Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Interaction of Podocytes, Glomerular Cells and Mesangial Cells in Glomerulopathies
*Corresponding author: Christian Pisrez Calvo, Universidad Libre de Barranquilla, Colombia.
Received: June 06, 2021; Published: September 23, 2021
DOI: 10.34297/AJBSR.2021.14.001974
Abbreviations: AKI: Acute Kidney Injury; ESRD: End-Stage Renal Disease; CKD: Chronic Kidney Disease; Cd2ap: CD2-AssZociated Protein; JAK2: Janus kinase 2; USP40: carboxyl Terminal Hydrolase 40; UCHL1: Carboxyl Terminal Hydrolase L1; MMP: 2.4.1. Matrix Metalloproteinases; ECM: Extracellular Matrix; MMP-9: Metallopeptidase 9
Introduction
Glomerular disease is the collective term to designate diseases that affect the structure and function of the glomerulus [1]. The causes of this entity are divided into primary or secondary. Generally, it is not an easy task to differentiate them because of their various forms of presentation, which vary in severity from asymptomatic urinary abnormalities to acute kidney injury (AKI) or end-stage renal disease (ESRD) [2] Primary glomerulopathies refer to those entities in which renal involvement is not the consequence of a more general disease and the clinical manifestations are restricted to the kidney. The five most important types of primary glomerulopathies are minimal lesions, focal segmental glomerulosclerosis, membranous nephropathy, IgA nephropathy and membranoproliferative glomerulonephritis. In this subset of glomerular diseases there are 3 factors that contribute to its genesis, however in most of them the antigen or the ultimate cause of the disease is unknown [1,3].
a) Immune System: Main trigger of glomerulopathies Primaries
b) Infectious Microorganisms: They can trigger abnormal immune responses or against microbial antigens
c) Genetic Factors: they can influence the predisposition to the development, progression and response to the treatment of glomerular injury.
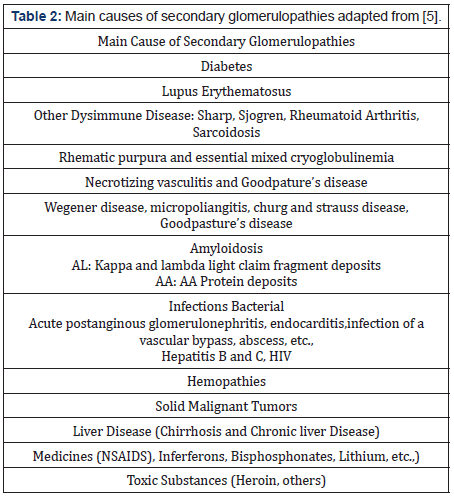
Compared to primary glomerulopathies, secondary glomerulopathies refer to those in which glomerular damage is part of a complex clinical picture, caused by different processes: immunological, tumors, hereditary, infections or drugs, the classic examples are triggered by the systemic lupus erythematosus (SLE), diabetes, among others See Tables 1&2 [4]. During years, glomerulopathies have been closely related to chronic kidney disease (CKD) [5], occupying the first places of CKD triggers according to the 2017 report of the “The United States Renal Data System” [6]. Taking into account only the primary glomerulopathies in the pediatric population generate 5%-14% of children with chronic kidney disease and 15%-29% of children with end-stage renal disease [7] Consequently, it is a pathology that contributes to the genesis of CKD, which in turn generates morbidity, mortality and annually high costs to the health system in many countries of the world. For all the above, glomerulopathies have historically had a special place in the medical community, especially in nephrologists, which is why these communities have been interested in understanding the pathophysiological mechanisms of glomerulopathies. Years ago, the management of this pathology was classified by the scientific community as “ordinary and inelegant” because steroids, cyclophosphamide, among other therapies were being used in little-known terrain. However, in recent years, knowledge of the pathophysiology of glomerular diseases has advanced rapidly. One of the topics that the medical community has emphasized is the role of important cells such as podocytes, mesangial cells and glomerular endothelial cells in glomerulopathies, which will be the objective of this article. See Figure 1 However, before focusing on the aforementioned topic, it is pertinent to mention the physiological role they play at the renal level [8].
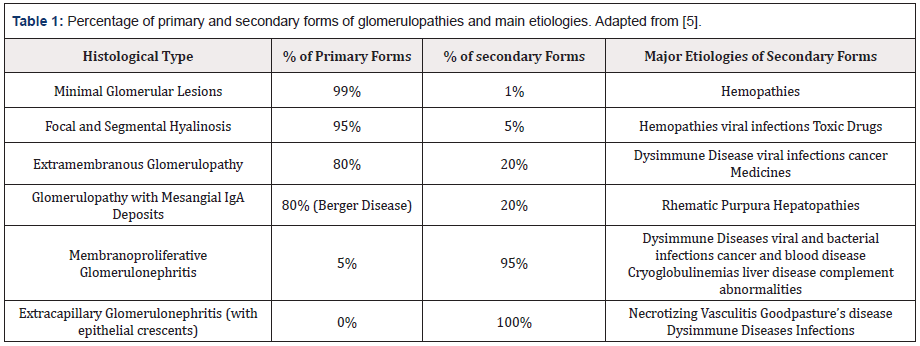
Table 1: Percentage of primary and secondary forms of glomerulopathies and main etiologies. Adapted from [5].
a) Glomerular Structure
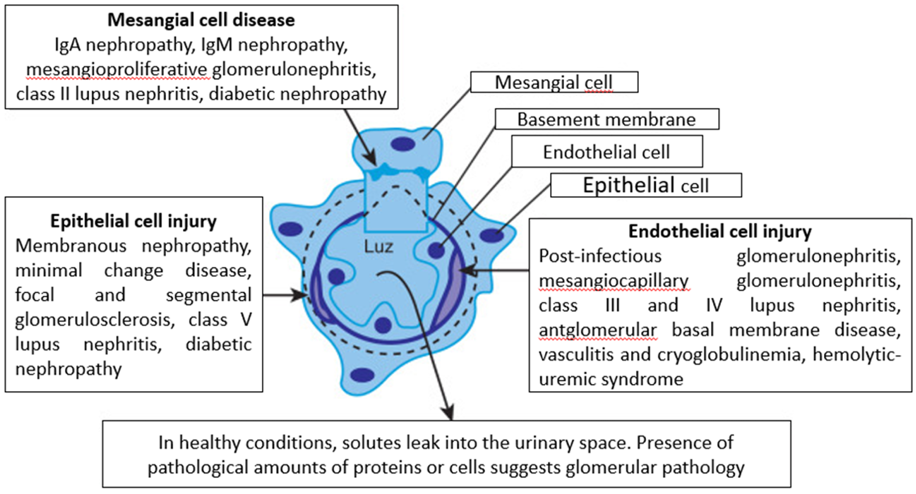
Within the nephron, the glomerular filtration barrier is responsible for the selective filtration of blood from the afferent arteriole to Bowman’s space. The filtration barrier includes 3 layers: glomerular epithelium, the basement membrane, and the slit diaphragms, which are made up of podocytes. The slit diaphragm is the final barrier that prevents the passage of proteins to the urinary filtrate. The filtrate that passes into Bowman’s space continues into the proximal tubule and loop of Henle for further processing [9].
b) Podocytes
In recent years we have witnessed novel advances in optical and electron microscopy techniques in the biomedical sciences. This has helped better understanding in multiple fields of medicine, a clear example is podocytes. These cells reside as specialized pericytes that cover the glomerular capillary endothelial cells into the parietal epithelial cells lining Bowman’s capsule. Together with the capillary endothelial cells, the podocytes form the glomerular basement membrane, which appears to be the decisive filtration layer in controlling the glomerular filtration barrier [11-13]. For decades, these cells have been related, given their importance in the glomerulus, with glomerulopathies, especially in nephrotic syndrome, focal and segmental glomerulosclerosis, diabetic and hypertensive nephropathy, where it has been found that the lesion and / or decrease in the number of these, sit contributes to the genesis of these pathologies [14-16]. As podocytes decrease in number, the glomerular filtration barrier is affected. In addition, the low capacity of these cells to regenerate makes them very prone to damage. In an experimental study carried out by Bryan L. Wharram, it was found that after 20% of the damage of these cells there was a higher proportion of glomeruli containing synechiae and segmental areas of podocyte depletion and glomerulosclerosis [17]. Multiple theories have been proposed on the mechanisms of injury and / or reduction of podocytes. However, the objective of this article is to review the mechanisms that have had greater progress in the scientific community in recent years and their relationship with glomerulopathies, among which we find:
c) Role of Podocyte Proteases
Proteolysis is an important mechanism that influences physiological and pathophysiological processes, such as differentiation, development, apoptosis, and cancer. In the case of podocytes, they play a prominent role in the degradation of proteins important to their architecture. Extracellular and intracellular proteases have been studied.
Intracellular Proteases
Cathepsins: Within the intracellular proteases we find this group. The first one related was cathepsin L, an intracellular cysteine protease, which has been related to multiple pathological glomerular states. For example, in the study carried out by Garsen M et al in relation to diabetic nephropathy [18,19]. Captesin L is believed to promote proteolysis of CD2-associated protein (Cd2ap) and synaptopodin. Furthermore, it leads to dynamic cleavage at an evolutionarily conserved site, resulting in reorganization of the podocyte actin cytoskeleton [20,21]. Other type of cathepsin that has been linked to glomerular pathologies is cathepsin D, it is an aspartic protease, which is important to maintain the integrity of podocytes, since mice deficient in cathepsin D develop cleavage diaphragm fragmentation, proteinuria and end-stage renal disease. [19,22]. Given the previously described findings, a study was carried out by Gregory Blass et al in which they wanted to verify the role of cathepsins in kidney damage by intravenous administration of the irreversible cysteine inhibitor cathepsin E-64 in animals. The study result did not reduce blood pressure or other aspects of glomerular injury that were induced by salt-sensitive hypertension [23]. Another study carried out by Tamadher A Alghamdi found that by inhibiting Janus kinase 2 (JAK2), in addition to other effects, it led to a down regulation in the expression of lysosomal genes and a decreased activity of the lysosomal enzyme, cathepsin D, generating thus reversal of lysosomal dysfunction and restoration of albumin selectivity permeability [24]./p>
Ubiquitin Proteases: they are a group of proteases that participate in maintaining the integrity of podocytes and participate in their proteostasis. Among those related to glomerular pathologies we find the ubiquitin carboxyl-terminal hydrolase 40 (USP40) and the ubiquitin carboxyl-terminal hydrolase L1 (UCHL1). USP 40 is a gene product which currently remains uncharacterized and its biological function is completely unknown. However, as a result of a study carried out by Takagi H et al. it was related to glomerular pathologies. In the study it was found that rodent glomeruli exhibit a specific expression of the USP40 protein, specifically in the cytoplasm of podocytes of the adult kidney. Likewise, this study found the reduction of USP40 in podocytes in patients in the proteinuric stage in the rat model of minimal change nephrotic syndrome. Finally, in USP40 depleted zebrafish they exhibited disorganized glomeruli with reduced endothelial cell attachment and effacement of podocytes [25].
Regarding UCHL1, it is a ubiquitin protease that is normally expressed in tubular and parietal cells of the kidney. Furthermore, it has also been found to be highly expressed in damaged human podocytes. A study was carried out by Naomi C Le et al, in which it was found that the positive regulation of UCHL1 in focal and segmental glomerulosclerosis associated with ACTN4 contributes to the proteasome. Also, that its deletion maintains the integrity of the podocyte cytoskeleton, thus protecting the glomerular filtration barrier [19,26-28]. Considering the aforementioned, associated with the vital role of podocytes in lupus nephritis, the idea has been raised that UCHL1 is a promising therapeutic target in the management of lupus nephritis. This could be used individually and with caution because this protease has abundant expression in human neurons and ß cells. Thus preventing patients from suffering from other diseases such as diabetes [29] In another area in which UCHL1 has been related is with respect to relapses of idiopathic nephrotic syndrome, in a study carried out by Agnès Jamin et al. Corroborated this because this protease was a target of autoantibodies in patients with this diagnosis with relapses [30].
Calpain: a calcium-dependent cysteine protease, it was originally identified as a protease that cleaved talin, an important focal adhesion molecule in the podocyte [19,31]. As a result, studies have shown evidence of an improvement of renal function in focal and segmental glomerulosclerosis. Therefore, the inhibition of this protease has been proposed as a possible future therapeutic target in the management of focal and segmental glomerulosclerosis [32.33].
Caspases: These proteases belong to a family of highly conserved aspartate-specific cysteine proteases and are members of the family of interleukin-1 beta converting enzymes, present in multicellular organisms. By Wang Y et al. [34,35] the role of protease-activated receptor 2 in the regulation of focal and segmental glomerulosclerosis was studied; the research group concluded that this receptor plays an important role in mediating injury. Kidney disease induced by glomerulosclerosis and even its inhibition has a protective effect at the renal level [35].
Extracellular Proteases
Matrix Metalloproteinases (MMP): proteins that are part of the extracellular matrix (ECM) which are capable, in their environment, of activating growth factors, surface receptors and adhesion molecules. In addition, they affect the degradation and exchange of the extracellular matrix protein, due to its action glomerular pathologies have been investigated [36,37]. In the study by Szu-Yuan Li, the relationship of metallopeptidase 9 (MMP-9) and diabetic nephropathy was studied. It was found to appear to play a role in the development of diabetic nephropathy. Likewise, it disrupted the integrity of podocyte cells, promoted the permeability of the podocyte monolayer to albumin, and the synthesis of extracellular matrix proteins [37].
Thrombin: it is a serine protease that interacts with receptors activated by protease (PAR). PARs are expressed throughout the body; At the renal level, G protein-coupled receptors have been found in mesangial cells and podocytes [38]. Likewise, elevated urinary thrombin is associated with glomerulonephritis and leads to overstimulation of PAR, increased intracellular calcium levels, proteinuria, and deterioration of the glomerulus [19]. Based on the findings described above, a study was conducted by Yu Guan et al in where they suggested that PAR-1 antagonists have therapeutic potential [35].
Oxidative Stress: Reactive oxygen species (ROS) are free radicals derived from oxygen, which are unstable chemicals and attack cell proteins and lipids, leading to cell dysfunction [39]. They play a crucial role in physiological processes and human pathophysiology; glomerular pathologies have not been the exception. These reactive oxygen species have been found to contribute to podocyte injury by different mechanisms, among them we find the study by Katalin Susztak et al in which podocyte apoptosis increased sharply with the appearance of hyperglycemia in mice due to the generation of ROS [40].
Role of Mitochondria
Mitochondria are essential organelles that meet the energy requirements of the cell through the generation of adenosine triphosphate (ATP). The kidney has the second highest density of mitochondria which helps meet the energy requirement to carry out essential functions, including blood filtration to remove waste, reabsorption of nutrients, maintenance of electrolyte and fluid homeostasis, secretion of hormones and blood pressure regulation [41]. Mitochondrial dysfunction leads to decreased ATP production, alterations in cell structure and functions, and loss of kidney function. When this is persistent, it plays a role in the early stages and progression of kidney diseases, such as acute kidney injury (AKI) and diabetic nephropathy, as it alters mitochondrial homeostasis and, therefore, normal kidney function [42]. This mechanism has been proposed because recent studies revealed that dysfunction of energy transduction in podocytes may be the basis of podocyte injury associated with numerous glomerular diseases, since these are very dynamic cells and require high energy to maintain the organization of the proteins of the cytoskeletal and extracellular matrix, its motility and remodeling, and their low capacity to regenerate makes them very prone to injury [41]. It was concluded in a study by Yoshifusa Abe that mitochondria play the main role in maintaining podocyte energy homeostasis. [43] Similarly, there are agents that coordinate the general regulation of biological programs at the mitochondrial level and play a fundamental role in podocyte energy homeostasis. These are peroxisome proliferator-activated receptors and peroxisome proliferator-activated receptor 1α (PGC- 1α) coactivator. The balance in mitochondrial biogenesis promoted by PGC-1α is believed to be critical to maintaining podocyte health. However, the increase in mitochondrial fusion by Dynamin-1 like protein (DNM1L) has a detrimental effect on podocytes under cellular stress, in this subject there are still many questions to answer which are still under study [41].
Glomerular Endothelial cells
Glomerular endothelial cells are characterized by fenestrations, circular transcellular pores 60-80 nm in diameter, essential for water permeability. The presence of fenestrations has meant that the glomerular endothelium contributes little to the macromolecular barrier. However, it is now widely accepted that the luminal surface of the glomerular endothelium is covered with a glycocalyx that constitutes a significant permeability barrier [44]. It has been found in certain pathologies, where endothelial injury leads to altered microvascular permeability and albuminuria [45]. Within pathologies in which the lesion of these cells is typical, we find rapidly progressive glomerulonephritis including ANCAassociated GN, anti-GBM GN, and class 3 and 4 lupus nephritis. Entities in which the decrease in the glomerular filtration rate is accompanied by a loss of the fenestral area. Among these we find preeclampsia observed in a study carried out by RA Lafayette et al. [46] and diabetic nephropathy observed in a study carried out by Masao Toyoda et al. [47].
It is believed that the mechanism by which diabetes affects endothelial cells is through endothelial dysfunction mediated by glucose and its by-products, generating an alteration of the glomerular filtration barrier by increasing the permeability of endothelial cells. glomerular cells, leading to the induction of endothelial cell apoptosis. Concomitantly, they impair endothelial repair capacity by reducing the number and function of endothelial progenitor cells [45-48]. Another of the related pathologies are those mediated by complement, within these we have the previously called atypical hemolytic uremic syndrome, currently called complementmediated thrombotic microangiopathy [49]. It has even been found that certain inflammatory stimuli such as histamine, thrombin, vascular endothelial growth factor, and activated neutrophils can cause dissociation of cell-cell junctions between endothelial cells, as well as contraction of the cytoskeleton, leading to an expanded intercellular space that facilitates transendothelial flow [50] Another of the components in which it contributes significantly to the glomerular filtration barrier is the endothelial glycocalyx. This dysfunction has been found to be related to the appearance of albuminuria and increased microvascular permeability [51,52] Diabetic nephropathy through the persistent hyperglycemia mechanism has been related to glycocalyx dysfunction and this in turn leads to the appearance of proteinuria in diabetics. In a study by Un Singh et al., The effects of high glucose on the biochemical structure of the glycocalyx were studied, where its damage was corroborated [53] Another mechanism of injury to the endothelial glycocalyx is through the generation of reactive species oxygen, a study by Anurag Singh confirmed his premise [54]. Given these findings, the scientific community has studied possible therapeutic targets that improve dysfunction at the level of these two cellular structures, especially in diabetic nephropathy. They find a group in which they act indirectly. Among these we find the new antidiabetic therapies, which have recently shown a specific benefit in diabetic nephropathy, probably because it involves the protection of the renal endothelium [48]. Studies have been carried out in the group that act in a specific way. A study carried out by Raina D Ramnath et al. Which was based on the fact that the detachment of syndecan 4 mediated by matrix metalloproteinase (MMP) is a mechanism of damage to the glomerular endothelial glycocalyx in vitro, which generates an increase in the permeability of albumin. Given which, the authors of the same used treatment with MMP inhibitors, a significant attenuation of the elimination of syndecan 4 from the glomerular endothelium and plasma was aimed and inhibited the activity of plasma MMPs. Thus, concluding that treatments aimed at protecting glucocalyx by inhibiting MMP may be beneficial in diabetic kidney disease [55,56] Another study by Sara Desideri et al. Suggests that the manipulation of VEGF receptor 2 signaling or a common endothelial glucocalyx biosynthesis pathway by these growth factors, can protect and restore the layer of the same, thus being a possible therapeutic target in the future for diabetic nephropathy [57].
Mesangial cells
Mesangial cells comprise approximately one third of the decapsulated glomerular cell population. These are responsible for maintaining the structural architecture of the glomerular capillary similar to the function of certain microvascular pericytes. They also contribute to mesangial matrix homeostasis, regulate the filtration surface area, and phagocytose apoptotic cells or immune complexes formed in or delivered to glomerular capillaries [58,59]. There is increasing evidence to support the role of glomerular mesangial cell proliferation and overproduction of extracellular matrix by mesangial cells in the development of glomerulopathies [60]. It was found that the B subunit of platelet-derived growth factor, which is a potent mitogen in these cells, is positively regulated in glomerular pathologies such as IgA nephropathy, diabetic nephropathy, lupus nephritis, among others [61,62]. This leads to the expansion of the mesangial matrix and the release of vasoactive mediators, thus resulting in a decrease in the glomerular surface area and an alteration in glomerular hemodynamics, with a decrease in GFR. If this persists, it leads to interstitial fibrosis, followed by glomerulosclerosis [45,63]. Another way in which these cells are related to glomerular pathologies is by serving as a deposit target. An example of this is the deposition of IgA in diabetic nephropathy. Another pathology in which accumulation at the mesangial level is evidenced is in lupus nephropathy [45,64]. Another pathology related to these cells is alport disease. In the study by Brianna Dufek et al, supported by the theory that this glomerulopathy is mediated by strain-sensitive biomechanical activation of mesangial actin dynamics. In this study, it was found that the activation of the endothelin A receptor in mesangial cells is a key event in the onset of the disease [65]. Through animal experiments, these cells have been studied as a possible therapeutic target. Among the therapeutic targets there are 3 that have been the most studied:
Nuclear Factor kappa B (NFκB): Is a transcription factor that plays a key role in coordinating many cellular responses. Ziad A. Massy et al. Verified the crucial role of NF-kB in the activation of mesangial cells. [66] Based on the above and its role in generating molecules such as interleukins, anti-inflammatory agents have been tested. Among these we have dehydroxymethyl-epoxyquinomycin (DHMEQ), a new inhibitor of NFκB activation, which was effective in the treatment of GN induced by anti-Thy1.1 antibodies in the study carried out by Kosaka T et al. [68] Likewise, this inhibitor showed a decrease in proteinuria, an improvement in creatinine clearance and a decrease in glomerular cell proliferation [67,68]. Another finding was in conventional treatments such as corticosteroids, it was evidenced that their beneficial effect in glomerular pathologies has been through the inhibition of NFκB [69].
Platelet-Derived Growth Factor (PDGF): It is a potent mitogen and a key survival factor for mesangial cells [67] In a study by C Zoja et al. [70] imatinib, a PDGF receptor tyrosine kinase inhibitor, was found to improve animal survival and renal manifestations in lupus mice. They also suggest the possibility of exploring whether Imatinib in the management of human lupus nephritis in the context of a steroid-sparing drug in human lupus nephritis [70].
Transforming Growth Factor beta: TGF-β is the prototype of the TGF-β family of growth and differentiation factors. It is encoded by 33 genes in mammals and comprises homo and heterodimers [71]. Binding of TGFβ to its receptor has been found to lead to phosphorylation of Smad2 and Smad3, this in turn translocates to the nucleus with Smad4 and upregulates collagen alpha transcription leading to increased collagen synthesis. Concomitantly, they lead to the phosphorylation of the early growth response protein 1 (Egr-1), through casein kinase 2 [67,72]. A study was carried out by Hirotaka Fukasawa, in which he showed that TGF-B causes an increase in the synthesis of mesangial cells, mesangial proliferation and glomerular fibrosis. Furthermore, in the same study an anti-TGF-β antibody was used resulting in amelioration of chronic progressive nephritis by inhibiting Smad / TGF-β signaling [67,73] Indirectly there is also evidence, the study carried out by Bernd Hohenstein et al., Used vardenafil, a specific inhibitor of the specific inhibition of phosphodiesterase type 5. It is known that it in turn activates TGF-B, thus finding the beneficial antiproliferative and antifibrotic effect in proliferative mesangial glomerulonephritis [74].
Conclusion
Glomerular diseases manifest as various clinical syndromes and etiologies that are not clearly understood. Glomerular changes are complex and affect all types of glomerular cells, including podocytes, glomerular endothelial cells, and mesangial cells. Understanding the role of each of these cells in glomerular pathologies helps the scientific community to have new therapeutic targets. Targeted therapies have been shown to reduce drug dosage and therefore minimize side effects. This is especially attractive in chronic kidney diseases that require treatment for long periods and could contribute in the future to reduce morbidities and mortality in the terminal stage of many glomerupathies such as CKD.
References
- (2021) Glomerulonefritis Primarias. Nefrología al día.
- Bose B, Cattran D (2014) Glomerular Diseases: FSGS. Clin J Am Soc Nephrol 9(3): 626-632.
- Glassock RJ, Cohen AH (1996) The primary glomerulopathies. Dis Mon DM 42(6): 329-383.
- de Sequera Ortiz P (2007) Glomerulonephritis secondary to systemic, inflammatory, infectious diseases and dysproteinemias. Med -Form Medica Contin Accredited Program 9(80): 5148-5156.
- R Binaut, N Maisonneuve, P Vanhille (2004) Glomerular nephropathies diagnostic orientation and evolution. ClinicalKey 1(2): 110-120.
- (2019) US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 75(1): 1-64.
- Rheault MN, Wenderfer SE (2018) Evolving Epidemiology of Pediatric Glomerular Disease. Clin J Am Soc Nephrol 13(7): 977-978.
- Jhaveri KD, Fishbane S (2014) Glomerular Diseases Entering a New Era. Clin J Am Soc Nephrol 9(3): 598-599.
- Garg P (2018) A Review of Podocyte Biology. Am J Nephrol 47(1): 3-13.
- (2021) Introduction to glomerular diseases-ClinicalKey.
- Nagata M (2016) Podocyte injury and its consequences. Kidney Int 89(6):1221-1230.
- Grahammer F (2017) New structural insights into podocyte biology. Cell Tissue Res 369(1): 5-10.
- Mathieson PW (2009) Update on the podocyte: Curr Opin Nephrol Hypertens 18(3): 206-211.
- Brinkkoetter PT, Ising C, Benzing T (2013) The role of the podocyte in albumin filtration. Nat Rev Nephrol 9(6): 328-336.
- Jauregui A, Mintz DH, Mundel P, Fornoni A (2009) Role of altered insulin signaling pathways in the pathogenesis of podocyte malfunction and microalbuminuria. Curr Opin Nephrol Hypertens 18(6): 539-545.
- Lal MA, Patrakka J (2021) Understanding Podocyte Biology to Develop Novel Kidney Therapeutics. Front Endocrinol 389-409
- Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, et al. (2005) Podocyte Depletion Causes Glomerulosclerosis: Diphtheria Toxin–Induced Podocyte Depletion in Rats Expressing Human Diphtheria Toxin Receptor Transgene. J Am Soc Nephrol 16(10): 2941-2952.
- Marjolein Garsen(2016) Cathepsin L is crucial for the development of early experimental diabetic nephropathy de 90(5): 1012-1022.
- Rinschen MM, Huesgen PF, Koch RE (2018) The podocyte protease web: uncovering the gatekeepers of glomerular disease. Am J Physiol-Ren Physiol. 315(6): 1812-1816.
- Sever S, Altintas MM, Nankoe SR, Möller CC, Ko D, Wei C, et al. (2007) Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest 117(8): 2095-2104.
- Keisuke S, Kohei M, Takuji E, Tomoki M, Yuichi M, Rina O, et al. (2020) Role of cathepsin L in idiopathic nephrotic syndrome in children. Med Hypotheses. 141: 109718.
- Yamamoto-Nonaka K, Koike M, Asanuma K, Takagi M, Oliva Trejo JA, et al. (2016) Cathepsin D in Podocytes Is Important in the Pathogenesis of Proteinuria and CKD. J Am Soc Nephrol JASN 27(9): 2685-2700
- Blass G, Levchenko V, Ilatovskaya DV, Staruschenko A (2016) Chronic cathepsin inhibition by E‐64 in Dahl salt‐sensitive rats. de mayo de 4(17): 12950.
- Alghamdi TA, Majumder S, Thieme K, Batchu SN, White KE, et al. (2017) Janus Kinase 2 Regulates Transcription Factor EB Expression and Autophagy Completion in Glomerular Podocytes. J Am Soc Nephrol JASN 28(9): 2641-2653.
- Takagi H, Nishibori Y, Katayama K, Katada T, Takahashi S, et al. (2017) USP40 gene knockdown disrupts glomerular permeability in zebrafish. Am J Physiol-Ren Physiol 312(4): 702-715.
- Meyer-Schwesinger C, Meyer TN, Sievert H, Hoxha E, Sachs M, et al. (2011) Ubiquitin C-Terminal Hydrolase-L1 Activity Induces Polyubiquitin Accumulation in Podocytes and Increases Proteinuria in Rat Membranous Nephropathy. Am J Pathol 178(5): 2044-2057.
- Yuan Liu, Huijuan Wu, Jiajing Wu, Suxia Wang, Ye Liu, et al. (2008) Detection of UCH-L1 expression by pre-embedding immunoelectron microscopy with colloidal gold labeling in diseased glomeruli. Ultrastruct Pathol 32(1): 5-9.
- Read NC, Gutsol A, Holterman CE, Carter A, Coulombe J, et al. (2014) Ubiquitin C-terminal hydrolase L1 deletion ameliorates glomerular injury in mice with ACTN4-associated focal segmental glomerulosclerosis. Biochim Biophys Acta 1842(7): 1028-1040.
- Cui J, Xie X (2017) UCH-L1 Expressed by Podocytes: a Potentially Therapeutic Target for Lupus Nephritis? Inflammation 40(2): 657-665.
- Jamin A, Berthelot L, Couderc A, Chemouny JM, Boedec E, et al. (2018) Autoantibodies against podocytic UCHL1 are associated with idiopathic nephrotic syndrome relapses and induce proteinuria in mice. J Autoimmun 89: 149-161.
- Xuefei Tian, Jin Ju Kim, Susan M Monkley, Nanami Gotoh, Ramiro Nandez, Keita Soda, et al. (2014) Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest 124(3): 1038-1113
- Verheijden KAT, Sonneveld R, Bakker-van Bebber M, Wetzels JFM, van der Vlag J, et al. (2018) The Calcium-Dependent Protease Calpain-1 Links TRPC6 Activity to Podocyte Injury. J Am Soc Nephrol JASN 29(8): 2099-2109.
- Tian X, Inoue K, Zhang Y, Wang Y, Sperati CJ, et al. (2020) Inhibiting calpain 1 and 2 in cyclin G associated kinase-knockout mice mitigates podocyte injury. JCI Insight 5(22): 142740.
- Chowdhury I, Tharakan B, Bhat GK (2008) Caspases an update. Comp Biochem Physiol B Biochem Mol Biol 151(1): 10-27.
- Wang Y, He Y, Wang M, Lv P, Wang J, et al. (2017) Role of Protease-Activated Receptor 2 in Regulating Focal Segmental Glomerulosclerosis. Cell Physiol Biochem 41(3): 1147-1155.
- Pereira Prado V, Asquino N, Apellaniz D, Bueno Rossy L, Tapia G, et al. (2016) Extracellular matrix metalloproteinases (mmps) in Dentistry. Odontostomatology18(28): 20-29.
- Li S-Y, Huang P-H, Yang A-H, Tarng D-C, Yang W-C, Lin C-C, et al. (2014) Matrix metalloproteinase-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Kidney Int 86(2): 358-369.
- Rondeau E, Vigneau C, Berrou J (2001) Role of thrombin receptors in the kidney: lessons from PAR1 knock‐out mice. Nephrol Dial Transplant 16(8): 1529-1531.
- Brieger K, Schiavone S, Miller Jr, Krause K (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142: 13659.
- Susztak K, Raff AC, Schiffer M, Böttinger EP (2006) Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55(1): 225-233.
- Gujarati NA, Vasquez JM, Bogenhagen DF, Mallipattu SK (2020) The complicated role of mitochondria in the podocyte. Am J Physiol Ren Physiol 319(6): 955-965.
- Bhargava P, Schnellmann RG (2017) Mitochondrial energetics in the kidney. Nat Rev Nephrol 213(10): 629-646.
- Abe Y, Sakairi T, Kajiyama H, Shrivastav S, Beeson C, et al. (2010) Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol 299(2): 464-476.
- Gnudi L, Long DA (2020) (Eds) Diabetic Nephropathy: Methods and Protocols.
- Kitching AR, Hutton HL (2016) The Players: Cells Involved in Glomerular Disease. Clin J Am Soc Nephrol 11(9): 1664-1674.
- Lafayette RA, Druzin M, Sibley R, Derby G, Malik T, et al. (1998) Nature of glomerular dysfunction in pre-eclampsia. Kidney Int 54(4): 1240-1249.
- Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M (2007) Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes 56(8): 2155-2160.
- Dou L, Jourde-Chiche N (2019) Endothelial Toxicity of High Glucose and its by-Products in Diabetic Kidney Disease. Toxins 11(10): 578.
- Nester CM, Barbour T, de Cordoba SR, Dragon Durey MA, Fremeaux-Bacchi V, et al. (2015) Atypical aHUS: State of the art. Mol Immunol 67(1): 31-42.
- Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, et al. (2009) Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med 11:19.
- Salmon AHJ, Satchell SC (2012) Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol 226(4): 562-574.
- Salmon AHJ, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, et al. (2012) Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol JASN 23(8): 1339-1350.
- Singh A, Fridén V, Dasgupta I, Foster RR, Welsh GI, et al. (2011) High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am J Physiol Renal Physiol 300(1): 40-48.
- Singh A, Ramnath RD, Foster RR, Wylie EC, Fridén V, et al. (2013) Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. Plos one 8(2): 55852.
- Ramnath RD, Butler MJ, Newman G, Desideri S, Russell A, et al. (2020) Blocking matrix metalloproteinase-mediated syndecan-4 shedding restores the endothelial glycocalyx and glomerular filtration barrier function in early diabetic kidney disease. Kidney Int 97(5): 951-65.
- Reine TM, Lanzalaco F, Kristiansen O, Enget AR, Satchell S, et al. (2019) Matrix metalloproteinase-9 mediated shedding of syndecan-4 in glomerular endothelial cells. Microcirc: 12534.
- Desideri S, Onions KL, Baker SL, Gamez M, El Hegni E Hussien H, et al. (2019) Endothelial glycocalyx restoration by growth factors in diabetic nephropathy. Biorheology 56(3): 163-179.
- Abboud HE (2012) Mesangial cell biology. Exp Cell Res 318(9): 979-985.
- Kurihara H, Sakai T (2017) Cell biology of mesangial cells: the third cell that maintains the glomerular capillary. Anat Sci Int 92(2): 173-186.
- Floege J, Johnson RJ, Couser WG (1992) Mesangial cells in the pathogenesis of progressive glomerular disease in animal models. Clin Investig 70(9): 857-864.
- Floege J, Eitner F, Alpers CE (2008) A New Look at Platelet-Derived Growth Factor in Renal Disease. J Am Soc Nephrol 19(1): 12-23.
- Reznichenko A, Korstanje R (2015) The role of platelet-activating factor in mesangial pathophysiology. Am J Pathol 185(4): 888-896.
- Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC (2008) Mechanisms of Glomerulosclerosis in Diabetic Nephropathy. Diabetes 57(6): 1439-1445.
- Cameron JS (1999) Lupus Nephritis. J Am Soc Nephrol 10(2): 413-24.
- Dufek B, Meehan DT, Delimont D, Cheung L, Gratton MA, et al. (2016) Endothelin A receptor activation on mesangial cells initiates Alport glomerular disease. Kidney Int 90(2): 300-310.
- Massy ZA, Guijarro C, O’Donnell MP, Kim Y, Kashtan CE, et al. (1999) The central role of nuclear factor-κB in mesangial cell activation. Kidney Int 56: 76-79.
- Scindia YM, Deshmukh US, Bagavant H (2010) Mesangial pathology in glomerular disease: targets for therapeutic intervention. Adv Drug Deliv Rev 62(14): 1337-1343.
- Kosaka T, Miyajima A, Kikuchi E, Horiguchi Y, Umezawa K, et al. (2008) The Novel NF-κB Activation Inhibitor Dehydroxymethyl-Epoxyquinomicin Suppresses Anti-Thy1.1-Induced Glomerulonephritis in Rats. Nephron Exp Nephrol 110(1): 17-24.
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M (1995) Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 270(5234): 286-290.
- Zoja C, Corna D, Rottoli D, Zanchi C, Abbate M, et al. (2006) Imatinib ameliorates renal disease and survival in murine lupus autoimmune disease. Kidney Int 70(1): 97-103.
- Morikawa M, Derynck R, Miyazono K (2016) TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect Biol 8(5).
- Carl M, Akagi Y, Weidner S, Isaka Y, Imai E, et al. (2003) Specific inhibition of Egr-1 prevents mesangial cell hypercellularity in experimental nephritis. Kidney Int 63(4):1302-1312.
- Fukasawa H, Yamamoto T, Suzuki H, Togawa A, Ohashi N, et al. (2004) Treatment with anti-TGF-β antibody ameliorates chronic progressive nephritis by inhibiting Smad/TGF-β signaling. Kidney 65(1): 63-74.
- Hohenstein B, Daniel C, Wittmann S, Hugo C (2008) PDE-5 inhibition impedes TSP-1 expression, TGF-β activation and matrix accumulation in experimental glomerulonephritis. Nephrol Dial Transplant 23(11): 3427-3436.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.