Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
OxyraseTM for Optimal Growth of Trichomonas vaginalis Isolates
*Corresponding author: JF Alderete, School of Molecular Biosciences, MC7520, College of Veterinary Medicine, Washington State University, Pullman, WA 99164.
Received: November 22, 2021; Published: November 30, 2021
DOI: 10.34297/AJBSR.2021.14.002055
Abstract
The inability of some fresh clinical isolates of Trichomonas vaginalis to grow and multiply when inoculated into the In-PouchTM trichomonas growth medium followed by death upon inoculation into the trypticase-yeast extract-maltose (TYM)-horse serum complex medium was investigated. Of fifty-one fresh clinical isolates derived from patients with trichomonosis, 11 (~20%) failed to grow to numbers after passage into the complex medium sufficient for cryopreservation (labeled Group 1). Isolates labeled Group 2 (~80%) thrived in both the In-PouchTM and complex media (Group 2). Experiments using the OxyraseTM scavenging system to remove oxygen from the medium revealed that Group 1 isolates grew to numbers equal to Group 2 isolate trichomonads. Group 1 isolates consisted of both dsRNA virus-negative and positive isolates, showing that absence or presence of virus was not responsible for the growth characteristics of the Group 1 isolate organisms. This further indicated that the Group 1 isolate parasites are unable to survive in a microaerophilic environment compared to Group 2 isolate trichomonads, suggesting that the patient vaginal environment was anaerobic. Analysis by one-dimensional sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of total proteins of T. vaginalis to compare Group 1 and Group 2 organisms showed no differences in protein patterns. Likewise, both Group 1 and Group 2 isolate parasites had similar total proteinase patterns by substrate gel analysis after electrophoresis. Comparative studies of the property of cytoadherence and amounts of adhesins also showed identical results for Group 1 organisms grown in OxyraseTM and Group 2 parasites in the presence or absence of OxyraseTM. These results are discussed in relation to the patient’s vaginal anaerobic environment as critical for establishment of infection by Group 1 isolate parasites. This work now allows for investigators to perform studies of T. vaginalis isolates heretofore unable to be cultured and cryopreserved.
Keywords: In-PouchTM; OxyraseTM; trichomonosis; Trichomonas vaginalis.
Abbreviations: HI: Heat-Inactivation; STI: Sexually Transmitted Infection; SDS-PAGE: Sodium Dodecylsulfate Polyacrylamide Gel Electrophoresis; TVV: Trichomonas vaginalis dsRNA virus; TYM: Trypticase-Yeast Extractmaltose Medium.
Introduction
Trichomonas vaginalis causes trichomonosis, the number one, non-viral and curable sexually transmitted infection (STI) worldwide [1]. This STI is responsible for numerous adverse health outcomes for women [2]. Numerous reports have established differences in virulence properties among trichomonads cultured in vitro over extended periods versus fresh clinical isolate organisms. For example, freshly-derived clinical isolate organisms grown in batch culture for three weeks lose the iron-upregulated synthesis of adhesins that mediate cytoadherence, a property preparatory for infection [4-6]. Likewise, the dsRNA viruses that infect some T. vaginalis isolates are lost after several weeks after batch cultivation in complex medium [6,7]. These and other observations make evident the need to be able to collect and preserve fresh clinical isolates to analyze and understand the properties important to the host-parasite interrelationship and to be able to identify trichomonad virulence factors. Such a knowledge base is prerequisite to any future interference strategies for this significant STI.
In this study the hypothesis that some T. vaginalis isolates that are difficult to grow to optimal density levels for cryopreservation was the result of the level of oxygen in the growth medium. The results from growth experiments showed that eleven of fifty-one (~20%) trichomonal isolates (labeled Group 1) freshlyderived from patients were lost after inoculation of the In-PouchTM trichomonal medium and the TYM-serum complex media [8]. This finding is in contrast with the forty (~80%) isolates called Group 2 that grew in both the In-PouchTM and the complex media. There are numerous reports of microorganisms requiring removal of oxygen from the growth medium by OxyraseTM for optimal growth and multiplication and for performing experiments [9-11]. Therefore, this study on the Group 1 isolate trichomonads show that addition of OxyraseTM to the In-PouchTM and the complex media [8] permitted achieving optimal cell densities equal to the Group 2 isolates, and these isolate organisms were able to be cryopreserved. The significance of these findings regarding the ability to cryopreserve in vitro growth-resistant fresh clinical isolates and the patient vaginal environment that is permissive for growth in vivo of these parasites is discussed.
Methods
Growth of vaginalis fresh clinical isolates and the long term-grown NYH 286 isolate using In-PouchTM and complex media with and without OxyraseTM
Patients with trichomonosis visited The University of Texas Health Science Center at San Antonio, and infection by T. vaginalis was confirmed by microscopic examination. Vaginal swab samples were obtained for inoculation of the In-PouchTM Trichomonas test kit (Biomed Diagnostics, Inc., White City, Oregon), which were then transported immediately to the laboratory for placement in a 37ᵒC incubator and monitored daily using the manufacturer’s protocol for parasite growth. After visualization of live organisms, samples of the InPouchTM medium were visualized by darkfield microscopy to confirm the T. vaginalis culture was axenic. Three milliliters of the In-PouchTM medium with live trichomonads of the fresh clinical isolates was then inoculated into T-25 tissue culture flasks (Millipore-Sigma, St. Louis, MO, USA) with 30ml of the TYM medium supplemented with 10% heat-inactivated horse serum (complex medium) [8,12]. A duplicate T-25 flask with complex medium was supplemented with 0.6ml OxyraseTM (Oxyrase, Inc., Mansfield OH, USA) (0.1ml per 5ml of medium) to remove oxygen as per the manufacturer’s instructions. Numbers of organisms were obtained daily for up to 7days using a hemacytometer. Isolates are referred to as Type I and Type II indicating the absence and presence of dsRNA virus, respectively, that infect some fresh clinical isolates and the long termgrown NYH 286 isolate trichomonads [6,7].
Total trichomonad proteins and protease analyses and levels of cytoadherence and amounts of adhesins
Total protein preparations of T. vaginalis proteins for sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were obtained by trichloroacetic acid precipitation as described before [12]. Conditions for one dimensional SDS-PAGE using 7.5% acrylamide gels and Coomassie Brilliant blue-staining of gels for comparing total proteins of trichomonads grown in the absence and presence of OxyraseTM was performed using established procedures [12]. The proteases of T. vaginalis organisms were analyzed by SDSPAGE that was performed with acrylamide copolymerized with gelatin as substrate as described previously [13].
The levels of cytoadherence to vaginal epithelial cells and the amounts of the specific adhesin proteins that mediate this property of attachment to host cells were also examined for Group 1 parasites grown in OxyraseTM medium and compared with the Group 2 organisms, as before [3-5,14].
Materials and Methods
Summary of vaginalis differentiated as Group 1 and Group 2 isolates
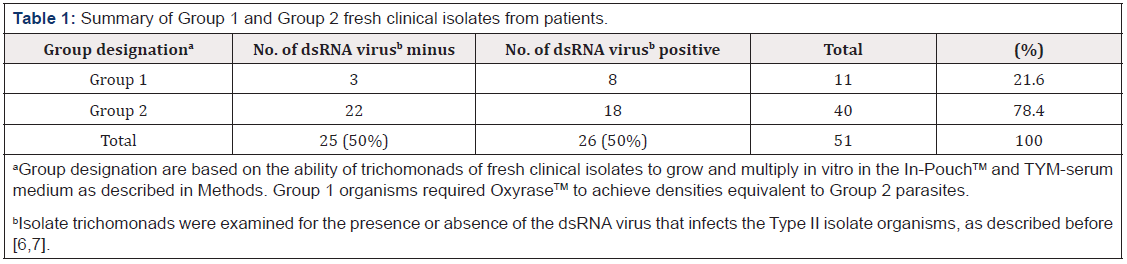
Table 1 presents the summary of the freshly-derived isolate trichomonads assigned to Group 1 and Group 2 based on the requirement of OxyraseTM to achieve optimal cell densities. A total of fifty-one isolates were examined, and eleven isolates (~20%) were found to require OxyraseTM for growth (Group 1) compared to the forty isolates (~80%) without an OxyraseTM requirement (Group 2). Further, as shown in Table 1, 50% of isolates were either positive or negative for the presence of the dsRNA virus as reported before [6,7], and the data show that the dsRNA virus had no effect on the growth kinetics of the organisms (Table 1).
OxyraseTM restores ability for in vitro growth of Group 1 trichomonads
Figure 1 shows the growth kinetics of T. vaginalis organisms from three of the eleven representative Group 1 isolates. All three isolates grew optimally by days 5 and 6 but only in the presence of OxyraseTM in the growth medium (open circles) compared to the absence of OxyraseTM (solid circles). These highly motile isolate parasites in the presence of OxyraseTM achieved densities of at least 6 x 106 trichomonads per ml. Isolates without OxyraseTM never recovered to densities needed for cryopreservation and were lost. Isolate UT99-16 without OxyraseTM grew to a density of 3 x 106 organisms per ml by day 3 but were not highly motile, after which the parasites subsequently died. Type I and Type II designations refer to the absence and presence of the dsRNA virus within the isolate trichomonads, respectively [6,7]. These data show the requirement of removing oxygen from the growth medium for optimal growth and multiplication of Group 1 isolate organisms.
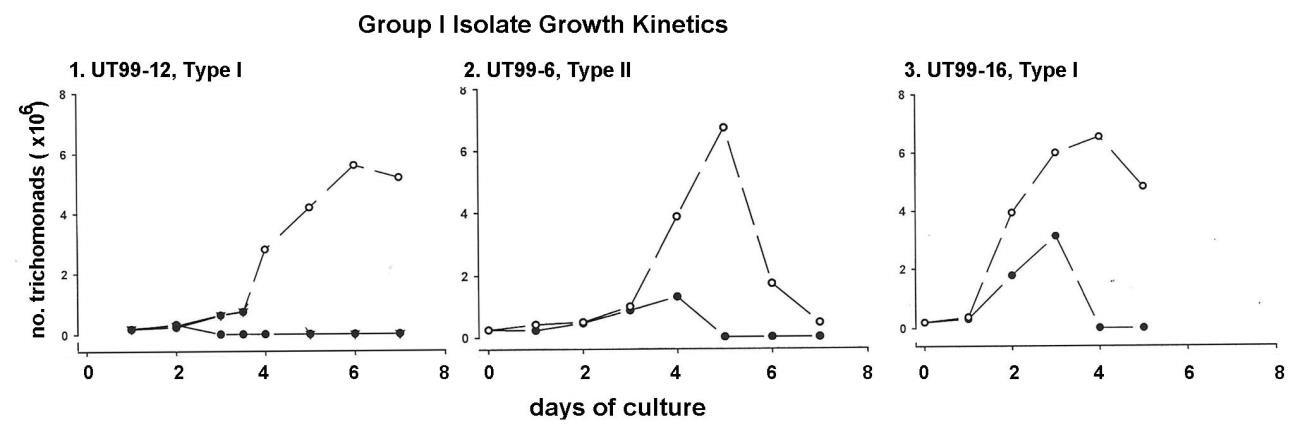
Figure 1: Representative growth kinetics of three of the eleven Group 1 T. vaginalis isolates grown in the TYM-serum medium (Methods) in the absence (solid circles) and presence (open circles) of OxyraseTM. Type I and Type II refer to isolate trichomonads without the dsRNA virus and with the dsRNA virus, respectively, as before [6,7]. Removal of oxygen from the medium by OxyraseTM significantly elevated the numbers of organisms per ml for each isolate.
Figure 2 illustrates the growth kinetics of a representative Type I dsRNA virus-negative isolate (UT9914) and a Type II virus-positive isolate (UT99-17) of the forty Group 2 isolates. Without OxyraseTM (solid circles) both isolates achieved densities of parasites of ~6 x 106 trichomonads per ml by days two and three and were highly motile. Although not requiring the oxygen scavenger, addition of OxyraseTM to the complex growth medium (open circles) kept the trichomonads highly motile up to 5 days. These data also affirm the importance of removing oxygen from the growth medium for optimal maintenance of healthy motile parasites for longer periods of time.
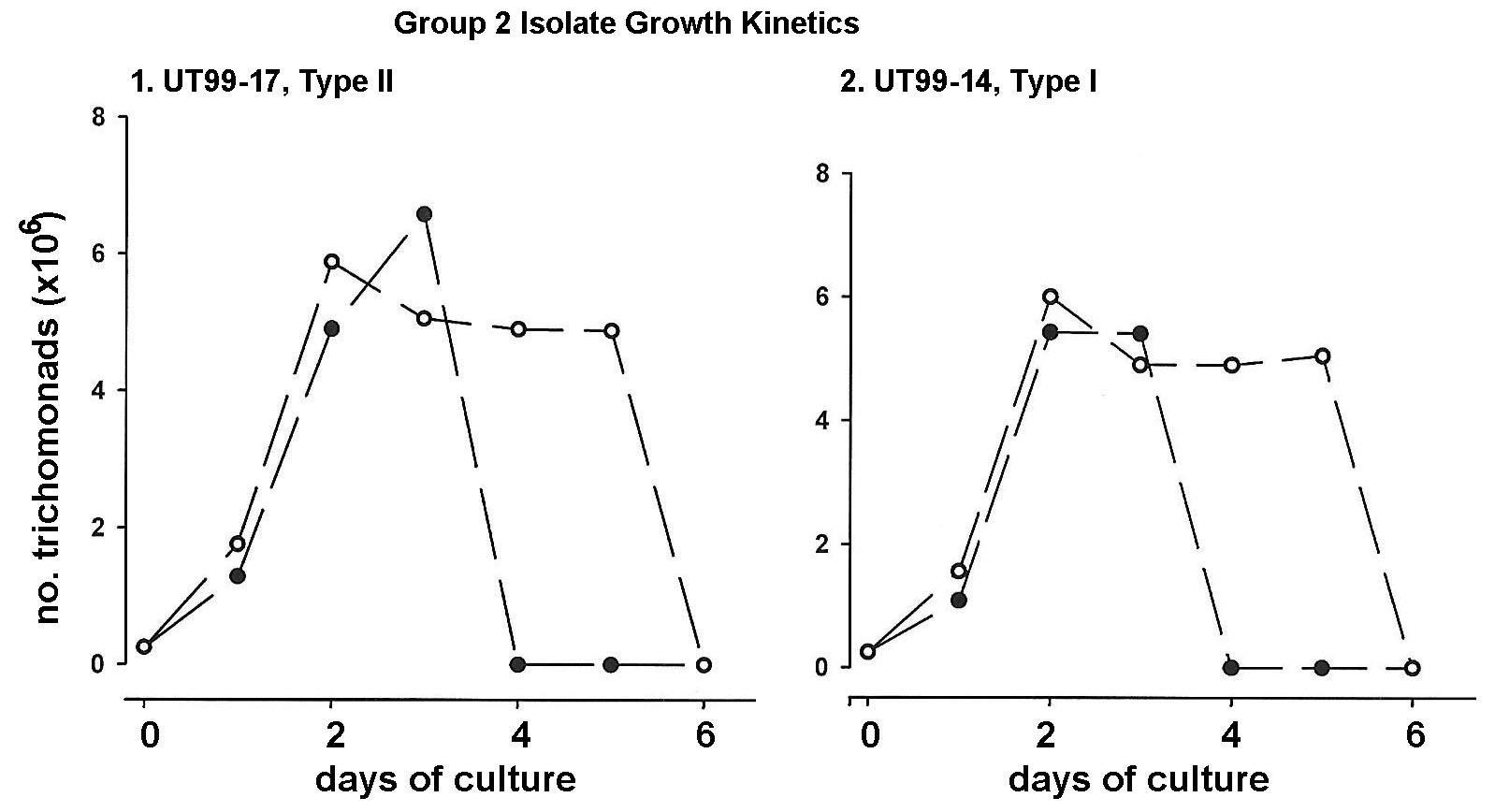
Figure 2: Growth kinetics of a virus-positive isolate (Type II) and virus-negative isolate (Type I) of the representative forty Group 2 T. vaginalis isolates grown in the TYM-serum medium without (solid circles) and with OxyraseTM (open circles) in the growth medium, as for Figure 1. Group 2 isolates grow and multiply to high densities without OxyraseTM. However, addition of OxyraseTM extended the motility and survival of organisms by two days compared to that observed of parasites without OxyraseTM.
Finally, we wanted to examine the representative Group 2 long term-grown isolate NYH 286 to compare with the fresh clinical isolates the growth kinetics in the presence (open circles) and absence (solid circles) of OxyraseTM in the growth medium. As can be seen in Figure 3, the batch culture in the complex medium under both conditions were nearly identical. It is noteworthy that for long term-grown isolate organisms, the high generation times for optimal densities were achieved by day two compared with the fresh clinical isolates of both Group 1 and 2 isolate trichomonads (Figure 3).
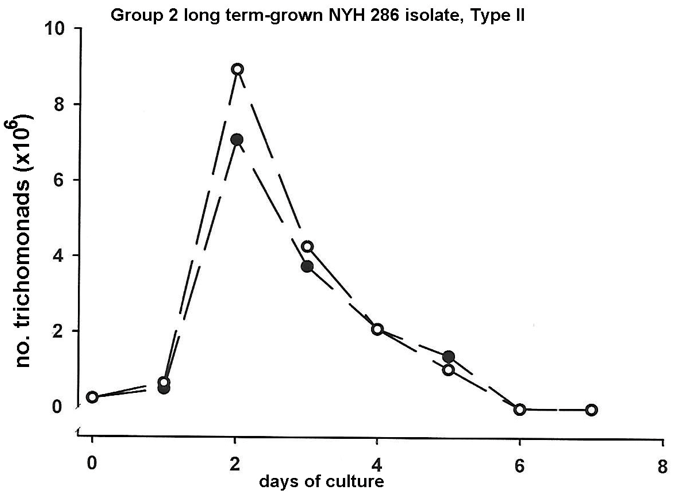
Figure 3: Representative growth kinetics of a virus positive (Type II) long term-grown Group 2 T. vaginalis isolate in relation to the growth medium without (solid circles) and with (open circles) OxyraseTM added to the growth medium. Long term-grown, laboratory-adapted isolate parasites have lower generation times and reach high densities sooner than fresh clinical isolate organisms.
Examination of total proteins and proteinases of Group 1 and Group 2 parasites
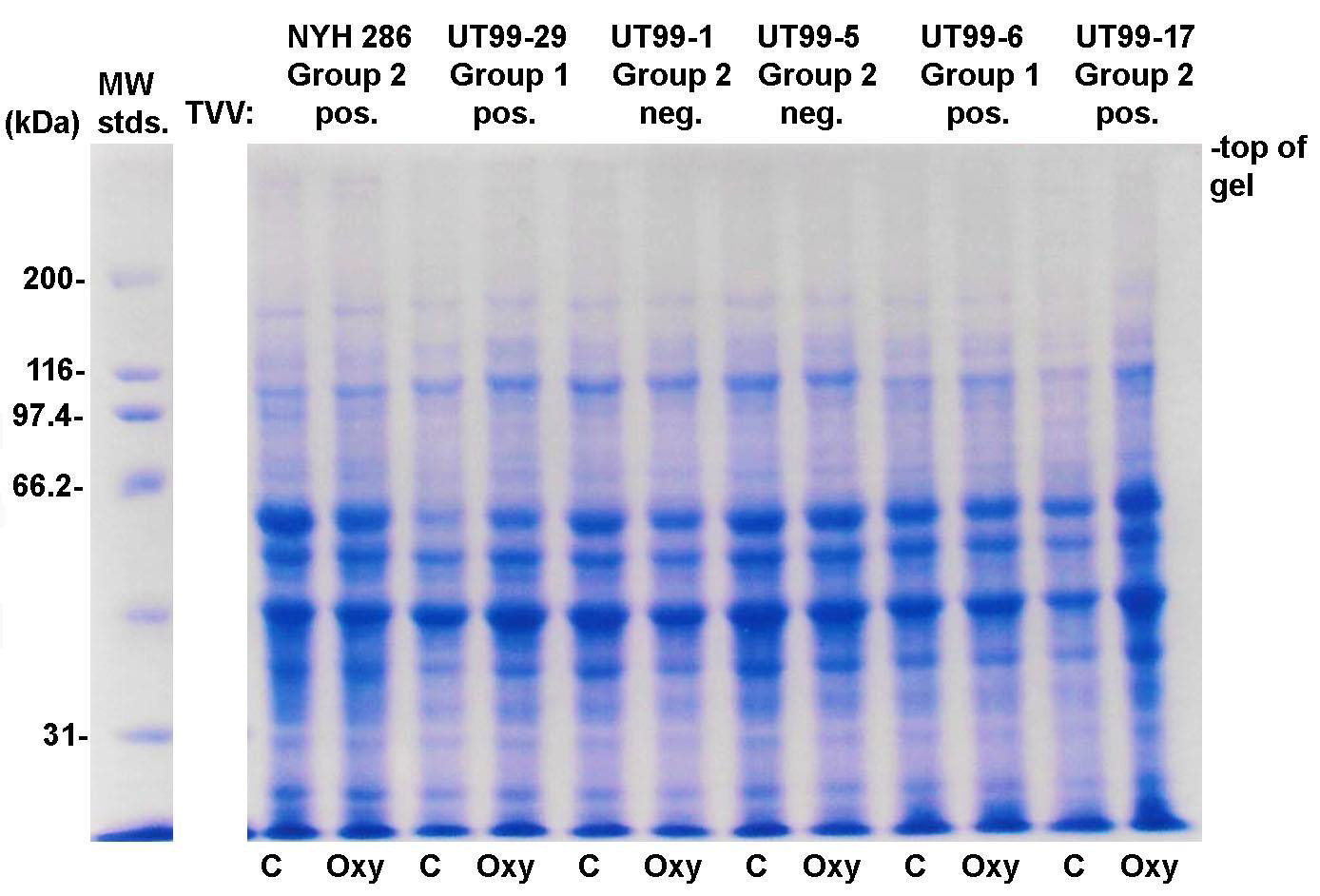
Figure 4: Representative Coomassie Brilliant-blue-stained protein patterns after SDS-PAGE of total proteins of T. vaginalis Group 1 and Group 2 isolates grown in the TYM-serum medium without (labeled C) and with OxyraseTM (labeled Oxy) (Methods). TVV refers to the T. vaginalis dsRNA virus to show that the presence of virus does not alter the patterns. All isolates are freshly-derived clinical isolates except for isolate NYH 286, which is a laboratory-adapted isolate that has been passaged over many years in batch culture. The preparation of total trichloroacetic acid-precipitated proteins of T. vaginalis is as previously published [12].
The Coomassie Brilliant, blue-stained gel of total proteins of two Group 1 and four Group 2 isolate trichomonads after SDS-PAGE is presented in Figure 4. To show that the dsRNA virus did not affect the protein patterns for both Groups 1 and 2, four isolates infected with the dsRNA virus and two isolates without the virus were analyzed. Each isolate was grown in the absence (lanes labeled C) and presence (lanes labeled Oxy) of OxyraseTM. For the Group 1 isolates without OxyraseTM with low numbers of organisms as shown in Figure 1, inoculation of multiple T-25 flasks was required to obtain the numbers necessary for total protein preparations as detailed in an earlier report [12]. As can be seen, except for the intensity of staining for some protein bands, the one-dimensional total protein patterns were nearly identical. These findings show that both the growth in OxyraseTM and the presence or absence of the dsRNA virus did not affect the protein composition of these Group 1 and 2 isolate parasites (Figure 4).
We then analyzed by substrate gel electrophoresis the total proteinases of the OxyraseTM-grown Group 1 and Group 2 isolate parasites, as before [13]. As with the total protein patterns shown above, there were no qualitative and quantitative differences in proteinases for organisms of either Group (data not shown).
Finally, we also performed standard adherence assays of the Group 1 and Group 2 isolate trichomonads grown in the presence of OxyraseTM using vaginal epithelial cells, as before [3-5,14]. Not unexpectedly for fresh isolate organisms both Group 1 and Group 2 organisms had similarly high levels of adherence and amounts of the adhesin proteins [3-5,14]. Also, as expected for fresh clinical isolates [14,15], the organisms for both Group 1 and Group 2 had up-regulated expression of the adhesin proteins when compared to the long term-grown NYH 286 isolate [3,4]. Collectively, these data indicate that, after correcting for the ability to grow in the TYM-serum complex medium via addition of OxyraseTM, all T. vaginalis trichomonads of both Group 1 and Group 2 were identical for the properties examined.
Discussion
The discovery of the OxyraseTM enzyme system derived from the E. coli cytoplasmic membrane that is efficient for scavenging oxygen in broth medium has been shown to enhance the growth of microorganisms [8-10]. Therefore, this study tested the hypothesis that oxygen in the growth medium for T. vaginalis restricted growth and multiplication of trichomonads of some fresh clinical isolates. These isolates (labeled Group 1) were unable to thrive in vitro and were subsequently lost when compared to Group 2 isolates with excellent growth kinetics that permitted cryopreservation. The data presented in Figure 1 indeed show that removal of oxygen from the growth medium by OxyraseTM permitted levels of growth and multiplication of Group 1 organisms equivalent to those observed for Group 2 parasites (Figure 2).
The data suggest that the Group 1 isolates are different from Group 2 isolates with respect to sensitivity to oxygen. This observation is significant when considering the host vaginal environment of some patients infected with T. vaginalis. This work suggests that some patients with a certain level of oxygen in the vagina may not be permissive for growth and multiplication of Group 1 isolate parasites. In this case the Group 1 isolate organisms require a more anaerobic vaginal environment. Indeed, it is known that vaginal oxygen concentrations affect the virulence of microbial pathogens, such as Staphylococcus aureus and group B streptococcus [10,11]. Further, increasing the vaginal oxygen level has been shown to have an antitrichomonosis effect [16], and this observation may be relevant to the Group 1 isolates unable to grow in medium without OxyraseTM removal of oxygen from the growth medium.
Importantly this report also examined both Group 1 and Group 2 isolate trichomonads for various established properties important to the T. vaginalis-host interrelationship [3,4,7,13-15]. Group 1 isolate parasites grown in OxyraseTM-supplemented medium and Group 2 organisms with and without OxyraseTM in the medium were analyzed for total protein profiles (Figure 4) [12], proteinases [13] and levels of cytoadherence and amounts of adhesin proteins [3-5,14]. Not unexpectedly for fresh clinical isolates, all Group 1 and Group 2 isolates possessed similar, if not identical, properties. It is noteworthy, however, that the comparative analysis of both Group 1 and Group 2 isolate parasites was possible only with the discovery that Group 1 isolate organisms could be grown upon OxyraseTM scavenging of oxygen from the medium.
Conclusion
This report shows for the first time that some fresh clinical T. vaginalis isolates require the removal of oxygen from the growth medium for optimal growth necessary for cryopreservation. This is important to be able to delineate and understand the biological properties, such as expression of proteins [12] and cytoadherence to vaginal epithelial cells [5], and virulence factors, such as adhesins [3,4] and proteinases [13], preparatory to infection of humans. The data regarding Group 1 isolate organisms further show the significance of the host vaginal anaerobic environment necessary for a permissive infection by T. vaginalis.
Acknowledgement
This study was supported by Public Health Service grants AI-39803 and AI-43940 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health while JFA was at The University of Texas Health Science Center at San Antonio. As per instructions by the International Committee of Medical Journal Editors on “Who is an Author?” (http://www.icmje.org/recommendations/browse/roles-andresponsibillities/defining-the-role-of-authors-and-contributors.html), I want to acknowledge the contribution of Jeanna Piper, MD of the Department of Obstetrics and Gynecology at The University of Texas Health Science Center at San Antonio (UTHSCSA) for providing the fifty-one fresh clinical isolates of T. vaginalis used in this study. The Institutional Review Board of the UTHSCSA approved obtaining T. vaginalis isolates from patients diagnosed with this STI.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.