Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Effect of Hydroxychloroquine on Acute Respiratory Syndrome (Covid 19) and its Role on Blood Biomarkers Is Hydroxychloroquine Beneficial for Covid-19 Patients?
*Corresponding author: Sarra Mohamed, Registrar in General Medicine Our Lady of Lourdes Hospital Drogheda, Ireland.
Received: February 03, 2022; Published: February 24, 2022
DOI: 10.34297/AJBSR.2022.15.002130
Abstract
Background: Covid pandemic has challenged the world for a curable option. Hydroxychloroquine [HCQ], due to its immunologic and anti-inflammatory effect, has been tried during the initial pandemic but received a controversial benefit.
Method: Cavan Monghan Hospital Group has performed a retrospective study of a total of 97 patients tested positive with Covid illness. The study aimed to measure the effect of hydroxychloroquine on respiratory system, certain inflammatory markers, and mortality risk. 49 patients received hydroxychloroquine and other 48 patients treated supportively.
Result: The group of HCQ showed a reduction in the need of respiratory support and increase discharge rate. Also, mortality rate among critically ill patients has decreased, although the overall mortality among all patients showed no superiority of HCQ over the supportive care. Finally, HCQ demonstrated dramatic decrease of certain inflammatory markers.
Conclusion: HCQ has shown a good anti-inflammatory property. Thus, we suggest this is the leading cause for earlier weaning of respiratory support and decrease hospital stay in comparison of other measure of standard care.
Introduction
Hydroxychloroquine [HCQ] has been widely used for various diseases due to its immunologic and anti-inflammatory response. COVID-19 disease has been declared as a pandemic by World Health Organization [WHO] in March 2020 [1]. During this rapidly evolving period HCQ received controversial issue on its efficacy in treating COVID-19 disease. In-vitro studies have showed a promising outcome in controlling the virus with HCQ, but they have failed to confirm benefit in-vivo trials. Given the sharing similarity of 80 % with severe acute respiratory syndrome coronavirus 1 [SARS-CoV1] and 34% of middle east respiratory syndrome [MERS], it has been hypothesized that the virus activates cytokines to release proinflammatory mediators which are responsible for immune dysregulation and multiorgan damage [2,3]. HCQ has been hypothesized that it interfere with the release of proinflammatory mediators [1,4]employed initially for the treatment of malaria, are now gaining worldwide attention due to the rapidly spreading pandemic caused by severe acute respiratory syndrome-coronavirus-2, named coronavirus disease (COVID. The first case of COVID 19 was reported in Ireland on the 1st of March 2020, followed by a rapid rise of cases. Border counties had high incidence and Cavan County was ranked the second highest rate per 100,000 people. Our hospital designed a local protocol to use Hydroxychloroquine in combination with Azithromycin for hospitalized COVID-19 patients who presented with severe disease and marked derangement of laboratory results. We reviewed COVID 19 patients who were admitted to our hospital over a onemonth period [March-April 2020]. The aim of this study focuses on the effect of HCQ on respiratory outcome, mortality rates and laboratory biomarkers such as lymphocyte, CRP, AST, ferritin, LDH and D-dimer.
Method
A comparative retrospective study in Cavan-Monaghan Hospital Group [CMHG], carried out from March 2020 until April 2020. A total number of 97 patient’s data were collected. This study compared the patients who received HCQ versus those who had not. All patients in this study were confirmed COVID-19 positive through naso-oropharyngeal swab. HCQ was given in combination with Azithromycin based on the guideline, which was issued at the early stages of COVID 19 outbreak. The dose of HCQ was 400 mg twice a day on the first day, followed by 200mg once daily for the next 4 days, between 24 to 48 hours after test confirmation. The dose of Azithromycin was 500 mg once on the first day followed by 250mgs daily for next five days. Both medications were started either on the second or third day of symptoms. As per hospital guidelines between 22nd of March 2020 and 16th of April 2020 those patients who received HCQ were either clinically ill or laboratory results signified severe disease. From 17th of April 2020, due to the uncertainty on the efficacy of HCQ, our hospital stopped using HCQ as per national guidelines. Severely ill patients who admitted after that period did not receive HCQ but rather received supportive treatment. The supportive treatment included antibiotics other than azithromycin, antipyretic and respiratory support. Mild cases were managed supportively. Data were collected from patient’s medical records, laboratory systems and radiology database. Statistical analysis was done using SPSS version 21.
Results
A total number of 97 patients confirmed COVID 19 were included in this study. Forty-nine patients received HCQ [HCQ group], and the other 48 patients treated by supportive care [non- HCQ group].
Basic Demographic and Clinical Status
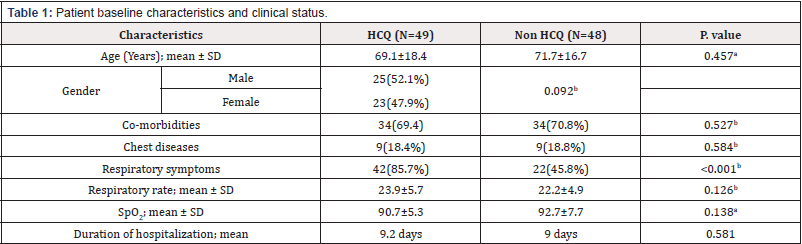
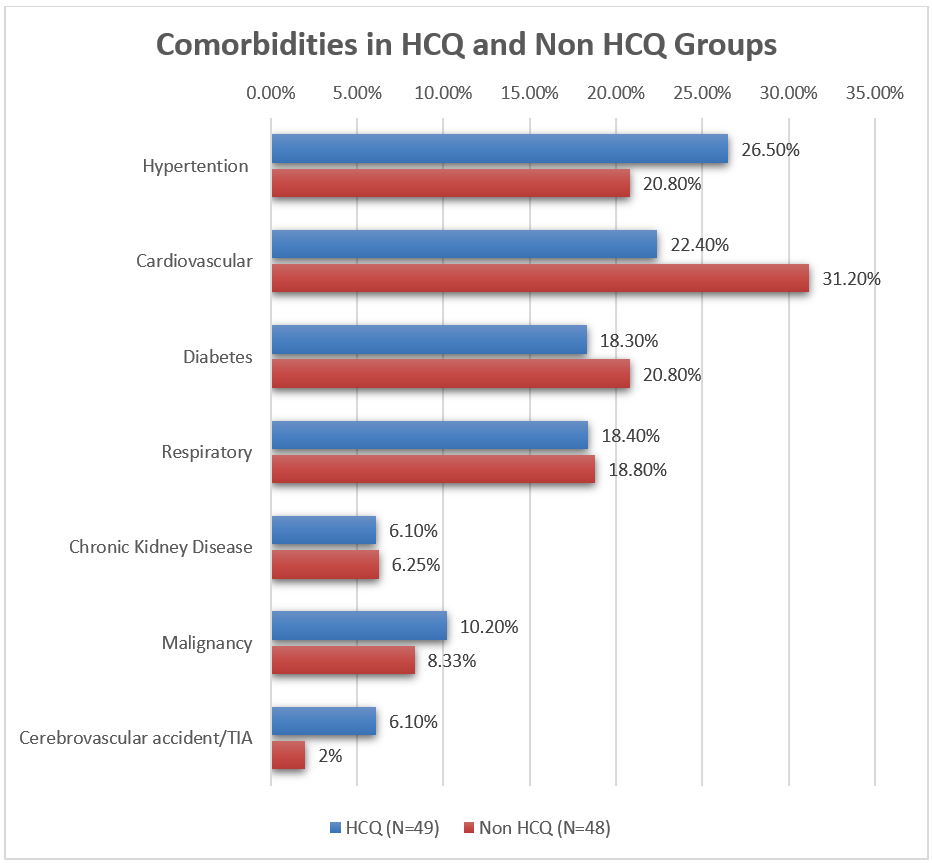
Patient’s characteristics are shown in [Table 1]. The mean age of patients was 69 years in HCQ group with male predominance [67%] and 71 years in the non-HCQ group with almost equal gender distribution. Co-morbidities and respiratory diseases shared a same percentage with slight difference in the commonest co-morbidity. Hypertension was the main co-morbid disease in HCQ-group [HCQ,26.5% vs Non HCQ, 20.80%], whereas in non- HCQ group it was cardiovascular disease risk factor [non-HCQ, 31.205% vs HCQ, 22.40%]. Respiratory diseases comprised of COPD, asthma, pulmonary fibrosis, and bronchiectasis [18.4% vs 18.8%]. Less common conditions observed were chronic kidney disease, malignancy, and cerebrovascular accidents. The main clinical presentation was respiratory symptoms which ranged from mild cough and flu like illness, to severe shortness of breath and respiratory distress. 85% in HCQ group presented initially with respiratory complains compared to 45% in non HCQ-group [P. Value=<0.001].
Radiographic Findings
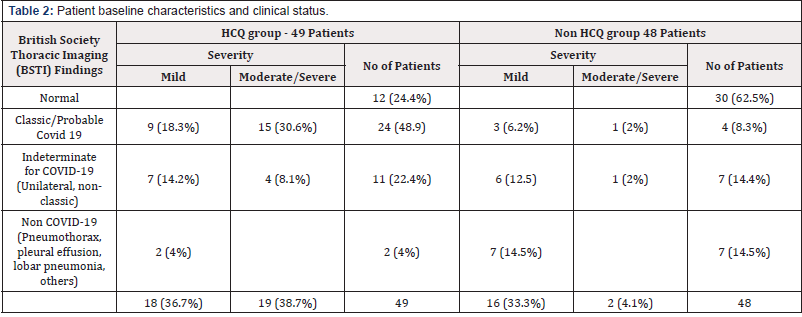
Chest X-rays obtained on admission were classified based on British Society Thoracic Imaging [BSTI] into classic COVID, indeterminate COVID, non-COVID, and normal. A comparison of both groups is shown in [Table 2] [supplementary appendix]. Overall, 55 patients had Covid 19 disease with CXR abnormalities and the majority were in the HCQ-group [37 patients]. Almost half of the patients in the HCQ group had classic bilateral peripheral CXR changes compared to four patients from non-HCQ group [48.9% vs 8.3%]. The rest of the CXR changes distributed in either indeterminate [non-classic e.g., unilateral] or non-COVID 19 such as pulmonary edema or definite lobar pneumonia. In non HCQ group, 62.5% had a normal CXR, whereas in HCQ-group 24.4% CXR were reported normal. The severity of pneumonitis on CXR has been classified into mild and moderate to severe. 19 patients in HCQ group had moderate to severe pneumonitis, 15 patients [30.6%] had classic and 4 patients [8.1%] had indeterminate CXR changes. Mild pneumonitis observed in 18/49. The distribution was as follow: 18.3% classic, 14.2% indeterminate and 4% noncovid sub-type findings. The non-HCQ group had only 2 patients with moderate/severe pneumonitis. The rest of the cases 16/48 had mild pneumonitis where the majority fall into non-covid and indeterminate [14.5% and 12.5% respectively] and classic accounted for 6.2 % CXR changes [Table 2].
Respiratory Support
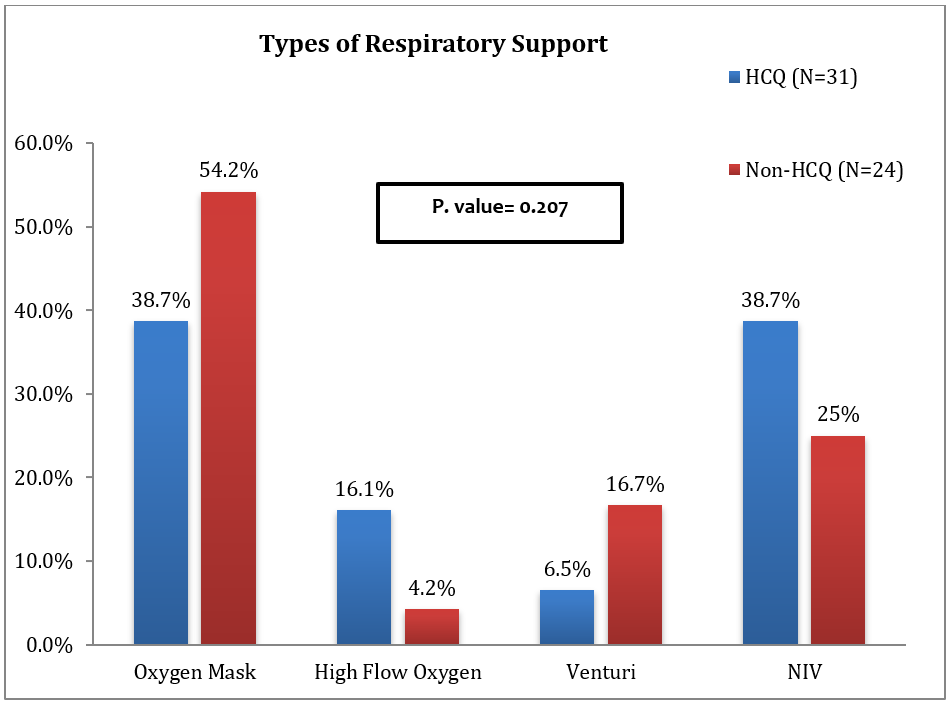
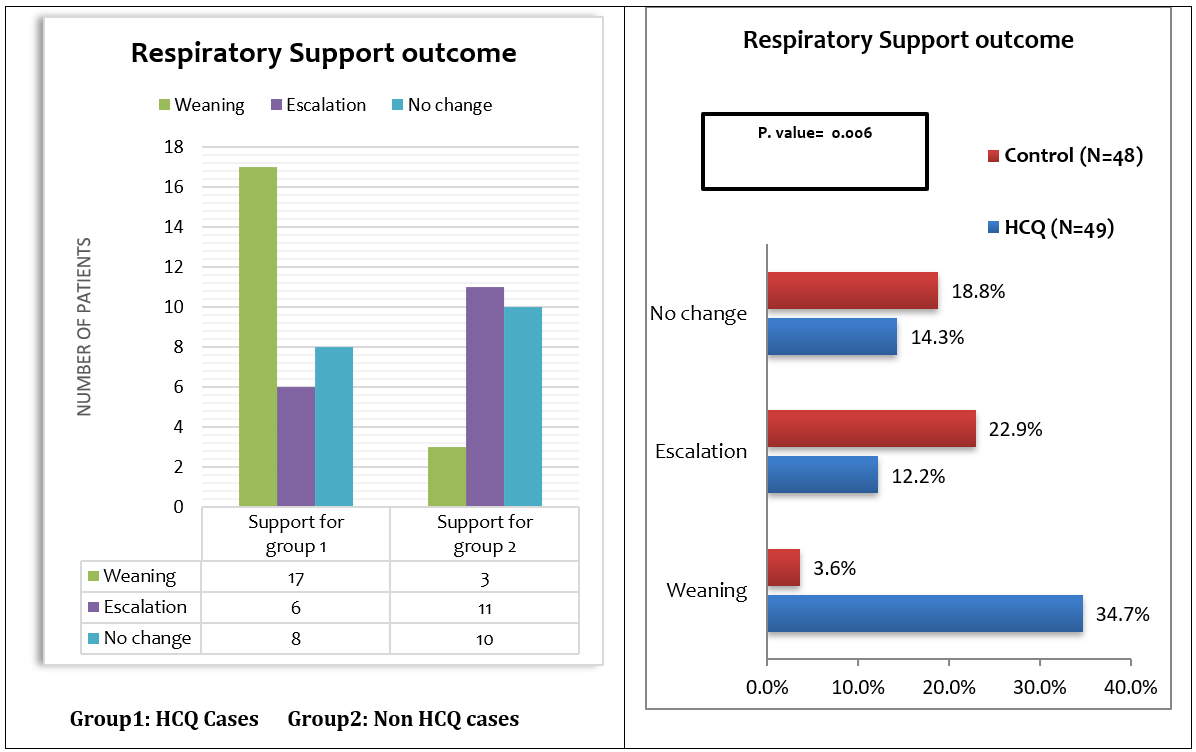
The total number of patients who needed respiratory support was 55. [Fig. 2] illustrated the types of respiratory support used in management. In thirty-one patients in HCQ group, the use of noninvasive ventilation [NIV] was equal to simple oxygen mask [38.7% for each] as initial requirement, followed by high flow oxygen [16.1%] and venturi mask [6.5%]. The need for higher respiratory care like NIV was much less in non-HCQ group and simple oxygen mask was the main required type of support [54.2 %]. The analysis of the result demonstrated that the use of HCQ had decreases the need for respiratory support compared with supportive care [Fig. 3]. The weaning rate from oxygen devices was higher in HCQ group and only 6 patients needed escalation of whom 4 were discharged eventually. In non-HCQ group, only 3 patients were weaned off oxygen, while 11 patients required escalation of their respiratory support in non-HCQ group. In addition, both discharge rate and mortality rate were significantly improved in HCQ group [P. Value=0.009]. [Fig.4].
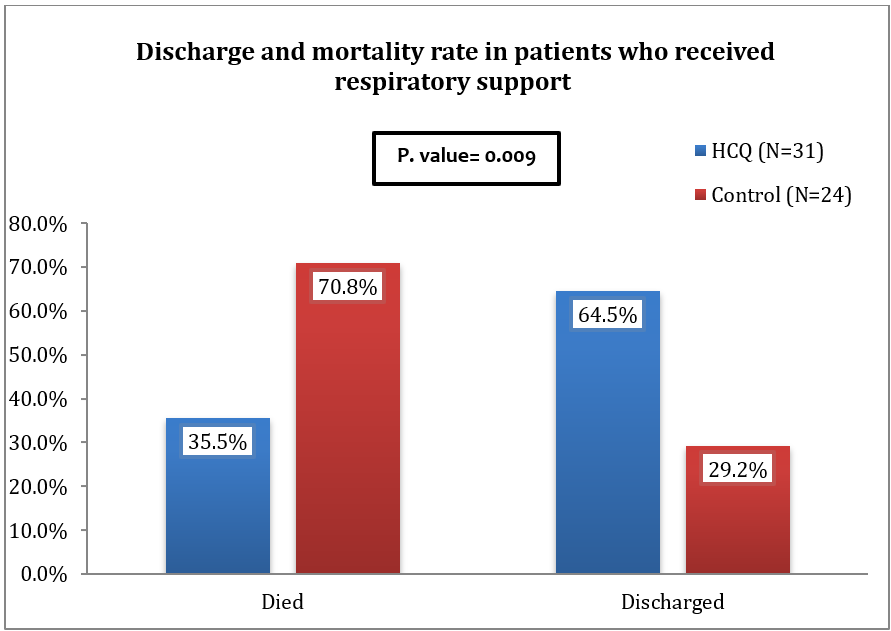
Figure 4: Percentages of patients who needed respiratory support according to result (discharge vs death).
Effect on Inflammatory markers:
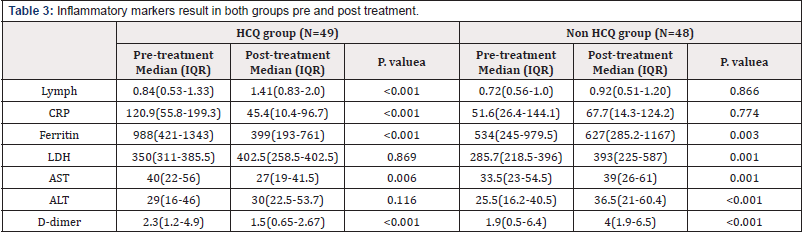
Biomarker blood results were obtained from the first result and the second one after treatment initiated during hospitalization from 6-15 days. Two analyses were done to compare the effect of HCQ. Table 4 [supplementary appendix] demonstrates all patients in the study, while table 5 [supplementary appendix] interprets the blood result in critical ill patients under respiratory care. Treatment with HCQ showed statistically significant improvement in inflammatory markers. In HCQ group lymphocytes count significantly improved [median:0.84 to median:1.41, P. Value=<0.001] compared to the mild improvement in non-HCQ group [median:0.72 to median:0.92]. Same result is noticed in the significant reduction of CRP and Ferritin levels under HCQ arm [P. Value=<0.001]. No significant difference is observed in D-dimer level as both groups showed same reduction in D-dimer level. LDH is the only parameter that improved significantly with supportive care compared to HCQ treatment.
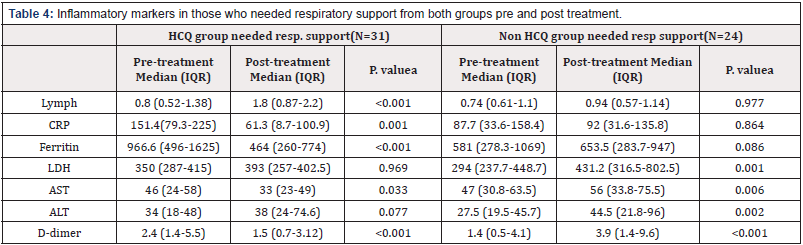
Table 4: Inflammatory markers in those who needed respiratory support from both groups pre and post treatment.
Result of all patients:
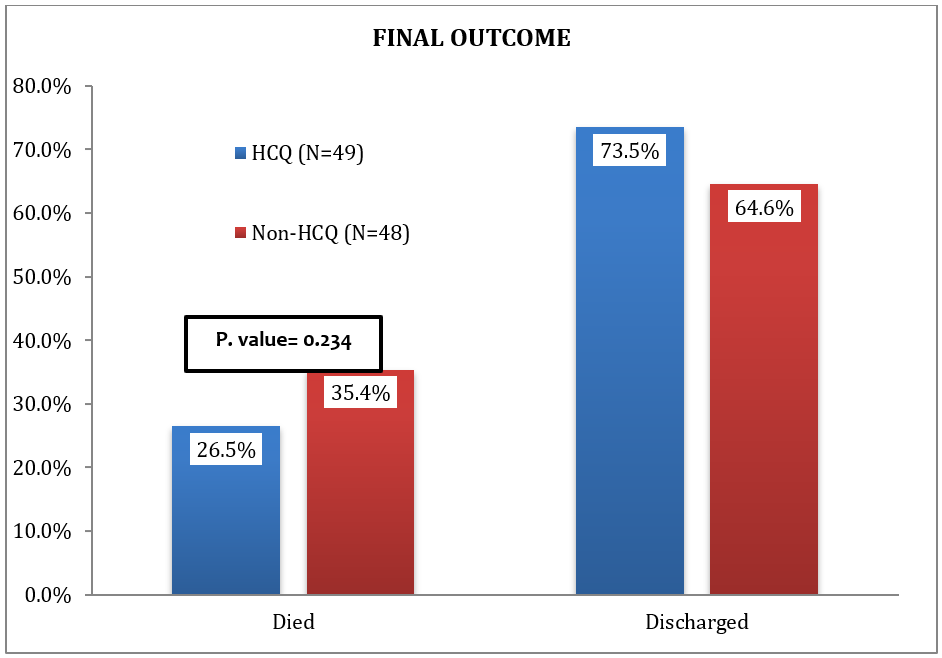
Overall mortality and discharge rate are illustrated in [Fig. 5]. The mean length of hospitalization was nine days in both groups. Our analysis showed that mortality in all patients was statistically insignificance between those received HCQ and those who did not [P. Value= 0.234]. Similar result is observed in the overall discharge rate.
Discussion
Today’s world faces a dilemma in choosing curative options for this highly contagious virus. After the initial trials that deemed hope in HCQ as a potential benefit, there is still no concrete evidence to embrace, nor to decline the use of HCQ as a potential effective therapy. The stormy event that placed our hospital under pressure, necessitated a day-by-day decision on management protocol according to the guidelines. Our hospital’s decision to discontinue the use HCQ was influenced by studies that suggest harm without strong scientific evidence. In addition, the retracted Lancet study had strengthened the view to halt treatment with HCQ. The article is testing to build evidence with other studies to help reach a global decision on curative options. Justification to treat with HCQ for COVID-19 illness is based on different studies perspective. Viral clearance and clinical coarse of the disease have been measured by different studies. The Pilot study enrolled 30 patients in an open randomized trial, demonstrated no difference in viral clearance, duration of disease and imaging improvement between those who received HCQ and those did not [1,5,6]while those in the control group were given conventional treatment only. The primary endpoint was negative conversion rate of COVID-19 nucleic acid in respiratory pharyngeal swab on days 7 after randomization. This study has been approved by the ethics committee of Shanghai public health clinical center and registered online (NCT04261517. Similar results was observed in Tang et al. study who used high dose of HCQ in 150 patients with mild and moderate disease but HCQ failed to achieve negative viral shedding [6,7]open label, randomised controlled trial. Setting 16 government designated covid-19 treatment centres in China, 11 to 29 February 2020. Participants 150 patients admitted to hospital with laboratory confirmed covid-19 were included in the intention to treat analysis (75 patients assigned to hydroxychloroquine plus standard of care, 75 to standard of care alone. Despite the high HCQ dose, gastrointestinal adverse event was a leading side effect rather than cardiac insult [6,7]open label, randomised controlled trial. Setting 16 government designated covid-19 treatment centres in China, 11 to 29 February 2020. Participants 150 patients admitted to hospital with laboratory confirmed covid-19 were included in the intention to treat analysis (75 patients assigned to hydroxychloroquine plus standard of care, 75 to standard of care alone. Paradoxically, the post analysis of Tang et al. study suggested improvement of symptoms and decrease of CRP in HCQ group [6]and to lesser extent chloroquine (CQ. A group of scientist in France published two different studies with variant sample sizes claiming positive viral eradication in patients who received HCQ compared to other treatment modalities along with good clinical improvement [6,8,9] the combination hydroxychloroquine (HCQ.
Although our study has not measure viral clearance and post treatment radiological follow up, our data included critical ill patients and the comparison was made between those who were under respiratory compromise and all patients with positive test. The analysis of the data suggested clinical and laboratory improvement under HCQ arm. In terms of respiratory composite, HCQ group patients were very sick, majority had classical chest X-ray findings of Covid 19, and required NIV on presentation. The combination therapy contributed to minimize the need for respiratory support, escalation of treatment, and reduced time span significantly compared to non-HCQ group [p value 0.006]. These results were in line with Chen et al. study that measured the overall clinical consequences without assessing viral clearance [10]. Although his study excluded critical ill patients from the analysis, his data in 62 patients illustrated not only clinical and radiological improvement, but HCQ had lessen the progression to severe disease [10]. Nevertheless, our study results do not match large sample size studies. M Mahévas et al, study suggested neither benefit of HCQ in decreasing admission to intensive care, nor increase in survival without acute respiratory distress syndrome [11]survival without acute respiratory distress syndrome, weaning from oxygen, and discharge from hospital to home or rehabilitation (all at day 21. In addition, 10% of patients who received HCQ developed electrocardiographic changes [11]survival without acute respiratory distress syndrome, weaning from oxygen, and discharge from hospital to home or rehabilitation (all at day 21. Same result was noticed in J Magagnoli et al study [12]. 412 patients who received HCQ either with or without Azithromycin, showed no reduction in the need for mechanical ventilation or mortality rate [12].
In addition, the interpretation of the result correlated well with other studies pointing to decrease inflammatory markers under HCQ treatment. In general, A dramatic improvement in lymphocyte, CRP, and D-dimer under HCQ group [P value of <0.001] compared to standard care which showed no improvement in those parameters but rather an increase in counts. These biomarkers have been linked to disease severity. In SARS-CoV2 infectivity, lymphopenia occurs due to several factors and indicates suppressed immunity [3]. C-reactive protein [CRP] correlates with the degree of infection, whereas studies points that D-dimer is used to predict disease severity [3,13]Hubei Province, China in December 2019. The etiologic agent is a novel coronavirus of presumed zoonotic origin with structural similarity to the viruses responsible for severe acute respiratory syndrome (SARS. This study also tested inflammatory markers of cytokines activation [ ferritin, LDH and AST ] that have been proposed to predict prognosis [3,13,14]Hubei Province, China in December 2019. The etiologic agent is a novel coronavirus of presumed zoonotic origin with structural similarity to the viruses responsible for severe acute respiratory syndrome (SARS. The results showed significant suppression of ferritin level in HCQ group [P value 0.001]. The improvement of AST was noticed in those needed respiratory care more than the other patients. AST elevation is a result of both tissue and hepatic injury by cytokine mediators and usually higher than alanine transferase [ALT] [15]. Of note, HCQ treatment had shown no effect on LDH levels. Mortality risk has been assessed in association with HCQ. In New York city, two large sample studies addressed the mortality issue and excluded the overall clinical improvement. Geleries et al, included 1446 patients from a single hospital, and Rosenberg et al. had 1475 patients from 25 different hospitals, both had a mortality rate of 25.1% and 20.3% respectively [16,17]excluding those who were intubated, died, or discharged within 24 hours after presentation to the emergency department (study baseline. On observation the patient who were treated with HCQ had more severe disease than non HCQ group [16,17]excluding those who were intubated, died, or discharged within 24 hours after presentation to the emergency department (study baseline. Both studies have suggested that HCQ does not improve the mortality or decrease progression to severe illness and admission to intensive care unit [16,17]excluding those who were intubated, died, or discharged within 24 hours after presentation to the emergency department (study baseline. Similar outcome was seen in Recovery trial, which stopped due to unwitnessed tangible effect in terms of decreasing death rate and escalation of care to intensive care unit [18]. The overall mortality rate in our study was 30.9% which is almost consisted to the studies conducted in NYC. However, the calculated mortality rate among respiratory support group had declined dramatically with the use of HCQ [P. Value 0.009]. This finding could be supported by Yu et al. study [19]. His study involved 48 patients out of 550 patients and showed that treatment with HCQ prolonged survival in critical ill patients and decrease death compared to those had not received the medication [19]. Another factor we measured in the study is length of hospitalization and discharge. The mean duration of hospital stay was similar in both groups. Nevertheless, given the fact that HCQgroup were sicker, we assume that HCQ could have contributed to reduce the duration of hospitalization. Furthermore, the discharge rate was significantly high among respiratory compromise patients who treated with HCQ compared to those received a standard care [P. Value 0.009]. Of note, the comparison of discharge rate among all patients showed not statistical difference among those who were under HCQ arm and the supportive care arm.
Conclusion
Despite our small sample size, the results demonstrated good ant-inflammatory properties of HCQ, and it was in line with other studies that suggest the same benefit. Our study suggests that the control of inflammation was the reason to improve respiratory distress and decrease the need for respiratory support. Perhaps studies should focus on adding a combination of anti-inflammatory drug like steroid to treat COVID 19. These results should be supported by high quality controlled randomized clinical trials which will ultimately be the definite evidence for hydroxychloroquine efficacy.
Limitation of the study
There are few limitations in this study. Firstly, the level of evidence could be low as our study is a retrospective observation study and not clinical or prospective cohort study. Furthermore, the sample size is small, especially, those who received the HCQ to draw a good reliable data. Moreover, treatment with HCQ was prescribed only for sick patient making the comparison between the two groups unequal. No radiological images were done post treatment to make the conclusion of respiratory improvement.
References
- Chowdhury MS, Rathod J, Gernsheimer J (2020) A Rapid Systematic Review of Clinical Trials Utilizing Chloroquine and Hydroxychloroquine as a Treatment for COVID-19. Acad Emerg Med 27(6): 493-504.
- Chuan Qin, Luoqi Zhou, Ziwei Hu, Shuoqi Zhang, Sheng Yang, et al. (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, Chin. J Chem Inf Model 71(15): 762-768.
- Evangelos Terpos, Ioannis Ntanasis Stathopoulos, Ismail Elalamy, Efstathios Kastritis, Theodoros N Sergentanis, et al. (2020) Hematological findings and complications of COVID-19. Am J Hematol 95(7): 834-847.
- Shukla AM, Archibald LK, Wagle Shukla A, Mehta HJ, Cherabuddi K (2020) Chloroquine and hydroxychloroquine in the context of COVID-19. Drugs Context. 9: 4-5.
- Jun Chen, Danping Liu, Li Liu, Ping Liu, Qingnian Xu, et al. (2020) A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang Univ (Medical Sci 49(2): 215-219.
- Eric A Meyerowitz, Augustin G L Vannier, Morgan G N Friesen, Sara Schoenfeld, Jeffrey A Gelfand, et al. (2020) Rethinking the role of hydroxychloroquine in the treatment of COVID-19. FASEB J 34(5): 6027-6037.
- Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. (2020) Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ 369(April): 1-11.
- Matthieu Million, Jean-Christophe Lagier, Philippe Gautret, Philippe Colson, Pierre Edouard Fournier, et al. (2020) Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis [Internet] 35(April): 101738.
- Philippe Gautret, Jean Christophe Lagier, Philippe Parola, Van Thuan Hoang, Line Meddeb, et al. (2020) Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 Covid-19 patients 34: 101663.
- Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. (2020) Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial 7.
- Mahévas M, Tran VT, Roumier M, Chabrol A, Paule R, Guillaud C, et al. (2020) Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: Observational comparative study using routine care data. BMJ 369:1-9.
- Joseph Magagnoli, Siddharth Narendran, Felipe Pereira, Tammy H Cummings, James W Hardin, et al. (2020) Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19. Med [Internet] 1(1):114-127.
- John L Frater, Gina Zini, Giuseppe d'Onofrio, Heesun J Rogers (2020) COVID-19 and the clinical hematology laboratory. Int J Lab Hematol. (1):11-18.
- Thirumalaisamy P Velavan, Christian G Meyer (2020) Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis [Internet] 95: 304-307.
- Akira Morimoto, Yozo Nakazawa, Eiichi Ishii (2016) Hemophagocytic lymphohistiocytosis: Pathogenesis, diagnosis, and management. Pediatr Int 58(9): 817-825.
- Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G et al. (2020) Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med 382(25): 2411-2418.
- Eli S Rosenberg, Elizabeth M Dufort, Tomoko Udo, Larissa A Wilberschied, Jessica Kumar, et al. (2020) Association of Treatment with Hydroxychloroquine or Azithromycin with In-Hospital Mortality in Patients with COVID-19 in New York State. JAMA - J Am Med Assoc 323(24): 2493-2502.
- Peter Horby, Marion Mafham, Louise Linsell, Jennifer L Bell, Natalie Staplin, et al. (2020) Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. medRxiv [Internet] 383(21): 2030-2040.
- Bo Yu, Chenze Li, Peng Chen, Ning Zhou, Luyun Wang, et al. (2020) Erratum to: Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci China Life Sci 63(10):1617-1618.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.