Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Development And Validation of An In-House Brucella Smooth Lipopolysaccharide as A Coating Antigen in ELISA For Serodiagnosis of Bovine Brucellosis
*Corresponding author: Central Laboratory for Evaluation of Veterinary Biologics, Agricultural Research, Egypt.
Received: January 20, 2022; Published: March 03, 2022
DOI: 10.34297/AJBSR.2022.15.002145
Abstract
Background: Brucellosis is an important zoonotic bacterial disease of global health importance affecting different animals and man. Brucellosis control and eradication procedures are highly depending on accurate diagnostic tools and effective and safe vaccination programs. Indirect ELISA, complement fixation tests, Buffered Acidified Plate Agglutination and the Rose Bengal plate agglutination are usually used for testing animals against bovine brucellosis.
The aim of the work is to evaluate and validate the diagnostic efficacy of in-house prepared S-LPS as an IELISA coating antigen in comparison to commercial ELISA kit used for diagnosis of bovine brucellosis.
Materials and methods: an Indirect Enzyme Linked Immunosorbent Assay (I-ELISA) was developed using in house produced and titrated Smooth Lipopolysaccharide (S-LPS) as coating antigen and compared with the commercial kit using 115 negative and positive bovine sera. The performance parameters for each test were estimated using Complement Fixation Test (CFT) as a gold standard test.
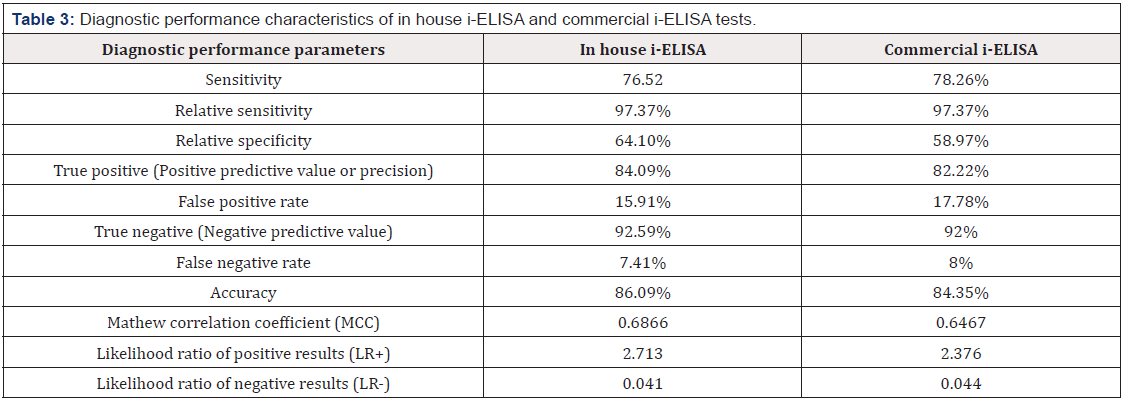
Results: the diagnostic performance characteristics for both ELISA tests were estimated to evaluate the diagnostic efficacy of in-house S-LPS. Brucella antibodies were detected in 88 (76.52%), 90 (78.26%) and 76 (66.09%), by in house i-ELISA, commercial i-ELISA and CFT, respectively. agreement for the positive result between the developed and commercial I-ELISA was in 14 positive sample and in 23 negative samples. Relative sensitivity was (97.37%) for both ELISA assay, while the specificity was 64.1% and 58.97% for developed I-ELISA and commercial ELISA kit respectively.
Conclusion: Results revealed that in house prepared, titrated, and validated S-LPS can be used as a coating antigen for IELISA test for bovine brucellosis diagnosis as a sensitive test. On other hand- in house test is cheaper than commercially available kits and so, it could be used for wide surveillance program.
Keywords: Brucella, Bovine Brucellosis, S-LPS, IELISA, CFT
Introduction
Brucellosis is a worldwide bacterial zoonotic disease of global importance affecting different mammals including man, cattle, camels, buffaloes, sheep, goats, swine, many species of wild animals, rodents, and marine mammals. The disease primarily affects the reproductive system of farm animals with significant loss of productivity (milk production) and re-productivity (fetal deaths) of affected animals. In human, the symptoms characterized by recurrent febrile episodes called undulant fever causing economic and public health importance. Due to lack of effective vaccines for man and the severity of human brucellosis, Brucella as an important agent for bioterrorism and eradication of human brucellosis has been largely dependent on the eradication of the disease in animals [1,2,3]. Control and eradication of animal’s brucellosis depend mainly on comprehensive vaccination, surveillance, and quarantine programs. Surveillance system depends mainly on an efficient, accurate and sensitive diagnostic tool especially serological test [4]. Serological tests used for diagnosis of bovine brucellosis such as Indirect enzyme-linked immunosorbent assay (IELISA) and slide agglutination tests are usually dependent on smooth lipopolysaccharides (S-LPS) as a diagnostic antigen [5,6,7] So, the aim of the work is to evaluate and validate the in-house prepared S-LPS as a IELISA coating antigen in comparison to commercial ELISA kit used for diagnosis of bovine brucellosis in bovine serum samples collected from brucellosis infected flock.
Material And Methods
Serum: Randomized bovine serum samples: 115 bovine sera collected from non-vaccinated animals of infected flocks. Brucellosis positive and negative sera were determined using conventional rose Bengal test as a screening pilot test for bovine brucellosis diagnosis [1,8,9].
In house prepared S-LPS: smooth lipopolysaccharide spleens (S-LPS, Hot Saline Extract) were prepared according to [9,10] and titrated to be used as a coating antigen using checkerboard method according to [9].
In house Indirect Enzyme-Linked Immunosorbent Assay (I-ELISA): was performed on a flat shaped microplate according to procedure described by [1,9] using in house prepared S-LPS and validated ELISA buffer.
Commercial-Validated ELISA: Commercial ELISA was done using Brucella abortus antibody test kit (IDEXX)
Complement Fixation test (CFT): Complement and hemolysin were prepared and preserved according to [9] and coping with [11] Stowell, personal communication, November 22, 2010). Sheep RBCs were collected on Alsever’s solution from an adult healthy ram serologically negative to brucellosis. These were standardized to 2% suspension in Veronal buffered saline (VBS). The proper test was performed according to the current American SOP by [12]. Warm fixation of complement at 37°C was adopted as cold fixation was unacceptably slow. The positive cutoff point for CFT was ≥ 20 ICFTU/ml. Quality control and quality assurance were fulfilled according to the requirements of the ISO/IEC 17025:2017 and the OIE guidelines [1]. With respect of CFT as a gold standard test [13] evaluation of performance of both i-ELISA tests in this study was carried out by calculating sensitivity, accuracy, positive predictive value or precision, negative predictive value, relative sensitivity, relative specificity, false positive rate, and false negative rate in addition to likelihood ratio of positive results and likelihood ratio of negative results. All above mentioned analysis were conducted using (http://vassarstats.net/clin1.html, https://ebmtools.knowledgetranslation.net/calculator/diagnostic/ and http://onlineconfusionmatrix.com/). Receiver Operating Characteristics (ROC) graph was plotted for cut off selection at its best accuracy. ROC was conducted using the statistical software MEDCALC.
Results And Discussion
Indirect ELISA and complement fixation tests, Buffered Acidified Plate Agglutination test and the Rose Bengal plate agglutination test are usually used for testing animals against brucellosis. The complement fixation test is the only test recommended for confirmation and international trade, but other tests as I-ELISA, competitive ELISA and Agar Gel Precipitation tests are used for confirmation purposes. S-LPS had been considered the most efficient diagnostic antigen for brucellosis (all species including human) during immune response and are the most important target for many immunological and serological research and studies. LPS can be easily extracted, standardized, and quantified as compared to other antigens. Isolation and identification of Brucella is performed as the gold standard for diagnosis of brucellosis [14].
The optimal working dilutions of S-LPS coating antigen was estimated with checkerboard titrations. In the present study, 115 bovine sera samples tested by commercial and developed IELISA and CFT. In house S-LPS have been successfully produced. This study differs from other performed research [15-18] in that the buffer used with in-house made ELISA was not in house prepared but it is commercially prepared and validated buffers, so, this study evaluated and validated the coating antigen S-LPS used for ELISA. Considering CFT as a gold standard test, evaluation of S-LPS antigen depend on measuring the performance of ELISA test using various performance parameters as sensitivity, relative sensitivity and specificity which were calculated using (http://vassarstats.net/clin1.html, https://ebm-tools.knowledgetranslation.net/calculator/diagnostic/, http://onlineconfusionmatrix.com/ and statistical software MEDCALC).
Out of 115 bovine serum samples, 88 (76.52%) (95%-CI: 67.52- 0.8371%), 90 (78.26%) (95%-CI: 69.4-85.19%) and 76 (66.09%) (95%-CI: 56.59-74.49%) were found positive for Brucella antibodies by in house i-ELISA, commercial i-ELISA and CFT, respectively (Table 1). *sensitivity %= positive animals/total number of tested animals X 100. A Venn diagram (Figure 1) expressed in detail the agreement of positive results among all serological tests. There was not a single positive case detected only by CFT, where at least one of two ELISA assays could identify CFT positive samples, also there was not a single positive case detected only by developed i-ELISA, where at least one of two other assays could identify in house i-ELISA positive samples. CFT, in house ELISA and commercial ELISA shared 72 positive samples where the total agreement between the three tests (positive samples+ negative samples) was in 95 samples. Various performance parameters were collectively measured and analyzed for diagnostic validation of serological test (Table 2 and 3).
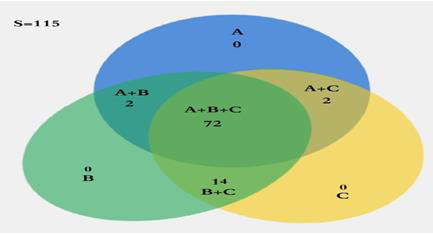
Figure 1: Venn diagram simply representing in detail the agreement of positive cattle results among serological tests and CFT.
A= CFT
B= in house ELISA
C= commercial ELISA
S= sample size
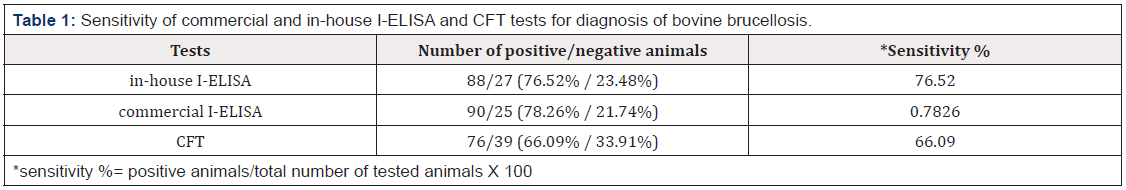
Table 1. Sensitivity of commercial and in-house I-ELISA and CFT tests for diagnosis of bovine brucellosis.
Each performance parameter was compared among all immunoassays to detect which assay behaved the best. In house ELISA was superior in 8 diagnostic parameters which were relative specificity, true positive, false positive rate, True negative, false negative rate, accuracy, Mathew correlation coefficient and Likelihood ratio of positive results while commercial ELISA scored the best superior in only three parameter which were Sensitivity, Relative sensitivity, and Likelihood Ratio of negative results (LR). The relative sensitivity of developed I-ELISA was comparable and equal with commercial I-ELISA (97.37%) whereas the specificity was a little bit compromised (64.1% and 58.97% respectively). Many serological and diagnostics tests are performed with different specificity and sensitivity, but they must be used in accordance with strict standardization rules and meet the requirements laid down by the OIE. For bovine brucellosis the OIE recommends i-ELISA (Sensitivity 97.2%, Specificity 97.1-99.8%) and CFT (Sensitivity 90- 91.8%, Specificity 99.7-99.9%) [19]. Evaluation of the two ELISA tests indicates that both had a valid sensitivity with approximately same degrees.
Receiver Operating Characteristics (ROC) graph for the comparison of home developed and commercial I-ELISA for detection of Brucella antibodies in bovine serum is shown in (Figure 2). The ROC curve showed the behavior of the sensitivity and specificity of both ELISA assay by using different cut-off points considering CFT as gold standard. This curve expresses the Area Under the Curve (AUC) which represent accuracy. The area under ROC curve (AUC) were 0.807 and 0.782 for in house i-ELISA and commercial i-ELISA respectively. The closer the curve follows the left-hand border and then the top border of the ROC space, the more accurate the test and the closer the curve comes to the 45-degree diagonal of the ROC space, the less accurate the test. Based on the ROC curve and difference between the two AUC (0.0256), result revealed that in house i-ELISA has better accuracy than that of test.
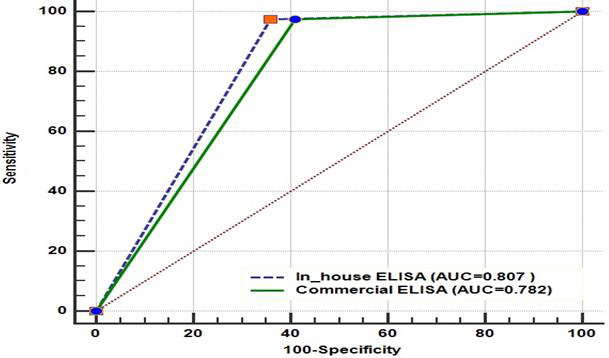
Figure 2: Receiver Operating Characteristics (ROC) graph for the comparison of homemade developed and commercial I-ELISA for detection of Brucella antibodies in bovine sera.
Difference in accuracy between two tests is not significant. In case of diseases control like brucellosis, it is preferable that the pilot or screening test is cheap, reliable and has sensitivity to detects almost all positive cases in a herd. Using i-ELISA, the high percentage of positivity was due to the ability of this test to detect very low levels of antibodies present in the early stage of infection, while RBPT and SAT cannot detect it [20]. The use of smooth LPS as antigen in the I-ELISA might be one of the reasons for higher sensitivity as the stronger immune responses are elicited against LPS in infected animals. Most bovine brucellosis ELISA kits available in market is very expensive and cannot used for national surveillance program especially in developing countries like Egypt. So, this developed test can be used for this wide surveillance as it cheaper than commercial ones and reliable with approximately same sensitivity as that of commercial kit.
Conclusion
The results of this study revealed that the sensitivity of developed I-ELISA proved to be equal to the sensitivity of commercial I-ELISA kit. The standardized developed I-ELISA could be easily available and developed, useful and cheap diagnostic test for detection of Brucella antibodies in bovine sera in comparison with the commercial kit especially where wider surveillance of large animals’ population is needed which economically impossible using commercial kits and particularly useful for a region where little epidemiological information is available about this disease and where surveillances is needed.
Conflict of Interest
None.
Acknowledgement
None.
References
- OIE, World Organization for Animal Health (2018) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, OIE, Paris, France.
- Cutler S J, Whitmore A M, Commander N J (2005) Brucellosis-new aspects of an old disease. J Applied Microbiol 98(6): 1270-1281.
- World Health Organization (1998) MZCP report on the third workshop on human and animal brucellosis, epidemiological surveillance in the MZCP countries. Damascus, Syrian Arab Republic, 4-5.
- Refai M (2002) Incidence and control of brucellosis in the Near East region. Vet Microbiol 90(1-4): 81-110.
- El Eragi AM, Manal H Salih, Mihad F E M Alawad, Mohammed K B (2016) Evaluation of immunochromatographic assay for serodiagnosis of bovine brucellosis in Gezira State, Sudan. Veterinary World, EISSN: 2231-0916.
- Nielsen K, Yu WL (2010) serological diagnosis of brucellosis. Sec Biol Med Sci 31(1): 65-89.
- Díaz R, Moriyón I (1989) Laboratory techniques in the diagnosis of human brucellosis Clin Microbiol Rev 33(1): 73-83.
- Blasco J M, Garin Bastuji B, Marin C M, Gerbier G, Fanlo J, et al. (1994) Efficacy of different rose bengal and complement fixation antigens for the diagnosis of Brucella melitensis infection in sheep and goats. Vet Rec 134(16): 415-420.
- Alton G G, L M Jones, R D Angus, J M Verger (1988) Techniques for the Brucellosis Laboratory. Institute National de la Recherche Agronomique, Paris 192: 195.
- Plackett P, G S Cottew, S J Best (1976) An indirect haemolysis test (IHLT) for bovine brucellosis. Aust Vet J 52(3): 136-140.
- Hennager S G (2004) Reagent Production Protocol Guinea Pig Complement Preparation for the Complement Fixation Test USDA, APHIS, National Veterinary Services Laboratories (NVSL)
- Hennager S G (2015) SOP-Complement Fixation Test for Detection of Antibodies to Brucella abortus and Brucella suis. USDA, APHIS, National Veterinary Services Laboratories (NVSL), ames, IA, USA.
- Yohannes M, Gill J PS, Ghatak S, Singh D K, Tolosa T (2012) Comparative evaluation of the Rose Bengal plate test, standard tube agglutination test and complement fixation test for the diagnosis of human brucellosis. Rev sci tech Off int Epiz 31(3): 979-984.
- OIE (World Organization for Animal Health) (2008) Bovine brucellosis. In: Manual of
Diagnostic Tests and Vaccines for Terrestrial Animals, 6th (edn). OIE, Paris, France 624-659. - Moustafa A Fadeel, Momtaz O Wasfy, Guillermo Pimentel, John D Klena, Francis J Mahoney, et al. (2006) Rapid enzyme-linked immunosorbent assay for the diagnosis of human brucellosis in surveillance and clinical settings in Egypt. Saudi Med J 27(7): 975-981.
- Munir R, Ur Rehman S T, Kausar R, Saqlan Naqvi S M, Farooq U (2008) Indirect Enzyme Linked Immunosorbent Assay for Diagnosis of Brucellosis in Buffaloes. Acta Vet Brno 77: 401-406.
- Ihsan Muneer Ahmed, Siti Khairani Bejo, Latiffah Hassan, Abdul Rani Bahaman and Abdul Rahman Omar (2015) Serological diagnostic potential of recombinant outer membrane proteins (rOMPs) from Brucella melitensis in mouse model using indirect enzyme-linked immunosorbent assay. BMC Veterinary Research 11(1): 275.
- Genc O, Cetinkol Y, Buyuktanır Yas O, Yurdusev N (2019) Validation of Two Rapid Serological Tests for Human Brucellosis Detection. Int J Med Dent Sci 8(1): 1682-1692.
- Wernery U (2014) Camelid brucellosis: a review. Rev sci tech Off int Epiz 33(3): 839-857.
- Guarino A, Fusco G, Serpe L, Gallo P, Di Matteo A, et al. (2001) Indirect ELISA for the diagnosis of brucellosis in water buffaloes (Bubalus bubalis) in Italy. Vet Rec 149(3): 88-90.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.