Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Nonalcoholic Fatty Liver Disease (NAFLD) and Extra Hepatic Malignancies
*Corresponding author: Amalou Khellaf, Department of gastro enterology, central military hospital Kouba, Algeria.
Received: November 16, 2021; Published: December 01, 2021
DOI: 10.34297/AJBSR.2021.14.002060
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease, its prevalence reaches 25 % in adults and about 10% in children (1-3). The prevalence of nonobese NAFLD ranged from 25% or less in some countries to higher than 50% in others (4). NAFLD’s disease spectrum varies from non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH), the most dangerous form with its complications of hepatic fibrosis, cirrhosis, and hepatocellular carcinoma (5,6). The existence of steatohepatitis and severe fibrosis are considered indicators of undesirable outcomes in patients with NAFLD and are associated with an increased risk for morbi-mortality by hepatic and extra hepatic complications (7,8). In decreasing order, mortality in NAFLD patients is due, first, to cardiovascular events and, second, to gastrointestinal (liver, intestine, esophagus, stomach, and pancreas) and extraintestinal (kidney in men and breast in women) malignancies, while end-stage liver disease represents the third cause of death (8,9). The hepatic manifestation of metabolic syndrome (Mets) is generally considered the NAFLD, and a remarkable body of literature shows an enhanced cancer risk in Mets subjects, especially in the gastrointestinal tract. In this environment, NAFLD may either express similar risk factors (i.e., obesity and millets diabetes). The frequency, prevalence, and severity of these complications are related to the histological severity of liver injury, signifying that NAFLD, but especially NASH, may also lead to low-grade inflammatory status by releasing multiple markers of inflammation, oxidative stress, and procoagulant factors (10,11). The aim of this narrative analysis was to synthesize recent evidence of NAFLD extrahepatic malignancies, based on the predominant prevalent incident/risk of such diseases in NAFLD patients. To date, an effective screening approach for extrahepatic malignancies has not yet been established. For patient management, collaborative care with relevant experts seems to be required because extrahepatic cancers can emerge across various organs (12).
Relationship between NAFLD and Colorectal Cancer (CRC)
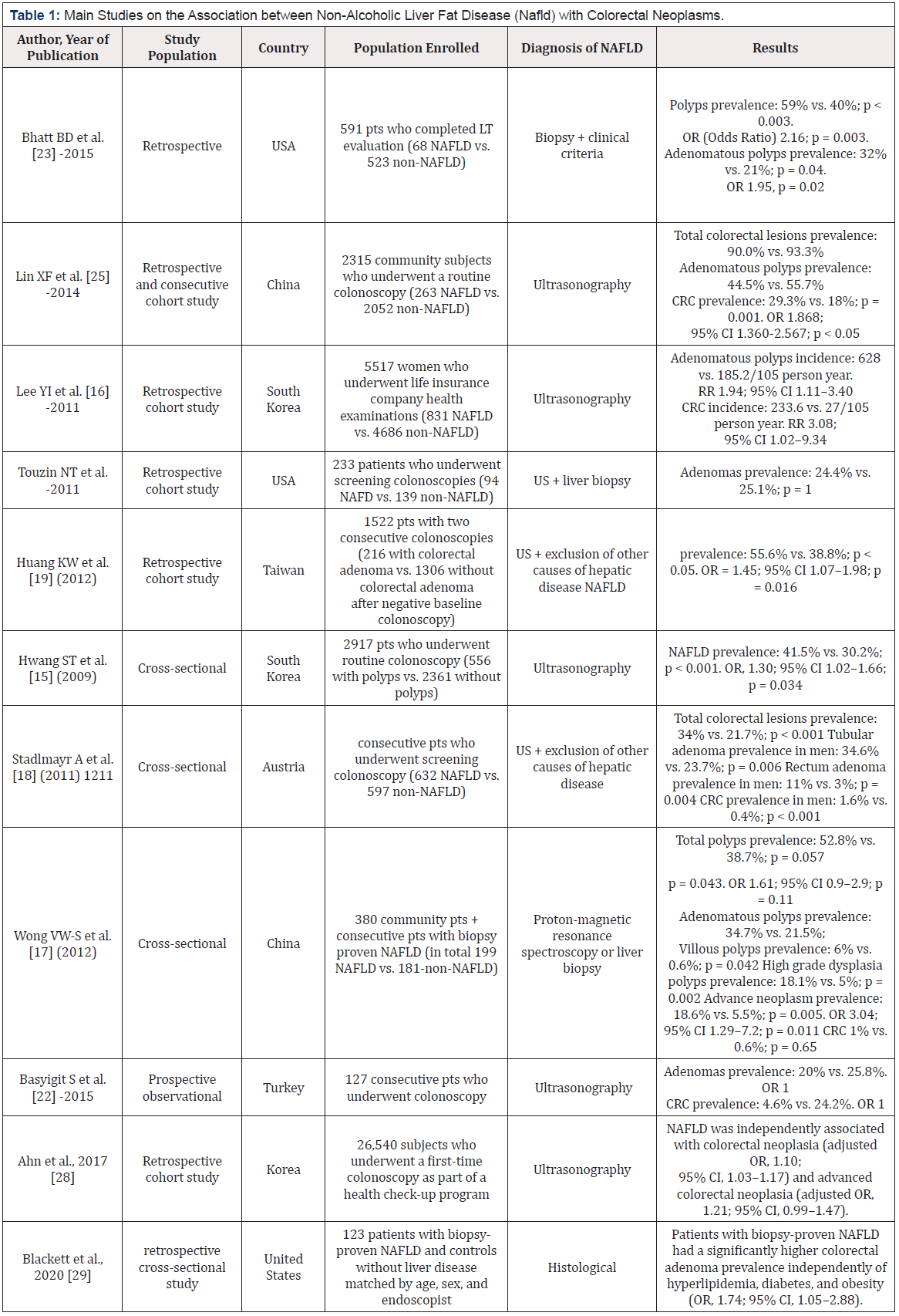
CCR is the third most widely diagnosed disease and the world’s fourth frequent cause of cancer-related deaths, responsible for about 1.4 million new cases, nearly 700,000 deaths in 2012(13). In literature, the relationship between NAFLD and CRC is the largest investigated, hence almost all studies have shown an increased prevalence of CCRs in patients with NAFLD, compared to those without (11). The first data showing the association of NAFLD with an enhanced risk of colorectal adenomatous polyps was reported by Hwang and collaborators. A population of 2917 patients was examined by colonoscopy, abdominal ultrasound, and liver tests in their study. The prevalence of NAFLD in the adenomatous polyp population was 41.5 % against 30.2 % in the control group. NAFLD was associated with a three-fold greater risk of colorectal adenomas (14). This original finding was confirmed in a large retrospective cohort study of 5,517 Korean women, which found a two-fold increase in the incidence of adenomatous polyps and a three-fold increase in CCR risk in patients with NAFLD compared to controls. The existence of NAFLD, nevertheless, did not affect the prognosis of CCR, and especially, on the recurrence of the cancer during follow-up (15). In NAFLD patients, the presence of histological lesions of NASH is a high-risk factor of for CRC. Patients with NAFLD diagnosed by both proton magnetic resonance spectroscopy and liver biopsy in a cross-sectional study had a substantially higher incidence of colorectal adenomas (34.7% vs. 21.5%) and advanced neoplasms (18.6% vs. 5.5%) than control subjects. CRC has been observed more commonly in NASH patients than in those with simple steatosis (51.0 % vs. 25.6 % and 34.7% vs. 14.0 %). NASH was associated with increased risk for both adenomas (Odds Ratio (OR) 4.89) and advanced neoplasms (OR 5.34) even after risk factors adjustment (16). Blackett et al in a retrospective cross-sectional study of 123 patients with biopsy-proven NAFLD who underwent colonoscopy and controls without liver disease matched by age, sex, and endoscopist, NAFLD had a substantially higher colorectal adenoma prevalence irrespective of hyperlipidemia, diabetes, and obesity (OR, 1.74; 95% CI, 1.05-2.88). Findings from crosssectional studies were also repeated longitudinally. In a prospective study where, coupled colonoscopies were performed in 1522 participants, Although the colonoscopy index was negative for all of them, the incidence of de novo adenoma occurrence in those with NAFLD increased by 45 % (17). A Danish prospective study assessing the general risk of cancer in hospitalized patient reported an increased risk of CRC in those with hepatic steatosis compared to the general population, but no difference was found between alcoholic and non-alcoholic fatty liver (18). Lastly, an et al in Korean study of 26,540 patients who underwent a first-time colonoscopy and ultrasonography as part of a health check-up program, NAFLD was independently associated with CRC (adjusted OR, 1.10; 95% CI, 1.03-1.17) and advanced colorectal neoplasia (adjusted OR, 1.21; 95% CI, 0.99-1.47) (19). A recent meta-analysis of observational studies revealed that NAFLD was identified to be associated with markedly elevated colorectal adenomas prevalence and cancer incidence (Odds Ratio (OR), 1.28 for prevalent adenomas and 1.56 for prevalent cancer; HR, 1.42 for incident adenomas and 3.08 for cancer) (20).
On the other hand, only two studies have failed to show an increased occurrence of colorectal adenomas in NAFLD patients compared with controls. The first was found to have a higher adenoma incidence in NAFLD patients, but the results did not meet statistical significance. The second revealed a significantly lower prevalence of CRC in NAFLD patients but an increased risk of CRC in the presence of insulin resistance, but it is well known that both increased levels of alanine aminotransferase (ALT) and ultrasound will underestimate the diagnosis of NAFLD (21,22). A wellknown risk factor for CCR is the existence of metabolic syndrome, including diabetes mellitus and obesity (23,24). Nevertheless, it is unclear if NAFLD is associated with an increased risk of CCR simply because of shared metabolic disorders or whether NAFLD itself could contribute to CCR growth. As for the current possibility, insulin resistance-induced hyperinsulinemia causes carcinogenesis by activating the process of proliferation via its effect on insulin receptors on tumor cells. Moreover, hyperinsulinemia raises insulin-like growth factor (IGF)-1 expression, which has more effective mitogenic and anti-apoptotic properties than insulin and can serve as a trigger for preneoplastic and neoplastic cell growth (12,23). Following the above possibility, adiponectin, which has anti-carcinogenic effects, has decreased blood levels in NAFLD patients. This process is due to the capacity of adiponectin to interrupt proliferation of colon cancer cells by the Amp-activated protein kinase (AMPK) and to cause a caspase dependent pathway that results in apoptosis of endothelial cells (11).
Esophageal and Gastric Cancer
Esophageal and gastric cancers are the 7th and 5th most prevalent cancers worldwide, with an estimated 572,000 and 1,000,000 cases in 2018 (25). In males, both cancer forms are more frequent than in females. The pathogenesis of esophageal cancer varies between adenocarcinoma and squamous cell carcinoma, the two major histological subtypes. Smoking and overweight are reported as risk factors for esophageal adenocarcinoma, while smoking and alcoholism are known risk factors for esophageal squamous cell carcinoma (26). Likewise, gastric cancers, known as gastric cardia and gastric non-cardia, tend to have different anatomical subsite pathologies. Smoking and obesity are recognized risk factors for gastric cardiac cancer, while the risk factors for gastric non-cardiac cancer are Helicobacter Pylori infection and smoking (27,28). The positive correlation between body mass index (BMI) and the risk of esophageal adenocarcinoma and gastric cardiac cancer has been documented in several observational studies. Turabi et al conducted a meta-analysis of 22 studies, including almost 8000 esophageal and gastric cardia adenocarcinoma cases. The overall RR was 1.71 (95% CI 1.50-1.96) for BMI between 25 and 30 and was 2.34 (95% CI 1.95-2.81) for BMI ≥ 30 kg/m2. The correlation was greater for esophageal adenocarcinoma (RR for BMI ≥ 30 kg/m (2) = 2.73, 95% CI 2.16-3.46) than for gastric cardia adenocarcinoma (RR for BMI ≥ 30 kg/m (2) = 1.93, 95% CI 1.52-2.45) (29). Hoya and al in a meta-analysis of 12 studies (8 North American, 3 European and 1 Australian) comprising 1997 esophageal adenocarcinomas cases, and 11 159 esophagogastric junction adenocarcinomas were assembled. The relationship between theme increased directly with growing BMI (P <0.001). Compared with individual’s BMI <25, BMI ≥40 was associated with both cancers (OR 4.76, 95% CI 2.96-7.66) and (OR 3.07, 95% CI 1.89-4.99) (30). Although, prospective cohort studies investigating abdominal obesity by subtype and subsite for esophageal and gastric cancer are marginal, with contrasting results (26). During 8.9 years of follow-up, Steffen et al documented 88 incident cases of esophageal adenocarcinoma EAC and 110 cases of esophageal squamous cell carcinoma ESCC. BMI, waist circumference, and waist-to-hip ratio (WHR) were correlated with EAC risk [relative risk (RR), 2.60; 95% confidence interval (95% CI), 1.23-5.51; P(trend) < 0.01; RR, 3.07; 95% CI, 1.35-6.98; P(trend) < 0.003; and RR, 2.12; 95% CI, 0.98-4.57; P(trend) < 0.004].
Conversely, BMI and waist circumference were negatively correlated to ESCC risk, whereas WHR showed no correlation with ESCC (31,32). In a Netherlands Cohort Study, Merry et al, after 13.3 years of follow-up, from 4552 sub cohort members, 133 esophageal and 163 gastric cardia adenocarcinomas were analysed. The RRs (95% CI) of esophageal adenocarcinoma were 1.40 (0.95 to 2.04) and 3.96 (2.27 to 6.88) for overweight (BMI 25.0-29.9 kg/m (2)) and obese subjects (BMI >or=30.0 kg/m (2)), respectively, related to subjects of average weight (BMI 20.0-24.9 kg/m (2)). For gastric cardia adenocarcinoma, these RRs were 1.32 (0.94 to 1.85) and 2.73 (1.56 to 4.79) (33). Associations between total and abdominal obesity with EAC and gastric adenocarcinoma among 218 854 patients in the prospective NIH-AARP cohort were studied, 253 incident EAC, 191 gastric cardia adenocarcinomas and 125 gastric non-cardia adenocarcinomas reported to the sample. Global obesity (BMI) was positively associated with EAC and gastric cardia adenocarcinoma risk (highest (≥35 kg/m (2)) vs referent (18.5-<25 kg/m (2)); HR 2.11, 95% CI 1.09 to 4.09 and HR 3.67, 95% CI 2.00 to 6.71, respectively). Waist circumference was also positively associated with EAC and gastric cardia adenocarcinoma risk (highest vs referent; HR 2.01, 95% CI 1.35 to 3.00 and HR 2.22, 95% CI 1.43 to 3.47, respectively) (34). In addition, to our findings, only one prospective study examined measurements of body fat composition that differentiate between adipose and non-adipose mass (evaluated using bioelectrical impedance) in relation to esophageal and gastric cancer risk (35). Among 41,295 people followed on average for 11.3 years, 30 cases with cancers in the gastric cardia or lower third of the esophagus and 68 cases with noncardiac gastric adenocarcinomas were ascertained via the population cancer registry. The risk of adenocarcinoma of the lower esophagus and gastric cardia was positively associated with BMI with a hazards ratio (HR) and (95% confidence interval) for people with BMI>or=30 kg/m2 compared with those<25 kg/m2, of 3.7 (1.1-12.4), an HR per 10 cm increase in waist circumference of 1.46 (1.05-2.04), and a HR per 10 kg increase on fat-free mass of 2.06 (1.15-3.69). Noncardiac gastric adenocarcinoma showed little relationship with body size. Obesity is related to upper gastrointestinal cancers by many possible biological processes. Obesity may contribute to metabolic disorders, such as higher levels of pro-inflammatory cytokines (such as tumor necrosis factor-alpha and interleukin-6), adipokines (such as glucose, insulin, and leptin), and endogenous sex steroids, which could increase the risk of cancer (26,36,37). There is significant evidence of gender differences in the distribution of body fat. Men appear to produce more visceral fat, while females in the subcutaneous depot hold more fat. In body fat distribution, sex hormones play an important role (38,39). Instead of the visceral adipose depot, estrogen facilitates the accumulation of fat in the subcutaneous depot and the reduction in estrogen levels in menopausal women is accompanied by an increase in visceral fat (39). In addition to controlling the spread of body fat, the occurrence of esophageal and gastric cancers in men relative to women can also be explained by sex hormones. Sex hormones, especially estrogens, have been suspected to protect against the development of esophageal and gastric malignancy (40,41).
Pancreatic Cancer
With more than 50,000 reported new cases in the United States in 2016, the incidence of pancreatic cancer is rising. Pancreatic cancer mortality is high, with a 5-year survival of 8%, considering the fact that most patients are diagnosed in final stages(42)the American Cancer Society estimates the numbers of new cancer cases and deaths that will occur in the United States in the current year and compiles the most recent data on cancer incidence, mortality, and survival. Incidence data were collected by the National Cancer Institute (Surveillance, Epidemiology, and End Results [SEER] Program. The World Cancer Research Fund Panel’s 2007 study notes that obesity is a major modifiable risk factor for pancreatic cancer(11). A meta-analysis recently published found a linear rise in the risk of pancreatic cancer and waist circumference, with a relative risk (RR) of 1.11 for every 10 cm (95% CI 1.05-1.18) and a waist-to-hip ratio of 1.19 (95% CI 1.09-1.31) for every 0.1 unit increase(43). Mets was reported as a neoplastic risk factor in a meta-analysis published in 2012, with an RR of 1.58 (p<0.0001) for female pancreatic cancer, likely influenced by reduced physical activity, high-calorie dense food consumption, high nutritional fat levels, low fiber intake, and oxidative stress(44). In an observational study conducted by Chang et al, NAFLD was an independent risk factor for pancreatic cancer (OR 2.63, 95% CI 1.24-5.58, p = 0.011), and patients without NAFLD had longer survival than patients with NAFLD (p = 0.005, log-rank test). Same with esophageal cancer, NAFLD, although no definitive proof is yet available, may be involved in this relationship.
Renal Cancer
Some of the elements of Mets, have been identified as risk factors in addition to smoking and alimentary habits, whose correlation with renal cancer is well recognized, and mentioned in several recommendations(45-48)treatment and follow-up”,”title-short”:”Renal cell carcinoma”,”volume”:”25 Suppl 3”,”author”:[{“family”:”Escudier”,”given”:”B.”},{“family”:”- Porta”,”given”:”C.”},{“family”:”Schmidinger”,”given”:”M.”},{“- family”:”Algaba”,”given”:”F.”},{“family”:”Patard”,”given”:”J. J.”},{“family”:”Khoo”,”given”:”V.”},{“family”:”Eisen”,”given”:”T.”},{“- family”:”Horwich”,”given”:”A.”},{“literal”:”ESMO Guidelines Working Group”}],”issued”:{“date-parts”:[[“2014”,9]]}}},{“id”:2952, ”uris”:[“http://zotero.org/users/local/bzZutjSz/items/E9APCJKF”],” uri”:[“http://zotero.org/users/local/bzZutjSz/items/ E9APCJKF”],”itemData”:{“id”:2952,”type”:”article-journal”,”abstract”:” OBJECTIVE: The multimodality approach to treating both localized and metastatic renal cell carcinoma has led to a demand for improved imaging evaluation. We review the information needed from the radiologic studies used to determine treatment strategies.\nCONCLUSION: Adequate preoperative radiologic assessment provides the treating physician with information critical in determining the sequence of treatments, role of nephron-sparing surgery, surgical approach, and timing of systemic therapy for metastatic disease.”,”container-title”:”AJR. American journal of roentgenology”,”DOI”:”10.2214/AJR.10.6249”,”ISSN”:”1546-31 41”,”issue”:”6”,”journalAbbreviation”:”AJR Am J Roentgenol”,”language”:” eng”,”note”:”PMID: 21606286”,”page”:”1255-1262”,”- source”:”PubMed”,”title”:”Renal cell carcinoma: what the surgeon and treating physician need to know”,”title-short”:”Renal cell carcinoma”,”volume”:”196”,”author”:[{“family”:”Chapin”,”- given”:”Brian F.”},{“family”:”Delacroix”,”given”:”Scott E.”},{“family”:” Wood”,”given”:”Christopher G.”}],”issued”:{“date-parts”:[[“ 2011”,6]]}}},{“id”:2954,”uris”:[“http://zotero.org/users/local/ bzZutjSz/items/7FJG3WRH”],”uri”:[“http://zotero.org/users/ local/bzZutjSz/items/7FJG3WRH”],”itemData”:{“id”:2954,”- type”:”article-journal”,”container-title”:”Annals of Oncology: Official Journal of the European Society for Medical Oncology”,” DOI”:”10.1093/annonc/mdq206”,”ISSN”:”1569-8041”,”journalAbbreviation”:” Ann Oncol”,”language”:”eng”,”note”:”PMID: 20555064”,”page”:”v137-139”,”source”:”PubMed”,”title”:”Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up”,”title-short”:”Renal cell carcinoma”,”volume”:” 21 Suppl 5”,”author”:[{“family”:”Escudier”,”given”:”B.”},{“- family”:”Kataja”,”given”:”V.”},{“literal”:”ESMO Guidelines Working Group”}],”issued”:{“date-parts”:[[“2010”,5]]}}},{“ id”:2959,”uris”:[“http://zotero.org/users/local/bzZutjSz/ items/2K5HBN5C”],”uri”:[“http://zotero.org/users/local/bz- ZutjSz/items/2K5HBN5C”],”itemData”:{“id”:2959,”type”:”article- journal”,”container-title”:”Annals of Oncology: Official Journal of the European Society for Medical Oncology”,”DOI”:”10.1093/ annonc/mdz056”,”ISSN”:”1569-8041”,”issue”:”5”,”journal- Abbreviation”:”Ann Oncol”,”language”:”eng”,”note”:”PMID: 30788497”,”page”:”706-720”,”source”:”PubMed”,”title”:”Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†”,”title-short”:”Renal cell carcinoma”,”volume”:”30”,”- author”:[{“family”:”Escudier”,”given”:”B.”},{“family”:”Porta”,”- given”:”C.”},{“family”:”Schmidinger”,”given”:”M.”},{“family”:”Rioux- Leclercq”,”given”:”N.”},{“family”:”Bex”,”given”:”A.”},{“family”:”Khoo”,” given”:”V.”},{“family”:”Grünwald”,”given”:”V.”},{“family”:”Gillessen”,” given”:”S.”},{“family”:”Horwich”,”given”:”A.”},{“literal”:”ESMO Guidelines Committee. Electronic address: clinicalguidelines@ esmo.org”}],”issued”:{“date-parts”:[[“2019”,5,1]]}}}],”schema”:” https://github.com/citation-style-language/schema/raw/ master/csl-citation.json”} . Visceral fat, measured by computed tomography (CT) scan in patients with cT1a renal cell carcinoma, is closely correlated with Fuhrman grade, the most commonly used neoplastic nuclear kidney grading model, and is an independent predictor of high-grade renal cell carcinoma (RCC)(49). Adiponectin levels are inversely related to the magnitude of the disease in a sample of 118 consecutive patients undergoing surgical procedure for RCC, with lower levels in patients with metastatic cancer(50). YH et al in a study included 706 patients with localized renal cell carcinoma who had undergone curative surgery. Visceral total adipose tissue was measured based on preoperative computerized tomography. The distribution of histological subtypes differed significantly among visceral adipose tissue percentage (VAT%) quartiles. The proportion of high-grade tumors increased as VAT% increased (OR 1.023, 95% CI 1.000-1.126, p = 0.037). A U-shaped correlation between VAT percent quartiles and the risk of disease recurrence was observed for all patients. Disease recurrence was substantially increased in the lowest (HR 3.198, 95% CI 1.765-10.040, p = 0.036) and highest (HR 4.760, 95% CI 2.937-13.210, p = 0.010) VAT% quartiles (51).
Breast Cancer
Breast cancer is the most common cancer in women and is the world’s leading cause of cancer-related death in women. Hormonal or reproductive causes are recognized risk factors for breast cancer, including early menarche age, nulliparity, late menopause and pregnancy ages (52,53). Related risk factors are identified between NAFLD and breast cancer, like metabolic disorders and obesity. NAFLD and breast cancer are both related to hyperinsulinemia, suggesting a potential conceptual correlation between the two diseases (54). However, limited studies have tested the relationship between breast cancer and NAFLD. A case-control analysis with a small sample size found that NAFLD was associated with breast cancer (55-57). In a pooled review of two case-control studies of 3869 postmenopausal women with breast cancer and 4082 cases of postmenopausal control, the authors reported a higher risk of neoplasia in women with Mets compared to those without it (OR 1.75; 95% CI 1.37-2.22). The odds ratios (ORs) of postmenopausal breast cancer were 1.33 (95% CI 1.09-1.62) for diabetes, 1.08 (95% CI 0.95-1.22) for hyperlipidemia, 1.19 (95% CI 1.07-1.33) for hypertension, 1.22 (95% CI 1.09-1.36) for waist circumference ≥88 cm and 1.26 (95% CI 1.11-1.44) for body mass index ≥30 kg/m(2). For women with Mets, the risk of postmenopausal breast cancer has been substantially increased. (OR = 1.75, 95% CI 1.37-2.22, for three or more Mets elements, P for trend for increasing number of elements < 0.0001)(58). In a study on 2092 patients treated for stage I-III invasive breast cancer, enrolled in eleven Italian centers 0-5 years after surgical treatment. The adjusted ORs for women with Mets versus women without any Mets traits were 2.17 (CI 1.31-3.60) overall, and 2.45 (CI 1.24-4.82) for distant metastasis. All Mets traits were positively associated with new events, and significantly so for low HDL and high triglycerides. Mets is an important prognostic factor (59). Additionally, one longitudinal study of 25,947 subjects, 8,721 (33.6%) had NAFLD showed that the cancer incidence rate of the NAFLD group was higher than that of the non-NAFLD group (782.9 vs. 592.8 per 100,000 personyears; hazard ratio [HR] 1.32; 95% (CI) 1.17-1.49; p <0.001). When demographic and metabolic factors were adjusted for, NAFLD showed a strong association with the breast cancer in females (HR 1.92; 95% CI 1.15-3.20; p = 0.01)(60). In a case-control study exploring the association between NAFLD and breast cancer, Kim et al reported that NAFLD was significantly associated with breast cancer (P = 0.046), and the subgroup analysis showed that NAFLD was significantly associated with breast cancer in the non-obese subgroup (odds ratio 3.04, 95% CI 1.37-4.32, P = 0.002) but not in the obese group (P = 0.163)(61).
Prostate Cancer
Prostate cancer (PCa) is the second most frequently diagnosed cancer in men worldwide and the sixth leading cause of cancerrelated mortality (62). The association between NAFLD and PC a has recently been suggested by increasing PCa and NAFLD incidences, suggesting that NAFLD is an important risk factor for PC a (63), but it remains controversial. In a systematic review and meta-regression analysis, including 31 cohort and 25 casecontrol studies, authors reported a 1.05 relative risk (95 % CI 1.01- 1.08), increased in patients with advanced diseases than localized diseases, for every 5 kg/m2 increase in BMI (64). The role of NALFD was systematically analyzed by two studies. NAFLD was reported to protect against neoplastic relapse in 293 consecutive patients following radical prostatectomy for prostate cancer (65). Compared to participants without NAFLD in both the training and validation sample, the NAFLD community reported slightly longer time-torecurrence (hazard ratio: 0.33 and 0.22; 95% CI 0.16-0.69 and 95% CI 0.11-0.43, respectively). In 1600 US-defined NAFLD patients and 1600 matched hepatitis C virus (HCV) infected subjects, the second one analyzed the development of cancers and the location of the illness: prostate cancer occurred in 12.6% of NAFLD compared to 3.5% in HCV patients, and the incidence of prostate malignance in NAFLD was higher than in the general population (11).
The Presumed Role of Insulin Resistance and Gut Microbiota in NAFLD in Extra-Hepatic Cancers Development
Even though data on the pro-inflammatory and procarcinogenic implications of insulin resistance (IR) are generally the most substantial evidence of a potential mechanistic between NAFLD and extra-hepatic oncogenesis, gut microbiota has recently been described as a new and fascinating striker in the development of obesity, NAFLD and many types of cancer. Dysbiosis is described in NAFLD patients, and the liver remains at the intersection of a complex relationship between modifications of microbiota, IR, inflammation, and carcinogenesis (66-69). Patients with colon cancer have been shown to have dysbiosis (70). Qualitative and quantitative changes of gut microbiota, through several pathways, contribute to increased intestinal permeability, including the control of tight junctions such as zonulin-1 and the occlusion in the ileum of toll-like receptor 2 (TLR2) (71-73). It is well established that the host diet has a profound effect on the microbial composition of the gut. The myeloid differentiation factor 88 (MyD88)- dependent pathway can mediate diet-induced NAFLD (74). This factor is a converter molecule that is important for TLR signaling. It is mobilized after interaction between microorganism-associated molecular patterns (MAMPs) and TLRs (particularly TLR4) and stimulates the transcription by activation of NF-B or c-Jun NH2- terminal kinase (JNK) contributing to IR initiation of many proinflammatory cytokines. Failure of mutation or knockout mice in TLR4 prevent obesity-induced IRs that underlie the essential role of this receptor in the regulation of the innate immune system (74). The key element of the axis of central obesity contains NAFLD and visceral adipose tissue. Low-grade chronic inflammation and insulin resistance (IR) establish a microenvironment appropriate for the cancer development in this environment by stimulating the insulin growth factor-1 (IGF-1) axis via hyperinsulinemia (75-77). Extra hepatic cancers have been associated with enhanced serum levels of IGF-1. Pertinently, the risk of Barrett’s esophagus and esophageal adenocarcinoma may be affected by the insulin/IGF system (78- 84), but this is not entirely agreed upon (85). In carcinogenic processes, multiple adipokines, implicated in the control of metabolism, inflammation and fibrogenesis, may also be involved. Adiponectin has anti-carcinogenic effects mediated by its power to stop the growth of colon cancer cells via the protein kinase (AMPK) triggered by AMPc and to induce a caspase-dependent pathway that results in apoptosis of endothelial cells. Tumor necrosis factor (TNF-), implicated in tumor cell proliferation and angiogenesis, may also be directly inhibited by Adiponectin. Given that NAFLD patients have reduced serum adiponectin levels, the pathways mentioned above reflect an important correlation between NAFLD and the development of disease at both the digestive and extraintestinal sites.
The pro-carcinogenic implications of leptin have been thoroughly explored, particularly in the presence of low rates of adiponectin (86,87). Leptin can promote motility and intrusiveness in human colon cancer cells through mitogen-activated protein kinase (MAPK) process induction. A case-cohort analysis in postmenopausal CRC women found that elevated plasma leptin levels were associated with an increased risk of CRC (88,89). The combination of high leptin and low adiponectin rates can also raise the risk of Barrett’s esophagus and esophageal adenocarcinoma in obese patients by increasing cell proliferation and decreasing apoptosis through extracellular signal-regulated kinase (90- 93). After that, resisting could also be related to malignancies associated with obesity by triggering the nuclear factor-B (NF-B) pathway and amplifying the procardiogenic actions of interleukin (IL)-1, IL-6 and TNF-ἀ, respectively. To present, in breast cancer, non-small cell lung cancer and in digestive tumors, a presumed role of resisting has been reported (94-96). IR-associated low-grade chronic inflammation also promotes the activation of macrophages and the large secretion into the systemic circulation of many proinflammatory cytokines, such as IL-6 and TNFἀ. Animal models have shown a correlation between TNF ἀ and various malignancies, like CCR (97-99). IL-6 has been related to carcinoma of renal cells, gastric cancer and CCR. By modulating many genes involved in proliferation, survival, and angiogenesis, cancer (100-105).
Conclusion
NAFLD is a complex and multifactorial disorder that is closely linked to obesity and type 2 diabetes and shares a substantially elevated risk of several cancer forms. Further than HCC risk, obviously induced by NASH, there is a clear epidemiological and biological argument, with the strongest support for colorectal tumors, for the correlation between NAFLD and certain extrahepatic cancers. However, before clear screening guidelines for cancer in NAFLD patients can be given, further studies are required, but we advise health care professionals who care for patients with NAFLD to be cautious about any signs and symptoms of malignancies, especially CCR, and to refer patients for further evaluation and management.
References
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L et al. (2016) Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes: hepatology 64(1): 73-84.
- Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R et al. (2012) Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol 56(1): 234-240.
- Kansra AR, Lakkunarajah S, Jay MS (2020) Childhood and Adolescent Obesity: A Review. Front Pediatr 8: 581461.
- Qing Ye, Biyao Zou, Yee Hui Yeo, Jie Li, Daniel Q Huang et al. (2020) Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 5(8): 739-752.
- Diehl AM, Day C (2017) Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med 377(21): 2063-2072.
- Musso G, Gambino R, Cassader M, Pagano G (2011) Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 43(8): 617-649.
- Rafiq N, Bai C, Fang Y, Srishord M, McCullough A et al. (2009) Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol 7(2): 234-238.
- Angulo P (2010) Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology 51(2):373-375.
- Shou Sheng Liu, Xue Feng Ma, Jie Zhao, Shui Xian Du, Jie Zhang et al. (2020) Association between nonalcoholic fatty liver disease and extrahepatic cancers: a systematic review and meta-analysis. Lipids Health 19(1): 118.
- Vanni E, Marengo A, Mezzabotta L, Bugianesi E (2015) Systemic Complications of Nonalcoholic Fatty Liver Disease: When the Liver Is Not an Innocent Bystander. Semin Liver Dis 35(3): 236-249.
- Sanna C, Rosso C, Marietti M, Bugianesi E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int J Mol Sci 17(5): 717.
- Tomeno W, Imajo K, Takayanagi T, Ebisawa Y, Seita K et al. (2020) Complications of Non-Alcoholic Fatty Liver Disease in Extrahepatic Organs. Diagnostics 10(11): 912.
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A et al. (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4): 683-691.
- Sang Tae Hwang, Yong Kyun Cho, Jung Ho Park, Hong Joo Kim, Dong Il Park et al. (2010) Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J Gastroenterol Hepatol 25(3): 562-567.
- Lee YI, Lim YS, Park HS (2012) Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol 27(1): 91-95.
- Vincent Wai Sun Wong, Grace Lai-Hung Wong, Steven Woon-Choy Tsang, Tina Fan, Winnie Chiu-Wing Chu et al. (2011) High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut 60(6):829-8 36.
- Huang KW, Leu HB, Wang YJ, Luo JC, Lin HC et al. (2013) Patients with nonalcoholic fatty liver disease have higher risk of colorectal adenoma after negative baseline colonoscopy. Colorectal Dis 15(7): 830-835.
- Sorensen HT, Mellemkjaer L, Jepsen P, Thulstrup AM, Baron J et al. (2003) Risk of cancer in patients hospitalized with fatty liver: a Danish cohort study. J Clin Gastroenterol 36(4): 356-359.
- Ahn JS, Sinn DH, Min YW, Hong SN, Kim HS et al. (2017) Non-alcoholic fatty liver diseases and risk of colorectal neoplasia. Aliment Pharmacol Ther 45(2): 345-353.
- Mantovani A, Nascimbeni F, Lonardo A, Zoppini G, Bonora E et al. (2018) Association Between Primary Hypothyroidism and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Thyroid 28(10):1270-1284.
- Touzin NT, Bush KNV, Williams CD, Harrison SA (2011) Prevalence of colonic adenomas in patients with nonalcoholic fatty liver disease. Therap Adv Gastroenterol 4(3):169-176.
- Basyigit S, Uzman M, Kefeli A, Sapmaz FP, Yeniova AO et al. (2015) Absence of non-alcoholic fatty liver disease in the presence of insulin resistance is a strong predictor for colorectal carcinoma. Int J Clin Exp Med 8(10):18601-18610.
- Sasazuki S, Charvat H, Hara A, Wakai K, Nagata C et al. (2013) Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci 104(11): 1499-1507.
- Moghaddam AA, Woodward M, Huxley R (2007) Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 16(12): 2533-2547.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6): 394-424.
- Sanikini H, Muller DC, Chadeau-Hyam M, Murphy N, Gunter MJ et al. (2020) Anthropometry, body fat composition and reproductive factors and risk of oesophageal and gastric cancer by subtype and subsite in the UK Biobank cohort. PLoS One 15(10): e0240413.
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F (2014) Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 23(5): 700-713.
- Thrift AP, El Serag HB (2020) Burden of Gastric Cancer. Clin Gastroenterol Hepatol 18(3): 534-542.
- Turati F, Tramacere I, La Vecchia C, Negri E (2013) A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 24(3): 609-617.
- Hoyo C, Cook MB, Kamangar F, Freedman ND, Whiteman DC et al. (2012) Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol 41(6): 1706-1718.
- Steffen A, Schulze MB, Pischon T, Dietrich T, Molina E et al. (2009) Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 18(7): 2079-2089.
- Steffen A, Huerta JM, Weiderpass E, Buenode Mesquita HBA, May AM et al. (2015) General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 137(3): 646-657.
- Merry AHH, Schouten LJ, Goldbohm RA, van den Brandt PA (2007) Body mass index, height, and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut 56(11): 1503-1511.
- O’Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC (2012) A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut 61(9): 1261-1268.
- MacInnis RJ, English DR, Hopper JL, Giles GG (2006) Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer 118(10): 2628-2631.
- Murphy N, Jenab M, Gunter MJ (2018) Adiposity and gastrointestinal cancers: epidemiology, mechanisms, and future directions. Nat Rev Gastroenterol Hepatol 15(11): 659-670.
- Karczewski J, Begier Krasińska B, Staszewski R, Popławska E, Gulczynska Elhadi K et al. (2019) Obesity, and the Risk of Gastrointestinal Cancers. Dig Dis Sci 64(10): 2740-2749.
- Palmer BF, Clegg DJ (2015) The sexual dimorphism of obesity. Mol Cell Endocrinol 402: 113-119.
- Aaron P Frank, Roberta de Souza Santos, Biff F Palmer, Deborah J Clegg (2019) Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res 60(10): 1710-1719.
- Chandanos E, Lagergren J (2008) Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer 44(16): 2397-2403.
- Chandanos E, Lagergren J. The mystery of male dominance in oesophageal cancer and the potential protective role of oestrogen. Eur J Cancer. 2009 Dec;45(18):3149-3155.
- Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA: A Cancer Journal for Clinicians 66(1): 7-30.
- Aune D, Greenwood DC, Chan DSM, Vieira R, Vieira AR et al. (2012) Body mass index, abdominal fatness, and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol 23(4): 843-852.
- Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D (2012) Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 35(11): 2402-2411.
- Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ et al. (2014) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol 30(5): 706-720.
- Chapin BF, Delacroix SE, Wood CG (2011) Renal cell carcinoma: what the surgeon and treating physician need to know. AJR Am J Roentgenol 196(6): 1255-1262.
- B Escudier, C Porta, M Schmidinger, N Rioux-Leclercq, A Bex et.al (2019) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol 30(5): 706-720.
- Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A et al. (2019) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up†. Ann Oncol 30(5): 706-720.
- Yao Zhu, Hong Kai Wang, Hai Liang Zhang, Xu Dong Yao, Shi Lin Zhang, et al. (2012) Visceral obesity, and risk of high-grade disease in clinical t1a renal cell carcinoma. J Urol 189(2): 447-453.
- Horiguchi A, Ito K, Sumitomo M, Kimura F, Asano T et.al (2008) Decreased serum adiponectin levels in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 38(2): 106-111.
- Yong Hyun Park, Jeong Keun Lee, Kwang Mo Kim, Ha Rim Kook, Hansol Lee et al. (2014) Visceral obesity in predicting oncologic outcomes of localized renal cell carcinoma. J Urol 192(4): 1043-1049.
- Suh JS, Yoo KY, Kwon OJ, Yun IJ, Han SH et al. (1996) Menstrual and reproductive factors related to the risk of breast cancer in Korea. Ovarian hormone effect on breast cancer. J Korean Med Sci 11(6): 501-508.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C et al. (2015) Cancer incidence and mortality worldwide: sources, methods, and major patterns in GLOBOCAN 2012. Int J Cancer 136(5): E359-386
- Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA (2015) Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350: g7607.
- Nseir W, Abu Rahmeh Z, Tsipis A, Mograbi J, Mahamid M (2017) Relationship between Non-Alcoholic Fatty Liver Disease and Breast Cancer. Isr Med Assoc J 19(4): 242-245.
- Bodai BI, Nakata TE (2020) Breast Cancer: Lifestyle, the Human Gut Microbiota/Microbiome, and Survivorship. Perm J 24.
- Lee YS, Lee HS, Chang SW, Lee CU, Kim JS et al. (2019) Underlying nonalcoholic fatty liver disease is a significant factor for breast cancer recurrence after curative surgery. Medicine (Baltimore) 98(39): e17277.
- Rosato V, Bosetti C, Talamini R, Levi F, Montella M et al. (2011) Metabolic syndrome, and the risk of breast cancer in postmenopausal women. Ann Oncol 22(12): 2687-2692.
- Berrino F, Villarini A, Traina A, Bonanni B, Panico S, Mano MP, et al. (2014) Metabolic syndrome, and breast cancer prognosis. Breast Cancer Res Treat 147(1): 159-165.
- Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ et al. (2017) Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol S0168-8278(17): 32294-32298.
- Min Sun Kwak, Jeong Yoon Yim, Ann Yi, Goh Eun Chung, Jong In Yang et al. (2019) Nonalcoholic fatty liver disease is associated with breast cancer in nonobese women. Dig Liver Dis 51(7): 1030-1035.
- Melissa M Center, Ahmedin Jemal, Joannie Lortet Tieulent, Elizabeth Ward, Jacques Ferlay et al. (2012) International variation in prostate cancer incidence and mortality rates. Eur Urol 61(6): 1079-1092.
- Hsing AW, Devesa SS (2001) Trends and patterns of prostate cancer: what do they suggest? Epidemiol Rev 23(1): 3-13.
- MacInnis RJ, English DR (2006) Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control 17(8): 989-1003.
- Won Mook Choi, Jeong Hoon Lee, Jung Hwan Yoon, Cheol Kwak, Young Ju Lee et al. (2014) Nonalcoholic fatty liver disease is a negative risk factor for prostate cancer recurrence. Endocr Relat Cancer 21(2): 343-353.
- Ohtani N, Yoshimoto S, Hara E (2014) Obesity, and cancer: a gut microbial connection. Cancer Res 74(7): 1885-1889.
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S et al. (2013) Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499(7456): 97-101.
- Hara E (2015) Relationship between Obesity, Gut Microbiome and Hepatocellular Carcinoma Development. Dig Dis 33(3): 346-350.
- Cani PD, Delzenne NM, Amar J, Burcelin R (2008) Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol Biol (Paris) 56(5): 305-309.
- Moran CP, Shanahan F (2014) Gut microbiota and obesity: role in aetiology and potential therapeutic target. Best Pract Res Clin Gastroenterol 28(4): 585-597.
- Mima K, Ogino S, Nakagawa S, Sawayama H, Kinoshita K et al. (2017) The role of intestinal bacteria in the development and progression of gastrointestinal tract neoplasms. Surg Oncol 26(4): 368-376.
- Louis P, Hold GL, Flint HJ (2014) The gut microbiota, bacterial metabolites, and colorectal cancer. Nat Rev Microbiol 12(10): 661-672.
- Keku TO, Dulal S, Deveaux A, Jovov B, Han X (2015) The gastrointestinal microbiota and colorectal cancer. Am J Physiol Gastrointest Liver Physiol 308(5): G351-363.
- Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC et al. (2009) Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 50(4): 1094-1104.
- Strong AL, Burow ME, Gimble JM, Bunnell BA (2015) Concise review: The obesity cancer paradigm: exploration of the interactions and crosstalk with adipose stem cells. Stem Cells 33(2): 318-326.
- Gilbert CA, Slingerland JM (2013) Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med 64: 45-57.
- Himbert C, Delphan M, Scherer D, Bowers LW, Hursting S et al. (2017) Signals from the Adipose Microenvironment, and the Obesity-Cancer Link - A Systematic Review. Cancer Prev Res (Phila) 10(9): 494-506.
- Grimberg A, Cohen P (2000) Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol 183(1): 1-9.
- Giovannucci E, Pollak MN, Platz EA, Willett WC, Stampfer MJ et al. (2000) A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev 9(4): 345-349.
- Schernhammer ES, Holly JM, Hunter DJ, Pollak MN, Hankinson SE (2006) Insulin-like growth factor-I, its binding proteins (IGFBP-1 and IGFBP-3), and growth hormone and breast cancer risk in The Nurses Health Study II. Endocr Relat Cancer 13(2): 583-592.
- Renehan AG, Painter JE, Atkin WS, Potten CS, Shalet SM et al. (2001) High-risk colorectal adenomas and serum insulin-like growth factors. BJS (British Journal of Surgery) 88(1): 107-113.
- Yu H, Spitz MR, Mistry J, Gu J, Hong WK (1999) Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst 91(2): 151-156.
- Yuanfeng Gong, Bingyi Zhang, Yadi Liao, Yunqiang Tang, Cong Mai et al. (2017) Serum Insulin-Like Growth Factor Axis, and the Risk of Pancreatic Cancer: Systematic Review and Meta-Analysis. Nutrients 9(4): 394.
- Harrison S, Lennon R, Holly J, Higgins JPT, Gardner M et al. (2017) Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? A systematic review and meta-analysis. Cancer Causes Control 28(6): 497-528.
- Sh S, Tl V, Jn L, R F, S L, et al. (2007) Longitudinal study of insulin-like growth factor, insulin-like growth factor binding protein-3, and their polymorphisms: risk of neoplastic progression in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev 16(11): 2387-2395.
- Uddin S, Hussain AR, Khan OS, Al Kuraya KS (2014) Role of dysregulated expression of leptin and leptin receptors in colorectal carcinogenesis. Tumor Biol 35(2): 871-879.
- Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M et al. (2011) Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 60(10): 1363-1371.
- Gloria Y F Ho, Tao Wang, Marc J Gunte, Howard D Strickler, Mary Cushman et al. (2012) Adipokines Linking Obesity with Colorectal Cancer Risk in Postmenopausal Women. Cancer Res 72(12): 3029-3037.
- Joshi RK, Kim WJ, Lee SA (2014) Association between obesity-related adipokines and colorectal cancer: a case-control study and meta-analysis. World J Gastroenterol 20(24): 7941-7949.
- Rubenstein JH, Morgenstern H, McConell D, Scheiman JM, Schoenfeld P et al. (2013) Associations of diabetes mellitus, insulin, leptin, and ghrelin with gastroesophageal reflux and Barrett’s esophagus. Gastroenterology 145(6): 1237-1244.e1-5.
- Thomas SJ, Almers L, Schneider J, Graham JE, Havel PJ (2016) Ghrelin and Leptin Have a Complex Relationship with Risk of Barrett’s Esophagus. Dig Dis Sci 61(1): 70-79.
- Chang ML, Yang Z, Yang SS (2020) Roles of Adipokines in Digestive Diseases: Markers of Inflammation, Metabolic Alteration and Disease Progression. Int J Mol Sci 21(21): 8308.
- Almers LM, Graham JE, Havel PJ, Corley DA (2015) Adiponectin May Modify the Risk of Barrett’s Esophagus in Patients With Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol 13(13): 2256-2264.e1-3.
- Tiaka EK, Manolakis AC, Kapsoritakis AN, Potamianos SP (2011) The implication of adiponectin and resisting in gastrointestinal diseases. Cytokine Growth Factor Rev 22(2): 109-119.
- Karmiris K, Koutroubakis IE, Kouroumalis EA (2008) Leptin, adiponectin, resistin, and ghrelin--implications for inflammatory bowel disease. Mol Nutr Food Res 52(8): 855-866.
- Lago F, Dieguez C, Gómez Reino J, Gualillo O (2007) Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol 3(12): 716-724.
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S et al. (2004) NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 431(7007): 461-466.
- Balkwill F (2009) Tumour necrosis factor and cancer. Nat Rev Cancer 9(5): 361-371.
- Balkwill F (2006) TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev 25(3): 409-416.
- Angelo LS, Talpaz M, Kurzrock R (2002) Autocrine interleukin-6 production in renal cell carcinoma: evidence for the involvement of p53. Cancer Res 62(3): 932-940.
- Hirohisa Kai, Yasuhiko Kitadai, Michiyo Kodama, Songde Cho, Tsuyoshi Kuroda et al. (2005) Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res 25(2A): 709-713.
- Lin WW, Karin M (2007) A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 117(5): 1175-1183.
- Alberto Mantovani, Paola Allavena, Antonio Sica, Frances Balkwill (2008) Cancer-related inflammation. J Clin Immunol 454(7203): 436-444.
- Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255): 539-545.
- Ancrile B, Lim KH, Counter CM (2007) Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev 21(14): 1714-1719.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.