarily describe and discuss the resulting
morphological changes of the material surface after 6 months of
clinical use,
c) To describe the patient’s own experiences of having a
silver tracheostomy tube for a prolonged period of time and
d) To study the release rate of metal ions into synthetic
biological fluids in vitro.
Materials and Methods
Study Design
The study has a descriptive and exploratory design with in vitro and in vivo part. This study is a complementary study of previously made studies concerning the material wear of polymeric tracheostomy tubes exposed to the environment of the trachea for 1, 3, and 6 months in patients with long-term tracheostomy [16,17]. Because silver tracheostomy tubes are commonly used for a prolonged period of time and are considered to be more sustainable than polymeric tubes, the authors decided to perform and describe a separate study over the material degradation process and mechanisms in detail for silver tracheostomy tubes.
Setting
The present descriptive/prospective study was conducted at the NRC, which is a clinic for outpatients with respiratory failure. The NRC has authorization from the Swedish Medical Agency to adapt and customize tracheostomy tubes to offer an optimal fit and increased comfort for the patients [1,16-18]. The Stockholm Ethical Regional Board in Sweden approved this project, and written informed consent was obtained from all patients involved in the study.
in-vivo study
Study Subjects:
a) Inclusion Criteria: Adult (>18 years of age) outpatients who
had a silver tracheostomy tube for more than 3 months were
eligible to participate in the study.
b) Exclusion Criteria: infectious hematogenous diseases,
antibiotic treatment at the start of the study, smoking,
life expectancy of <10 months, cognitive dysfunction, or
a tracheostomy tube with other modifications besides a
customized fenestration.
c) In total, 6 patients were Included in the study: 3 patients
having a tracheostomy tube from the brand La Barré® (Th. La
Barré GmbH, Wangen, Germany) and 3 having a tracheostomy
tube from the brand and Bauer Häselbarth® (Bauer &
Häselbarth, GmbH, Schleswig-Holstein, Germany). All devices
consisted of an outer tube with a flange (neck plate) and an inner tube. The characteristics of the participants are presented
in Table 1. Ten patients met the inclusion criteria in the initial
phase of the study, but due to their individual needs for further
modification of the tracheostomy tubes, 4 participants had to
be excluded from the study, leaving 6 patients to complete the
entire study.
Data collection-in-vivo study
At the initiation of the study, each patient received a new sterling silver tracheostomy tube, which was inserted into the trachea by an anesthesiologist. During the following 6 months, each patient visited the NRC monthly. Before inspection and cleansing of the tube, bacterial sampling was done from the inside of the tracheostomy tube. Inspection and cleansing of the tube were then performed according to standardized procedures, (i.e., mechanical cleansing of the tube with detergent and warm water, ultrasonic cleansing in salty water for 4 minutes, immersion in 70% ethanol for 1 minute, and rinsing in saline solution [NaCl 0.9mg/mL] for 1 minute) [1,16,35]. If the tube passed the visual inspection of the physician after cleansing, it was reinserted into the trachea of the patient. During the study period, the patients were instructed to not clean the outer tube but to take the inner tube out routinely for cleansing at least twice a day. During the study period, the patients visited the NRC at 7 occasions. After 6 months of exposure to the trachea, the tubes were removed, cleansed according to the standardized procedures [1,16,35] and put in separate sample bags and preserved in a dry environment to awaiting analysis at the Royal Institute of Technology (KTH) in Stockholm, Sweden. One new tube of each brand was also sent for analyses as reference tubes. At the patient´s visits to the NRC, bacterial samples from the tracheostomy tubes were taken before removal of the tube and sent for microbiological analysis to the laboratory at Danderyd Hospital, Stockholm. The analyses were performed with standardized methods.
The patients were also given a study specific questionnaire to fill in about the treatment at NRC and experiences of living with long-term tracheostomy. With the intention of obtain a complete picture of each patient’s experiences with long-term tracheostomy, as well as to appreciate the influence that the condition of the tube material had on the quality of life of the patient, a study-specific questionnaire was used. The questionnaire was developed by the authors in collaboration with two respiratory nurses with more than 20 years of experience working with tracheostomy patients. The questions used a Lickert scale 1-4 as response alternatives, where 1 was the lowest and 4 the highest rating. The questionnaire was pre-tested in a group of 4 patients and no alterations were made. The questionnaire consisted of 12 items, and participants had the option to make additional comments. The questionnaire was sent to the patients at the end of the study period and was administrated by postal mail with a prepaid return envelope. The results were compiled and are presented at group-level due to the low number of participants and content analysis was performed.
Material Surface Analysis
A total of 3 samples with a size of approximately 4x4 mm were cut from 3 different locations of the tracheostomy tubes (Figure 1). Samples from Areas 1-3 were also cut from reference tubes of the same brand for a comparative study. Prior to analyses, the samples were rinsed with 70% ethanol and distilled water. All analyses were carried out blinded using standardized equipment. Only the outer surface of the outer tube was studied in all cases. Scanning electron microscopy (SEM) was used to study the morphology of the material surface of all samples. The samples were examined twice: once directly after being received from the hospital to study the presence of microorganisms, and again after cleansing to study the surface changes of the material. The degree of surface change was scored on a scale from 1 to 4 on the material degradation index (1=no visible changes, 2=tendency to surface changes, 3=visible surface changes, and 4=major surface changes with cracks, pores, and pits) [16,35]. As the severity of the surface changes varied within level 4 regarding the size of the pores and cracks, the values 5, and 6 were used as well (with 6 representing the most severe condition).
Statistical Analysis of Material Degradation in-vivo
Non-parametric statistical tests were applied for comparison of the two tube brands concerning the in-vivo degradation. The homogeneity of the two tube materials was assessed using Kruskal Wallis test. To determine whether the homogeneity hypothesis of the two materials could be rejected at a significance level of p<0.05, pairwise tests were performed between the different brands and locations of the tubes, respectively, using the Mann-Whitney U test. The analyses were performed with (SPSS Version 27).
Qualitative Analysis of Patient Experiences
The answers from the questions in the study specific questionnaire was summarized and are displayed in a table. Regarding the open-ended questions and comments, a content analysis inspired by [36] was adopted. The open-ended questions and comments were read through and meaning units were derived and coded into three categories. Two of the researchers were initially part of this process, but the results were discussed in the whole research group and consensus were reached through discussions.
in-vitro study
Study material: The sterling silver tracheostomy tubes investigated in the present study were from two different brands: La Barré® (Th. La Barré GmbH, Wangen, Germany) and Bauer Häselbarth® (Bauer & Häselbarth, GmbH, Schleswig-Holstein, Germany). The elemental composition of the tube materials was analyzed using energy-dispersive X-ray spectroscopy (EDX).
Aging test: To investigate the release rate of metal ions from sterling silver tracheostomy tubes, samples were exposed to simulated biological fluids (SBF) over a prolonged period (i.e., from 1 to 84 days). New tracheostomy tubes (reference tubes) were cut into 5mm long ring-shaped samples. The samples were all wet ground on both sides by using a 1200 grit silicon carbide paper. Prior to exposure to the SBFs, the samples were ultrasonically cleaned for 4 minutes in distilled water, immersed in 70% ethanol for 3 minutes, and finally rinsed with distilled water and air-dried overnight. Two different SBFs were used: Gamble’s solution with a PH of 7.4, representing the interstitial fluid of the deep lung and artificial lysosomal fluid (ALF) with a PH of 4.5, simulating the lung condition under inflammatory conditions. The chemical compositions of both fluids are presented in Table 2. To replace proteins, citrate was used in both solutions. In the case of Gamble’s solution, acetate was used to substitute the organic acids present in the body environment [37]. All samples were exposed to 10% HNO3 for 24 hours to remove any contamination on the surface before being stored in polyethylene containers. The solutions were added, and the containers were closed and sealed to avoid any leakage. The surface area/solution volume ratio of each sample was approximately 1cm2/mL-1. The containers were stored in an incubator kept at 37 °C ± 2 °C under dark conditions and gently rocked at 30RPM during the testing period. The exposure periods for the samples ranged from 1 day to 84 days, and all tests were performed on triplicate samples to ensure reproducibility.
The exposed samples were removed from the solutions after 1, 3, 7, 14, 21, 28, 35, 56, 70, and 84 days and preserved in a dry environment prior to surface analysis. The liquid samples were acidified with HNO3 to reduce the pH to ≤2 and refrigerated at 4 °C awaiting chemical analysis. A reference solution was prepared for each of the exposure times and for each of the two SBFs used. The liquid references samples were stored under the same conditions as all the other liquid samples and used as a correction for the background metal concentration.
Metal Analysis
Flame atomic absorption spectroscopy (FAAS) was used for analyzing the liquid samples to determine the concentration of released silver and copper ions from the samples. The apparatus used was a Perkin Elmer Analyst 800 with a detection limit of 6μg/l for copper in both ALF and Gamble’s solutions, while the detection limits for silver were 4μg/l and 1μg/l, respectively. The metal concentration of each reference solution was subtracted from the values detected for the corresponding liquid samples if above the detection limit; otherwise, it was considered to be zero [38,39]. The surface changes of the 54 exposed metallic samples were characterized using scanning electron microscopy (SEM).
Ethical Considerations
The authors assert that all procedures contributing to this work comply with the ethical standards of Stockholm Ethical Regional Board, Sweden and with the Helsinki Declaration of 1975, as revised in 2008. All participants gave their written informed consent before the study start and the participation was voluntary and confidentiality.
Results
EDX analysis of the material matrix showed that both brands of tracheostomy tubes investigated in this study (La Barré® and Bauer Häselbarth®) consisted of ~92wt% Ag (silver) and ~8wt% Cu (copper). The texture of the material surfaces clearly revealed the presence of irregularities in the form of polishing lines, scratches, and pores. Probably due to differences in the manufacturing procedure, evident disparities in the surface finish of the material were observed on the new reference tubes (Figure 3).
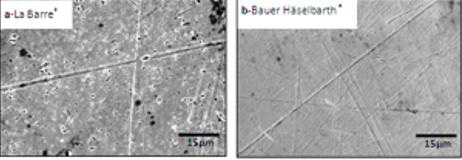
Figure 3: SEM micrographs of the material surfaces of reference tubes of the brand (a)La Barré® and (b)Bauer Häselbarth®. In both cases the images are representative for the material studied (the bars represent 15μm).
in-vitro study
For the samples exposed to SBFs, the concentration of silver and copper ions released from the samples was measured by FAAS. The results revealed that the concentration of copper ions released from the samples was significantly higher than the concentration of silver ions in both solutions (Figures 4&5). As noted in Figure 4, a substantial concentration of silver ions was initially released in the ALF solution, after which time a relative stability was obtained. The concentration of silver ions released into the Gamble’s solution increased gradually throughout the entire exposure period. The behavior of copper ions showed the opposite trend when exposed to Gamble’s and ALF solutions respectively, demonstrating a higher release rate in the ALF solution (Figure 5). The total average concentration of silver ions released in the ALF solution was established as ~90μg/l, compared to ~130μg/l in the Gamble’s solution. For the copper ions released, the average concentration was found to be ~17mg/l in the ALF solution and ~3mg/l in the Gamble’s solution. The SEM investigation of the metallic samples exposed to SBFs revealed that neither the solutions nor the exposure time had any visible influence on the material surface.
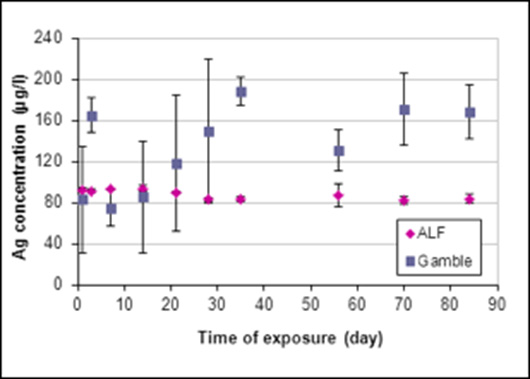
Figure 4: The concentration of silver ions released in two different SBFs, i.e., the ALF solution (PH =4.5) and the Gamble’s solution (PH =7.4). Each data point represents the average concentration of the triplicate samples at each time period.
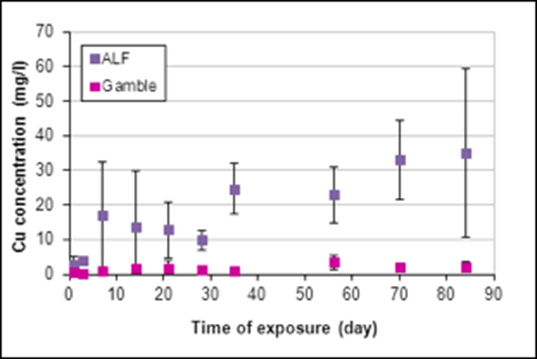
Figure 5: The concentration of copper ions released in two different SBFs, i.e., the ALF solution (PH=4.5) and the Gamble’s solution (PH =7.4). Each data point represents the average concentration of the triplicate samples at each time period.
in-vivo study
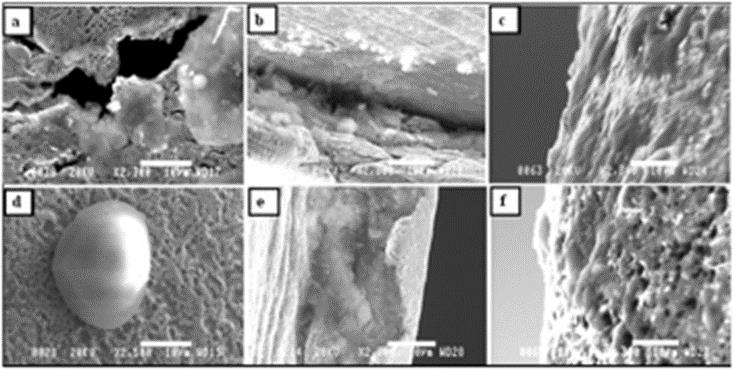
Figure 6: SEM micrographs of samples retrieved from tracheostomy tubes exposed to the trachea for 6 months. Micrographs (a), (d), and (f) utilize
the brand La Barré®, and (b), (c), and (e) utilize Bauer Häselbarth®.
a) 6a: Area 1 of tube No. 3-local bacterial colonies covered in biofilm and attached around holes in the material
b) 6b: Area 2 of tube No. 4-organic substances inside a groove close to the fenestration site
c) 6c: Area 3 of tube No. 6-a thin organic film (biofilm) has covered the surface
d) 6d: Area 1 of tube No. 2-a bacterial colony covered in biofilm and attached to the porous surface of the material
e) 6e: Area 2 of tube No. 6-similar conditions as in micrograph
f) 6f: Area 3 of tube No. 3-organic substances attached to the porous surface. In all micrographs, the bars represent 10μm.
Material surface changes: No pores or cracks were detected with the naked eye during visual inspection of the cleaned tracheostomy tubes exposed to the trachea for 6 months. In Figures 6a&6b, representative SEM micrographs of the material surface of different samples, as well as different locations on the samples, are presented. As demonstrated in Figure 6c & 6f, an organic film partly covers the device’s surface. The presence of probably local bacterial colonies was also detected as noted in Figure 6d. It is, however, important to point out that the presence of bacteria on the surface of the tube material has not been confirmed by conventional methods. Results from the microbiological analysis of samples taken from the tracheostomy tubes before removal showed the presence of Haemophilus parainfluenzae, Neisseria species, Pseudomonas aerigunosa, Staphylococci, and Streptococci species, which are all recognized as normal flora of the airways in this patient group [18,35,40]. Thus, no pathogenic species were found, and no antibiotic-resistance testing was therefore carried out. The material analyses, i.e. SEM, showed severe changes in the surface topography, resulting in a rougher and more porous texture, as can be seen in Figures 6a&6f. The presence of scratches and grooves around the fenestration site of the tubes are apparent in Figures 6b&6e. It is, however, believed that the scratches around the fenestration site are the result of machining during the construction of the customized fenestration, and they should not be considered a result of the material’s exposure to the trachea. In Figure 7, micrographs of both the reference samples and the samples retrieved from the exposed tubes are presented for comparison. Regarding the reference tubes, the texture of the material surfaces clearly reveals the presence of irregularities in the form of scratches, polishing lines, and/or other surface defects, see Figures 7a-7e. However, the surface irregularities were more severe for the reference tubes of the brand La Barré®. For the exposed tubes, different degrees of degradation were established (i.e., cracks, pits and voids), as well as an increased surface roughness, as noted in Figure 7.
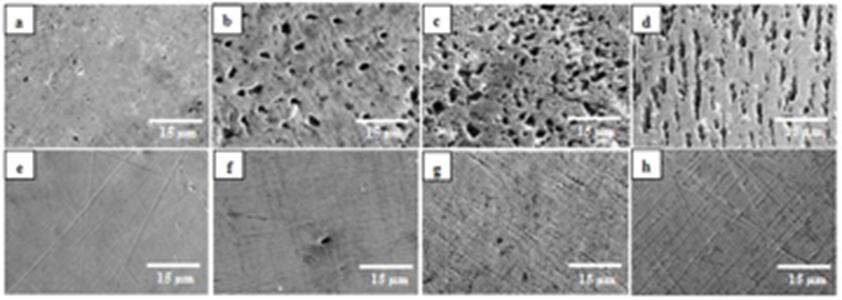
Figure 7: SEM micrographs of samples retrieved from the unexposed tracheostomy tubes (reference tubes), and from the tubes exposed to
trachea for 6 months.
Micrographs (a)-(d) are of the brand La Barré®:
7a: Area 2 of the reference tube-small pores/pits that originates from the manufacturing process
7b: Area 1 of tube No.1-pitting
7c: Area 2 of tube No.2-severe pitting (an increase in size and number)
7d: Area 3 of tube No.3- severe pitting (elongated pits).
Micrographs (e)-(h) are of the brand Bauer Häselbarth®:
7e: Area 2 of the reference tube-scratches and polishing lines that originates from the manufacturing process
7f: Area 1 of tube No.4-a limited number of cavities (>3μm)
7g: Area 2 of tube No.5-pits and fissures
7h: Area 3 of tube No.4-an increased number of pits and fissures. In all micrographs the bars represent 15μm.
Material degradation index
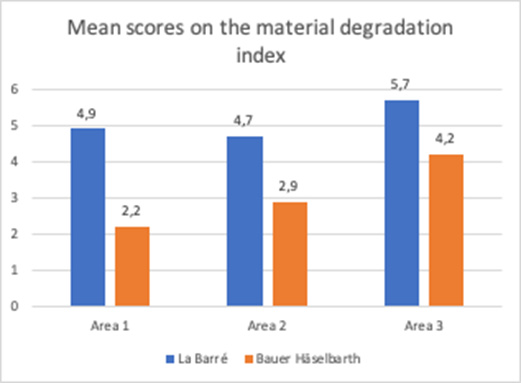
Figure 8: The material degradation index for each of the two brands of tracheostomy tubes investigated, i.e., La Barré® and Bauer Häselbarth®, presented as mean values of the scores obtained for Area 1, Area 2 and Area 3, (P<0.001).
There was severe material degradation for both tracheostomy tube brands after patient use. The degradation was not homogeneously distributed on the tubes, and comparison of the degradation of the different locations of the tubes (area 1-3) using Kruskal-Wallis test, revealed that area 3, the tip of the tube, was the most affected part. This was statistically significant (p=.03). There were differences between the tube brands regarding scores on the material degradation index [2,35]. When comparing the SEM results on the material degradation index (all areas) the difference between the tube brands was significant (p<.0001), i.e. the La Barré® tubes revealed more extensive material degradation compared to Bauer-Häselbarth® tubes. For the La Barré® tubes, 41% of the samples were scored with the highest score (6) and 89% were scored with 4 or above; for the Bauer-Häselbarth® tubes, 52% were scored with 4 or 5 and none with 6, Figure 8.
Difference within the Tube Brands
Concerning the homogeneity of the material, for both brands, area 3 (distal end of the tube), displayed the highest score on the material degradation index; La Barré® Mn=5.67 and for Bauer- Häselbarth® Mn=4.22, which was statistically significant (p=.019) for Bauer-Häselbarth®, but not for La Barré® (p=.072), see Table 3. The Mann-Whitney tests revealed significant differences in material degradation between La Barré tubes and Bauer Häselbarth® tubes with regard to area 1 (Stoma) average (p<.0008) and area 3 (tip) (p<.0036). La Barré displayed higher material degradation for area 1 (stoma) (mean rank = 5) than Bauer Häselbarth® (mean rank=2). A similar pattern was observed for area 2 (mean rank La Barré®=5, mean rank Bauer Häselbarth®=3(p<.0387) in Table 3.
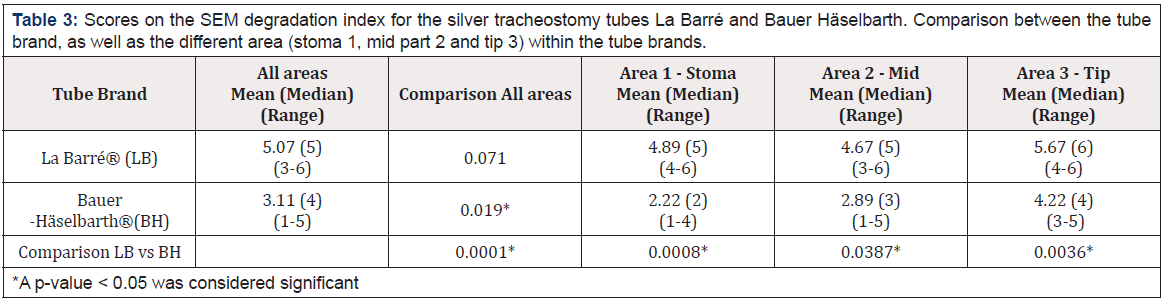
Table 3: Scores on the SEM degradation index for the silver tracheostomy tubes La Barré and Bauer Häselbarth. Comparison between the tube brand, as well as the different area (stoma 1, mid part 2 and tip 3) within the tube brands.
Further SEM Observations
In some samples extreme cases of local degradation/corrosion was observed, see Figure 9. The material degradation index of these samples was not considered in the evaluation of the overall results (only results representative for the whole sample surface were considered).
Patient Experiences
All participants answered the study-specific questionnaire, so a 100% response rate was obtained. Overall, the patients were very content with the treatment they received at the NRC, as well as with their tracheostomy tube and all participants except one found the tracheostomy tube comfortable, see Table 4. Further, there were three open ended questions in the questionnaire: Which are your positive experiences of having a long-term tracheostomy? Which are your negative experiences of having a long-term tracheostomy; Do you have any suggestions for improvement of the care you receiv. To live a life with a chronic tracheostomy has a great impact on a person’s life. The possibility for own comments in the questionnaire was used by all participants on several occasions. From the comments and answers from the open-ended questions categories emerged; relief, limitations and leading one’s life.
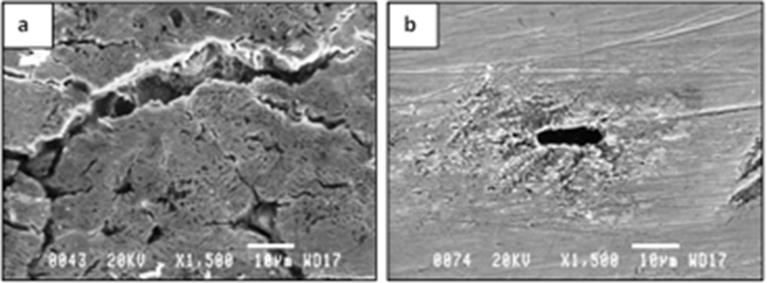
Figure 9: SEM micrographs of samples retrieved from the tubes exposed to trachea for 6 months. Both micrographs represent extreme cases of local degradation/corrosion, i.e., (a) a rather deep crack, as well as several small cracks, was found on the surface of one of the samples from tube No.3 (brand La Barré®), and (b) the surface is locally corroded and a large void/cavity of about 11μm in length was found on the surface of one of the samples from tube No.4 (brand - Bauer Häselbarth®).
Relief
Having had the possibility to receive a properly fitted tube
has been essential in continuing a decent life. The participants
experienced an improvement in quality of life and were happy to
be alive. This shows how the individual’s wellbeing over time may
be impacted by the tracheostomy tube. Some of the patient´s own
comments are displayed below:
a) I am alive!
b) It was my possibility to survive
c) The quality of life has substantially increased
Limitations
However, one of the patients occasionally experienced pain,
secretions, and coughing due to the tracheostomy tube, and
another patient had some irritation of the tissue around the stoma.
Two patients felt challenged when speaking in large groups or in
gatherings with a higher noise level. Losing the ability to swim and
ski were also mentioned as negative experiences.
a) There are negative experiences; pain, coughing, the speaking
valve comes off
b) It’s impossible to go sailing (cannot be at the sea)
c) It’s difficult to speak in larger social situations
Leading one’s life
Overall, a properly fitted tracheostomy tube was pointed
out as essential for having a positive treatment experience and
promoting tube comfort. It was also important that the tube was
easy to care for. The patients agreed that after receiving a properly
fitted tracheostomy tube, and thereby having a free pathway for
breathing. Living with a tracheostomy tube has improved their life,
but they could feel stigmatized sometimes.
a) It improved my breathing
b) Can breathe without problem
c) Curious, staring people-children are more honest-they ask,
‘what do you have in your throat?
Concerning removing the entire tracheostomy tube (both inner
and outer tubing) for cleansing at home, three of the patients were
uncomfortable with this method for different reasons. Finally, the
patients thought that regular tube cleansing and check-up had a
positive effect on their wellbeing. Overall, the patients were content
with the service offered by the NRC in the form of monthly visits for
inspection of the trachea and stoma, as well as cleansing and visual
inspection of the tube.
a) I find that the function of the tube is better after cleansing
b) I have dropped the tube once, and I’m afraid not to be able to
replace it again
c) I only take out the inner tube for cleansing
Discussion
The main aim of the present study was to evaluate and describe the performance and durability of sterling silver tracheostomy tubes, to preliminarily describe and discuss the resulting morphological changes of the material surface after 6 months of clinical use, and to describe the patient’s own experiences of having a silver tracheostomy tube for a prolonged period of time. The release rate of metal ions into synthetic biological fluids was also studied in vitro. Our main finding was that silver is not an inert material during prolonged (i.e., months of) clinical use as a tracheostomy tube. This was not entirely surprising, as previous case reports have described corrosion and fractures when using this material [21,23-27,29,30,34,]. Due to a university collaboration between our institutes in medicine and technology, respectively, it was possible for us to describe in better detail how the material wear of silver developed over time. All participating patients fared well during the study period (6 months x 6 patients = 36 months in total), and no adverse event was noted.
in-vitro Results of Metal Analysis, Composition
The SEM study conducted on the reference tubes of the brand Bauer Häselbarth® revealed an “acceptable” surface structure, while tubes of the brand La Barré® revealed a defective surface with a rough and porous structure. EDX analysis did not show any difference in the chemical composition of the tube materials used in either brand or the proportion of metals (copper versus silver) was identical between the two brands investigated. Thus, we conclude differences exist in the manufacturing process employed. It has been reported in the literature that oxidation of copper during melting and/or casting can result in a poor surface finish, which may be the case regarding this study’s findings [39-43].
The Occupational Safety and Health Administration (OSHA) standard has recommended an exposure limit of 0.01 milligrams of soluble silver compound per cubic meter of air, averaged over an 8-hour work shift. The American Conference of Governmental Industrial Hygienists has also established limit values of 0.01mg/m3 and 0.1mg/m3 for soluble and metallic forms of silver, respectively [38,44]. In the present study, the amount of silver released in both SBFs was above 80μg/l after one day of exposure, which is much higher than the recommended thresholds. Additionally, the amount of metal released in vivo is expected to be more significant due to the higher degree of degradation observed with the in-vivo exposed tubes (Figures 7&8). It should be noted no patients were diagnosed directly with toxicity during the 6 months of study. Although the established exposure limits are thought to be quite conservative, the toxicological effect of exposure to both silver and copper should be taken into consideration for cases of long-term tracheostomy [38,44]. The scattering of the obtained immersion data and the variations between the results obtained for the triplicate samples investigated appear to be due to the different surface conditions of initial samples. In other words, some samples suffer from more irregularities and defects, resulting in higher release rates of metal ions, while others have a more uniform surface structure. However, no significant surface changes could be observed on the samples exposed in the different SBFs.
in-vivo results
The SEM study conducted on the reference tubes of the brand Bauer Häselbarth® revealed an “acceptable” surface structure, while tubes of the brand La Barré® revealed a defective surface with a rough and porous structure. The SEM results from in-vivo study of the tubes exposed in the trachea for 6 months showed a severely porous structure overall. These results correspond to the findings from the same authors about material wear of polymeric tracheostomy tubes [16-18]. Further, it has been described in the literature that the junction between the tube and the neck plate is a sensitive are [21], which our results as well confirm, although the study period was rather short, i.e. 6 months.
However, the deterioration of the tube material in the present study is believed to have occurred quickly due to the initial defects in the material surface. The pores probably acted as nests, in which microorganisms were protected from the regular cleaning procedure, giving them the opportunity to deepen the pores by accelerating the rate of degradation. Moreover, as sterling silver contains copper and copper is less noble than silver, corrosion of copper-rich fractions of the surface may have occurred after being induced by the pores and other initial defects. The highest degree of degradation was observed at area 3 (the tip of the tube) in all tubes investigated. This is probably because the highest concentration of attached secretion is at the distal end of the tube. The extreme local degradation observed on some tubes, see Figure 9, might have been initiated from a local defect present on the material surface and propagated by a local stress during handling of the tube or as a result of unintended mishandling by the patient. The effect of specific bacteria on the material wear, as well as the pH in the trachea of the patient, has not been considered in this study, but the cultures taken from the patients showed only normal tracheal bacterial flora. Results from the microbiological analysis taken from the tracheostomy tubes before removal showed the presence of Pseudomonas aerigunosa, Haemophilus parainfluenzae, Neisseria species, Staphylococci, and Streptococci species, which are all recognized as normal flora of the airways in this patient group [1,40]. Thus, no pathogenic species were found, and no antibioticresistance testing was carried out.
Comparison of in-vitro vs in-vivo
After comparing the in-vivo SEM results with those obtained from the in-vitro investigation, it was evident the trachea builds a significantly harsher environment for the material in the presence of enzymes, proteins, and microorganisms. Considering the severe degradation established on all exposed tubes, the released metal ions were expected to be much higher in patients than what was measured from the in-vitro immersion tests. The extreme local degradation observed on some tubes, see Figure 9, may have been initiated from a local defect present on the material surface. The immersion tests performed have given clear information about the bio-accessibility of the metals present in the tube material. FAAS data revealed a significantly higher release of copper ions than of silver ions, even though copper constitutes no more than ~8wt% of the alloy. The results obtained confirm the proportion of released alloy constituent can significantly differ from the alloy composition. Based on the results of the immersion tests, it was confirmed copper releases to a higher degree in a more acidic solution (ALF), while silver showed the opposite release trend. This is due to the greater susceptibility of silver to the higher concentration of chloride and sulfide in the Gamble’s solution. The severely porous structure observed in the in-vivo exposed samples is thought to be partly due to the dissolution of copper.
Patient Experiences
Regarding patient´s experiences, participants in this study found the care at the NRC to be good and expressed the importance of a properly fitted tracheostomy tube. These results are in line with previous research about patients with long-term tracheostomy [41], where patients, with invasive ventilation (at night), had a better quality of life than those with non-invasive ventilation. Likewise, a retrospective study performed at a dedicated respiratory clinic specializing in modifying tracheostomy tubes for long-term use has shown that patients with tracheostomy, with or without ventilation, have a low rate of hospitalization and an unaffected life expectancy [1,18].
Conclusion
The present study indicates the service time of a silver
tracheostomy tube is clearly affected by the environment of the
trachea, as well as by the manufacturing process of the tube. A
more extensive study is needed to clarify the significance of these
findings, but the results should be considered in the context of the
long-term clinical use of silver tracheostomy tubes. Many patients
consider the tubes to be comfortable during use, although they are
less flexible than polymeric tubes. If the tube is properly fitted in
the trachea, the patient will experience fewer complications.
The most important findings of this work can be summarized
as follows:
a) An evident difference in the surface finish of the tube material
with regard to both topography and texture was established to
exist on new tubes. The texture of the material surface clearly
revealed the presence of surface defects such as scratches,
polishing lines, and pores and small cavities.
b) The initial surface irregularities/defects provide optimum
conditions for the addition of an organic film and/or local
bacterial colonies, and they are the main cause for the
accelerated deterioration rate obtained in the tube material
when exposed to the complex environment of a trachea. The
deterioration is also partly due to the dissolution of copper
ions.
c) Initial surface irregularities/defects are clearly related to
the manufacturing process of the tube material. Additional
measures must be taken to promote good practice and to avoid
the oxidation of copper during the melting and/or casting step
of the manufacturing process.
d) The distal end of the tubes was found to be the most susceptible
area to degradation.
e) There were significant differences in material degradation
between the different brands in-vivo.
f) The amount of silver ions released in the in-vitro studies,
simulating the body of the patient, was established to be far
above the recommended exposure limits, suggesting the need
for performing toxicity measurements on patients with longterm
silver tracheostomy tubes.
g) Overall, the patients were content with the treatment they
received and with their tracheostomy tube. Patients affirmed
the importance of having a properly fitted tracheostomy tube
and experienced improved wellbeing when provided with a
well-fitted tube.
Acknowledgement
The authors are thankful to all colleagues at KTH, KI, and The Swedish Red Cross University college, as well as the staff at the NRC in Stockholm, Sweden for helping with planning the present study, caring for the patients, and collecting the tracheostomy tubes investigated. Financial support from the Stockholm County Council and the Sophiahemmet Research Foundation are gratefully acknowledged.
References
- Björling G, Johansson UB, Andersson G, Schedin U, Markstrom A, et al. (2006) A retrospective survey of outpatients with long-term tracheostomy. Acta Anaesthesiologica Scandinavica 50(4): 399-406.
- Björling G (2009) Long-term tracheostomy: How to do it. Breathe 5(3): 204-210.
- Epstein SK (2005) Late complications of tracheostomy. Respir Care 50(4): 542-549.
- Hess DR, Altobelli NP (2014) Tracheostomy tubes. Respir Care 59(6): 956-973.
- Klemm E, Nowak A (2019) Regarding Mortality associated with tracheostomy complications in the United States: 2007-2016. The Laryngoscope 129(6): E198.
- Patton J (2019) Tracheostomy care. Br J Nurs 28(16): 1060-1062.
- Bull S (2000) Materials degradation and its control by surface engineering. Tribology international 33(2): 144-146.
- Halliwell B, Cross CE (1994) Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect 102(Suppl 10): 5-12.
- Koelling A, Johnson, RA, Ballintyn NJ (2000) Infections in Orthopaedics. In: Sawan S (Ed.)., Antimicrobial/anti-infective materials: principles, applications and devices. Technomic Publishing Co, Inc, Pennsylvania, USA.
- Williams D, Williams RL (2004) Degradative effects of the biological environment on metals and ceramics. In: Ratner B, Hoffman AS, Schoen FJ, Lemons JE (Eds.) Biomaterial Science: an introduction to materials in medicine. (2nd)., Elsevier Academic Press, UK, pp. 430-438.
- Niederman MS, Levine SA (1991) Impact of anaesthetic procedures on colonization defences of the tracheobronchial tree. Bailliere's Clinical Anaesthesiology 5(1): 39-59.
- Swain R (2000) Anti-infective devices in otolaryngology: current applications and future opportunities. In: Sawan S, Manivannan G (Eds.) Antimicrobial/anti-infective materials: principles, applications and devices. (1st edn)., Technomic Publishing Co, Inc, USA.
- Thorarinsdottir HR, Kander T, Holmberg A, Petronis S, Klarin B (2020) Biofilm formation on three different endotracheal tubes: a prospective clinical trial. Crit Care 24(1): 382.
- Rassekh CH, Zhao J, Martin ND, Chalian AA, Atkins JH (2015) Tracheostomy complications as a trigger for an airway rapid response: analysis and quality improvement considerations. Otolaryngology-Head and Neck Surgery 153(6): 921-926.
- Allsopp D, Seal KJ, Gaylarde CC (2004) Introduction to biodeterioration. (2nd )., Camebridge University Press, Cambridge, UK.
- Backman S, Björling G, Johansson UB, Lysdahl M, Markström A, et al. (2009) Material wear of polymeric tracheostomy tubes: A six‐month study. Laryngoscope 119(4): 657-664.
- Björling G, Belin AL, Hellström C, Schedin U, Ransjö UM, et al. (2007) Tracheostomy inner cannula care: A randomized crossover study of two decontamination procedures. AJIC: American Journal of Infection Control 35(9): 600-605.
- Frostell C, Björling G, Strömberg E, Karlsson S, Aune RE (2017) Tracheal implants revisited. The Lancet 389(10075): 1191.
- Kraus M, LeRiger M (2016) Aspiration of a fractured tracheostomy cannula in a pediatric patient. Can J Anaesth 63(12): 1376-1377.
- Parida PK, Kalaiarasi R, Alexander A, Saxena SK (2020) Factors associated with fracture and migration of tracheostomy tube into trachea in children: a case series. Iran J Otorhinolaryngol 32(113): 379-383.
- Bo LJ, Yu PX, Qi X, Kang RT (2019) Anesthetic management of a patient with an unusual broken tracheostomy tube: a case report. J Int Med Res 47(2): 718-721.
- Bowdler DA, Emery PJ (1985) Tracheostomy tube fatigue. An unusual cause of inhaled foreign body. J Laryngol Otol 99(5): 517-522.
- Gupta SC, Ahluwalia H (1996) Fractured tracheostomy tube: an overlooked foreign body. J Laryngol Otol 110(11): 1069-1071.
- Majid AA (1989) Fractured silver tracheostomy tube: a case report and literature review. Singapore Med J 30(6): 602-604.
- Songern A, Boonsarngsuk V (2016) Fractured metallic tracheostomy tube: A rare complication of tracheostomy. Respir Med Case Rep 19: 46-48.
- Sood RK (1973) Fractured tracheostomy tube. J Laryngol Otol 87(10): 1033-1034.
- Wilson W, Nagaraja DM, Dias A, Murty S (2018) Fracture and aspiration of tracheostomy tube: A case report. Hong Kong journal of emergency medicine 25(6): 371-374.
- Afzal M, Mutairi AH, Chaudhary I (2013) Fractured tracheostomy tube obturator: a rare cause of respiratory distress in a tracheostomized patient. World J Anesthesiol 2(3): 30-32.
- Gana PN, Takwoingi YM (2000) Fractured tracheostomy tubes in the tracheobronchial tree of a child. Int J Pediatr Otorhinolaryngol 53(1): 45-58.
- Gupta SL, Swaminathan S, Ramya R, Parida S (2016) Fractured tracheostomy tube presenting as a foreign body in a paediatric patient. BMJ Case Rep 2016: 1-3.
- Loh TL, Chin R, Flynn P, Jayachandra S (2014) Fracture and aspiration of a tracheostomy tube. BMJ Case Reports 2014:bcr2013203232.
- Lynrah ZA, Goyal S, Goyal A, Lyngdoh NM, Shunyu NB, et al. (2012) Fractured tracheostomy tube as foreign body bronchus: Our experience with three cases. Int J Pediatr Otorhinolaryngol 76(11): 1691-6195.
- Parida PK, Kalaiarasi R, Gopalakrishnan S, Saxena SK (2014) Fractured and migrated tracheostomy tube in the tracheobronchial tree. Int J Pediatr Otorhinolaryngol 78(9): 1472-1475.
- Piromchai P, Lertchanaruengrit P, Vatanasapt P, Ratanaanekchai T, Thanaviratananich S (2010) Fractured metallic tracheostomy tube in a child: a case report and review of the literature. J Med Case Rep 4(1): 234.
- Björling G, Axelsson S, Johansson UB, Lysdahl M, Markström A, et al. (2007) Clinical use and material wear of polymeric tracheostomy tubes. The Laryngoscope 117(9): 1552-1559.
- Elo S, Kyngäs H (2008) The qualitative content analysis process. J Adv Nurs 62(1): 107-115.
- Herting G, Wallinder IO, Leygraf C (2006) Factors that influence the release of metals from stainless steels exposed to physiological media. Corrosion Science 48(8): 20-32.
- ACGIH (2015) Documentation of the threshold limit values and biological exposure indices, 7th (edn.)., American Conference of Governmental Industrial Hygienists ACGIH, Cincinatti, Ohio.
- Nisaratanaporn E, Wongsriruksa S, Pongsukitwat S, Lothongkum G (2007) Study on the microstructure, mechanical properties, tarnish and corrosion resistance of sterling silver alloyed with manganese. Materials Science & Engineering A 445: 663-668.
- Harlid R, Andersson, G, Frostell, CG, Jörbeck, HJ, Örtqvist (1996) Respiratory tract colonization and infection in patients with chronic tracheostomy. A one-year study in patients living at home. Am J Respir Crit Care Med 154(1): 124-129.
- Markström A, Sundell K, Lysdahl M, Andersson G, Schedin U, et al. (2002) Quality-of-life evaluation of patients with neuromuscular and skeletal diseases treated with noninvasive and invasive home mechanical ventilation. Chest 122(5): 1695-1670.
- Moss OR (1979) Simulants of lung interstitial fluid. Health Phys 36(3): 447-448.
- Procter N, Louw C (2016) A broken fenestrated tracheostomy tube fragment removed from the right main bronchus. Southern African Journal of Anaesthesia and Analgesia 22(6): 195-197.
- OSHA (1989) United States Code of Federal Regulations Occupational Safety and Health Administration. Air contaminants, final rule, 29CFR Part 1910.1000.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.