Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
One-Pot Efficient Synthesis of 1,4-Bis (2,2′:6′,2′′-Terpyridin-4′-Yl) Benzene Ditopic Bridging Ligand
*Corresponding author: Ramin Zibaseresht, Department of Chemistry and Physics, Faculty of Sciences, Maritime University of Imam Khomeini, Noshahr, Iran; Biomaterials and Medicinal Chemistry Research Centre, Aja University of Medical Sciences, Tehran, Iran.
Received: August 24, 2021; Published: August 30, 2021
DOI: 10.34297/AJBSR.2021.14.001947
Abstract
The ditopic bridging 1,4-bis(2,2′:6′,2′′-terpyridin-4′-yl) benzene (I) was prepared in a controlled one-pot condensation reaction of acetylpyridine, benzene-1,4-dicarbaldehyde and ammonium acetate in basic medium in EtOH in high yield. The product was characterised using conventional methods.
Keywords: 2,2′:6′,2′′-terpyridine, Symmetrical bridging ligands, Synthesis
Introduction
In the line of part of our studies, we conducted research projects involving preparation of poly-pyridine ditopic bridging ligands for the purpose of preparation of heterodinuclear Ru (II)-Co (III) complexes [1-6] In our studies, we have been interested in the preparation of bridging ligands in which at least one binding site was based on 2,2’:6’,2”-terpyridine ligand binding domain.
2,2’:6’,2”-terpyridine ligand was appealed at one end of the bridging ligands, because of its geometry for being planar in addition to its tridentate binding site which can bind to a transition metal ion such as ruthenium ion [7-21]. In our previous studies, we have reported preparation of terpyridine types of bridging ligands with two different binding sites, [1,4&5] because planar terpyridine binding domain ensures a meridional arrangement of the nitrogen donor atoms which leads to the reduction of the number of possible isomers when an octahedral metal coordination forms at this site. Additionally, the different binding sites within the ligands provide coordination discrimination in which different metal ions can be selectively coordinated to the appropriate site.
In this study, we took the opportunity for the preparation of symmetrical terpyridine bridging ligands for the purpose of our investigation to accomplish comparison studies with which nonsymmetrical ligands were studied. To conduct our investigation, we have re-visited the preparation of a symmetrical ligand 1,4-bis(2,2′:6′,2′′-terpyridin-4′-yl) benzene (I) which was reported earlier by Constable and his group [22]. The ligand (I) is well known in the literature and available commercially. X-ray structure of the ligand was also reported [23] (I) figure.
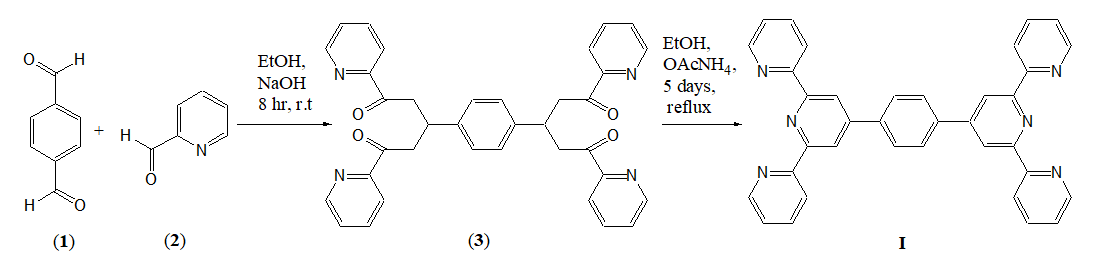
Constable, et al. reported the preparation of the ligand (I) (Scheme 1) which involved a two-step reaction of benzene-1,4- dicarbaldehyde (1) with 4.5 equivalents of 2-acetylpyridine (2) results in the formation of a tetraketone 1,4-bis[1,5-dioxo-1,5-bis(2- pyridyZ)pentan-3-yl]benzen (3) in 64% yield and the reaction of (3) with ammonium acetate in 5 days to obtain the ligand in 39% yield (Scheme 1).
Following the procedure reported by Constable, et al. [22] we found that, although the tetraketone (3) is isolable and characterisable, without separation and isolation of the compound (3), by addition of ammonium acetate to the reaction mixture and refluxing for 72 hours, the target ligand (I) can be obtained in 84% overall yield (Scheme 2).
Scheme 2: One-pot synthesis of ligand 1,4-bis(2,2′:6′,2′′-terpyridin-4′-yl) benzene (I) (this study).
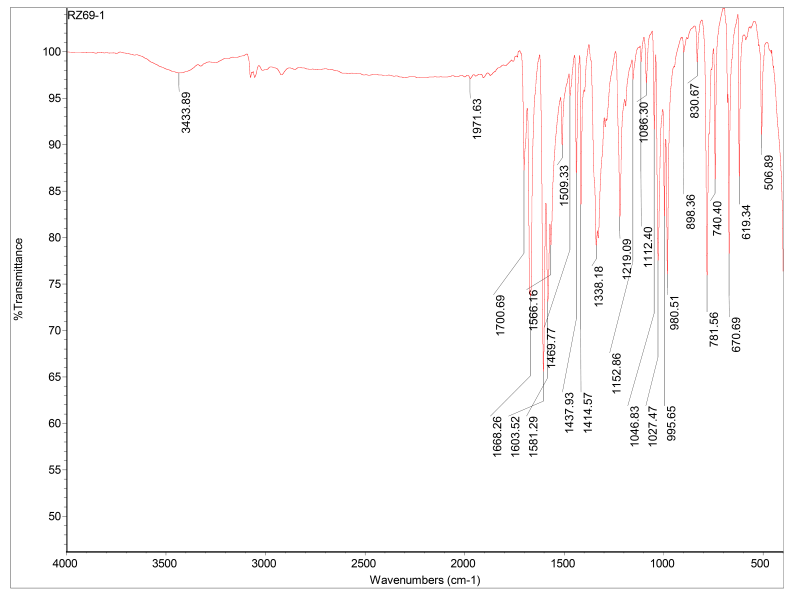
To monitor the reaction progression, after about 8 hours of the reaction of (1) and (2) in basic EtOH at r.t, required amount of the white precipitate which was formed was taken, washed and dried. Characterisation of the isolated tetraketon (3) using NMR was not achieved due to poor solubility of the compound and presumably because of possible keto-enol tautomerisation on the NMR time scale which was also observed by Constable et.al in their previous studies [22, 24]. However, characterisation of compound (3) in solid state was performed using FTIR spectrophotometry and elemental analysis. The FTIR spectrum of (3) in Figure 1 shows a peak at 1700.69 cm-1 which can be assigned for υC=O stretching frequency as a characteristic of the non-conjugated carbonyl group. These observations were consistent to those reported earlier [22] The hydroxyl stretching mode, υO-H 3433.89 cm-1 is assigned for OH group in a possible occurrence of keto-enol tautomerism (Figure 1).
Colour change was observed upon addition of ammonium acetate to the reaction mixture. The mixture was allowed to reflux for 72 hours during which time an off-white solid was precipitated in a dark solution. The precipitate was separated by filtration, washed with EtOH and air-dried to afford ligand (I) in 84% yield. The product which was obtained was fully characterised using NMR, Mass, IR and elemental analysis (Figures 2-4). All data were consistent to those reported by Constable and his research group [22] (Figure 2-4).
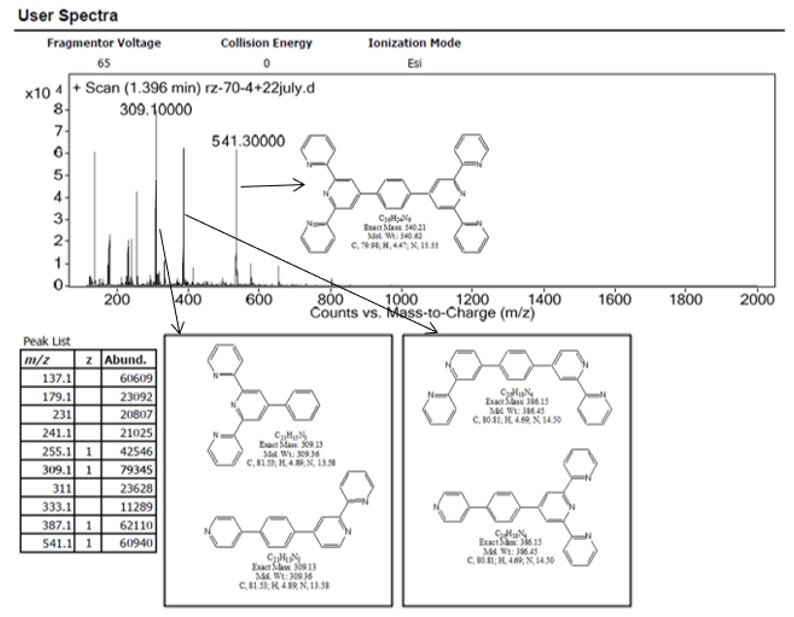
Figure 4: The LC-MS spectrum of (I): m/z 541.3 ([M+H]+), 387.1 ([(M-2py+H)]+), and 309.1 ([(M-3py+H)]+).
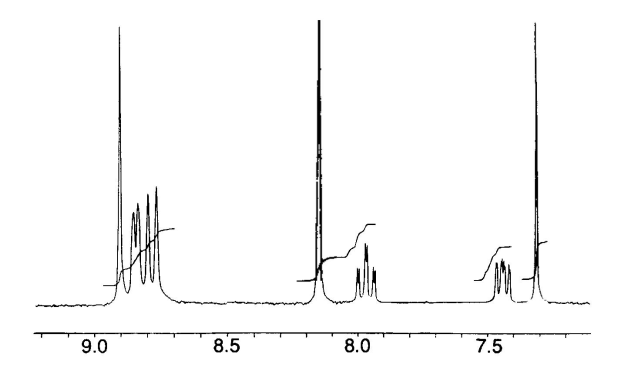
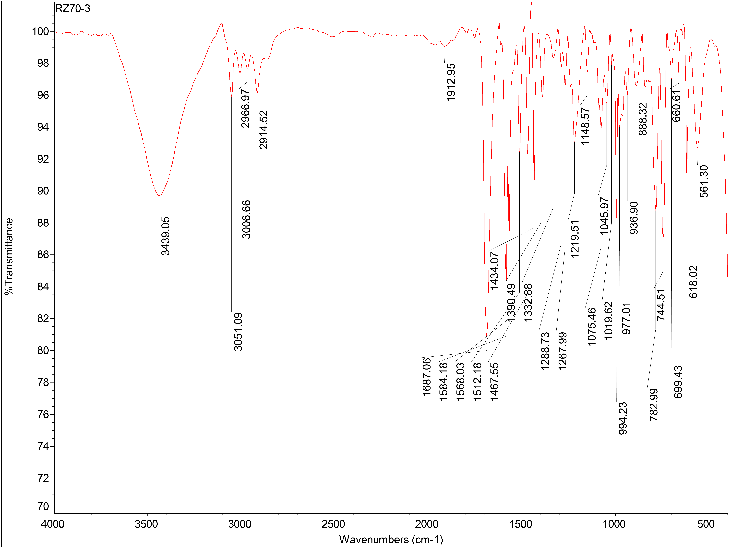
The 1H NMR spectrum corresponding to the ligand (I) (Figure 2) was consistent to the data reported earlier. The singlet peak at σ 8.86 is a characteristic signal associated to the four equivalent of single H3′ in pyridine rings in the middle of terpyridyl domains (2-pyridyl) and the singlet peak at σ 8.08 assigned to the four equivalent protons of the benzene ring in the middle. The FTIR spectrum of ligand (I) (Figure 3) shows peaks at 1687 cm-1 and 1568 cm-1 assigned for aromatic C=N double bond stretching frequency in pyridine rings. The broad peak at 3439 cm-1 also assigned for the presence of EtOH which is consistent with the elemental analysis of the compound. LC-MS spectrum of the ligand (I) (Figure 4) also shows three significant peaks at m/z 541.3 ([M+H]+), 387.1 ([(M-2py+H)]+), and 309.1 ([(M-3py+H)]+).
Experimental
Reagent grade commercial compounds were used as starting materials, and their purity was checked by 1H and 13C NMR. The 400 MHz 1H NMR spectra were acquired on a Bruker-400 spectrometer. 1H NMR chemical shifts are reported relative to tetramethylsilane. Infrared spectra (400-4000 cm-1) were obtained using a Shimadzu 8201PC Series FTIR interfaced with an Intel 486 PC operating Shimadzu HyperIR software. Spectra were obtained using diffuse reflectance method in solid KBr. GC-MS, LC-MS and Microanalyses were performed at Shahid Beheshti University of Medical Sciences. Melting points of organic compounds were determined using Electrothermal IA 9200 with microprocessor heating oven control melting point apparatus without corrections.
Synthesis of 1,4-bis(2,2′:6′,2′′-terpyridin-4′-yl)benzene (I): To a 500 mL double-necked round bottom flask containing benzene- 1,4-dicarbaldehyde (2.68 g, 20 mmol) in 200 mL EtOH was added 2-ccetylpyridine (10.0 mL, 90 mmol) dropwide with stirring at r.t. The reaction mixture was allowed to stir for about 8 hrs. during which a white precipitate was formed in orange solution. The small amount of the precipitate was taken for characterisation. The white compound which was taken from bulk mixture was washed thoroughly with EtOH and dried for FTIR and elemental analyses. The compound was characterised as tetraketone 1,4-bis[1,5- dioxo-1,5-bis(2-pyridyZ) pentan-3-yl]benzen (3). Anal. Calc. for C36H30N4O4 (582.23): C 74.21, H 5.19, N 9.62%; found: C 74.09, H 5.32, N 9.74%. IR spectrum, υ, cm-1: 3433.89 (O-H), 1700.69. To the reaction mixture was added ammonium acetate (5.0 g) over a period of 10 min. The reaction mixture was connected to reflux and allowed to stir at refluxing temperature for 72 hrs. The mixture was cooled to r.t and the precipitate was collected by filtration then washed with EtOH and dried to obtain the desired ligand as an off-white solid (84%, 9.07 g, 16.79 mmol). Mp > 200 oC. 1H NMR (CDCl3) δ 8.86 (s, H3′), 8.79 (d), 8.73 (d), 8.11 (s, benzene ring), 7.94 (dd), 7.41 (dd). MS: m/z 541.3 ([M+H]+), 387.1 ([(M-2py+H)]+), 309.1 ([(M-3py+H)]+). Anal. Calc. for C36H24N6•C2H5OH (586.25): C 77.79, H 5.15, N 14.32%; found: C 77.61, H 5.29, N 14.51%. IR spectrum, υ, cm-1: 3439 (presence of molecular EtOH), 1687 cm-1 and 1568 cm-1 (C=N).
Acknowledgments
The authors appreciate Maritme University of Imam Knomeini and Aja University of Medical Sciences for their support to this work.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Zibaseresht R, Hartshorn R M (2005) Coordination chemistry of a terpyridine-tris(pyrazolyl) ditopic ligand. Dalton Trans (24): 3898-3908.
- Zibaseresht R, Hartshorn R M (2005) The Nickel (II)/2,4,6-Tris(2-pyridyl)-1,3,5-triazine System: Synthesis and Crystallographic Characterization of a Series of Complexes. Aust J Chem 58(5): 345-353.
- Zibaseresht R, Downward A M, Hartshorn R M (2010) Coordination Chemistry of Some Terpyridyl-Polyamine Bridging Ligands. Aust J Chem 63(4): 669-679.
- Zibaseresht R (2019) Synthesis and characterization of a new ditopic bipyridine-terpyridine bridging ligand using a Suzuki cross-coupling reaction. Arkivoc vi: 277-287.
- Zibaseresht R (2020) Efficient synthesis of a new ditopic bipyridine–terpyridine bridging ligand. Synth Commun 2020, 50(6): 904-915.
- Zibaseresht R (2006) PhD Thesis, University of Canterbury, New Zealand.
- Hjelm J, Handel RW, Hagfeldt A, Constable EC, Housecroft CE, Forster RJ (2005) Inorg Chem 44: 1073–1081.
- Benniston AC, Grosshenny V, Harriman A, Ziessel R (2004) Dalton Trans 1227–1232.
- Baranoff E, Collin JP, Flamigni L, Sauvage JP (2004) Chem Soc Rev 33: 147–155.
- Amini A, Harriman A, Mayeux A (2004) Phys Chem 6: 1157–1164.
- Schubert US, Hofmeier H, Macromol (2002) Rapid Commun. 23: 561–566.
- Gohy JF, Lohmeijer BGG, Schubert US (2002) Macromolecules 35: 4560–4563.
- Schubert US, Eschbaumer C, Andres P, Hofmeier H, Weidl C H, Herdtweck E, Dulkeith E, Morteani A, Hecker NE, Feldmann J (2001) Synth Met 121: 1249–1252.
- Laurent F, Plantalech E, Donnadieu B, Jimenez A, Hernandez F, Martinez-Ripoll M, Biner M, Llobet A. (1999) Polyhedron 18: 3321–3331.
- Kelch S, Rehahn M (1999) Macromolecules 32: 5818–5828.
- Craig DC, Scudder ML, McHale WA, Goodwin HA (1998) Aust. J Chem 51: 1131–1139.
- Albano G, Balzani V, Constable EC, Maestri M, Smith DR (1998) Inorg Chim Acta 277: 225–231.
- Islam A, Ikeda N, Yoshimura A, Ohno T (1998) Inorg Chem 37: 3093–3098.
- Chotalia R, Constable EC, Hannon MJ, Tocher DAJ (1995) Chem Soc, Dalton Trans 3571–3580.
- Constable EC, Thompson AMWC, Tocher D A, Daniels MAM (1992) Synthesis, characterisation and spectroscopic properties of ruthenium(II)-2,2':6',2''-terpyridine coordination triades X-ray structures of 4'-(N,N-dimethylamino)-2,2':6',2''-terpyridine and bis(4'-(N,N-dimethylamino)-2,2':6',2''-terpyridine)ruthenium(II) hexafluorophosphate acetonitrile solvate. New J Chem 16(8-9): 855–867.
- Beley M, Collin J P, Sauvage J P, Sugihara H, Heisel F, et al. (1991) Photophysical and photochemical properties of ruthenium and osmium complexes with substituted terpyridines. J Chem Soc Dalton Trans 11: 3157–3159.
- Constable E C, Thompson A M W C (1992) Multinucleating 2,2′ : 6′,2″-terpyridine ligands as building blocks for the assembly of co-ordination polymers and oligomers. J Chem Soc Dalton Trans 24: 3467-3475.
- Fernandes Jose A, Almeida Paz F. A, Lima .P. P, Alves Jr. S, Carlos L. D., Acta Cryst. 2010, E66, o3241–o3242.
- Constable E C, Ward M D (1990) Synthesis and co-ordination behaviour of 6′,6″-bis(2-pyridyl)-2,2′ : 4,4″ : 2″,2″′-quaterpyridine; ‘back-to-back’ 2,2′ : 6′,2″-terpyridine. J Chem SOC Dalton Trans 4: 1405.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.