Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Effect of Monosodium Glutamate Substitutes on Physiochemical, Microbiological and Sensory Properties of Fried Chicken Breast Strips
*Corresponding author: Fahim Shaltout, Food control, Faculty of Veterinary Medicine, Benha University, Egypt.
Received: January 19, 2022; Published: February 28, 2022
DOI: 10.34297/AJBSR.2022.15.002140
Abstract
The effect of replacement monosodium glutamate (MSG) with 1:1 ratio mixture of sugar and Salton physiochemical, microbiological, and sensory properties deep fat fried chicken breast strips during frozen storage (-18°C) for 90 days was investigated. Results showed that control samples had statistically higher moisture, carbohydrate, and ash contents than the treated fried samples, moisture, carbohydrate, and ash contents for all treatments and slightly decreased as storage period progressed. The crude protein and fat contents of deep fat fried chicken breast strips decreased by replacing MSG with a mixture of 1:1 sugar and salt. The crude protein content of all treatments slightly increased as storage period progresses, while fat content of all treatments slightly decreased as storage period progressed. Treatment contains mix of sugar and salt in ratio of 1:1 as MSG alternative had a higher, WHC, cooking loss, PH and lower TVBN, and TBA values than treatment containing MSG (control). The obtained results also showed that control chicken breast strips had the highest counts of total bacterial count and lowest counts of total coliform count, then other treatment. E. coli and Salmonella were not detected in both treatments until the end of storage period. Control chicken breast strips had the lowest counts of total Staph. aureus, yeast and mold and total psychrophilic bacteria counts than other treatments. Treatment containing MSG (control) had higher sensory proprieties (color, taste, crispness, odor and acceptability) than that treatment containing mix of sugar and salt as MSG replacer.
Keywords: WHC; Cooking Loss; PH; TVBN; TBA
Introduction
Several major manufacturers have announced to move away from using artificial ingredients and flavors in their products. Monosodium Glutamate (MSG) is one such ingredient that has been controversial for decades. It is one of the ingredients that some companies have committed to remove from products [1]. MSG is a flavor enhancer commonly added to processed food products like chicken to boost the palatability. Its remarkable effects on the sensory appeal have been proven in various studies [2,3]. Removal of this ingredient is very likely to cause reduced consumer acceptability. Using MSG substitute is a promising approach to compensate for the sensory satisfaction loss caused by MSG elimination. The flavor enhancement effect of MSG is mainly from glutamate which contributes to umami or savory taste sensation. Besides glutamate, there are several other umami eliciting components such as aspartate and 5’ribonicleotides. Among nucleotides, Inosinate (IMP) and Guanylate (GMP) significantly contribute to flavor and taste enhancement [4]. Theoretically, substances that are naturally rich in umami components have the potential to replace MSG in food products. Consumers preferred natural extracts such as yeast extract, mushroom extract, and tomato extract as MSG substitute in chicken products [5].
Sugars may also contribute to umami taste characters in the form of glutamate glycoconjugates [6]. Furthermore, salts of potassium are also responsible to enhance umami taste strength. However, during boiling process, significant levels of potassium leach out from potatoes [7,8]. Sodium chloride is an important ingredient added to most of foods which contributes to flavor enhancement and food preservation [9]. MSG is a flavor enhancer that is found in some processed foods and Chinese cuisine. To avoid this sodium product there are some potential substitutes can be used as substitutes for MSG. Use 1:1 ratio mixture of sugar and salt as a substitute ingredient to your recipe instead of MSG. This is safer to use, especially if you have children at home. MSG is a food additive used as a flavor enhancer [10]. The advantage of MSG goes to those who easily lose their appetite.
This is a very common ingredient in fast foods and food seasonings. MSG is harmless but too much consumption would cause headaches, and this is not good for people who have vertigo (a sensation of spinning) [11]. Currently, there is limited research comparing the enhancement effects of MSG with these natural extracts in food products. Given the capability of salty taste enhancement, MSG substitute may also be able to increase the sensory appeal of meat products with reduced salt content. Previous study indicated that used of yeast extract successfully enhanced the taste of fermented sausage [12]. Ground mushroom has also been reported to improve the flavor of taco blend [13]. To replace MSG, it is necessary to conduct more research to compare the performance between MSG and its alternatives in salt-reduced food matrix. The aim of the current study was to investigate the effect of replacement MSG with 1:1 ratio mixture of sugar and salt on physiochemical, microbiological, and sensory properties deep fat fried chicken breast strips during frozen storage (-18°C).
Materials and Methods
Materials
Fresh boneless skinless chicken breast strips 74.33% moisture 20.72% protein, 2.26% fat, 1.18% carbohydrate, 1.18 Ash and PH 5.09, were obtained after 8 h of slaughtering from Cairo Poultry Processing Company, Sharkia, Egypt. The samples were transferred under cooling conditions to Food Technology Department Laboratory (Zagazig University, Egypt) and saved in freezer at -18°C for 3 months until processing.
Methods
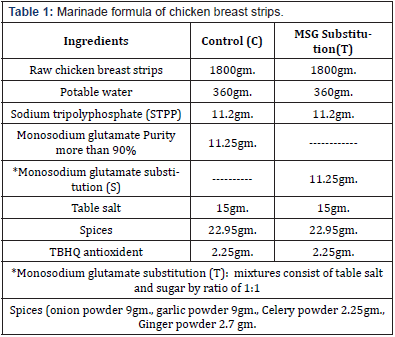
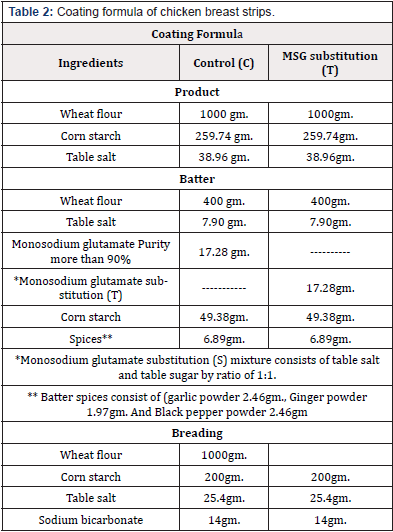
Preparation of Chicken Breast Strips: After preparation of chicken breast strips as described (Tables 1&2), samples divided into two groups: control group containing MSG (C) and the other containing MSG substitution (T).
Preparation of Marinade Solution: The amount of water below 5°C was placed in a bag of high-density polyethylene, after that the amount of food grade Sodium Tripolyphosphate (STPP) was dissolved in it, followed by dissolving the table salt and McGinn the case of control or MSG substitution (a mixture of table salt and table sugar in a ratio of 1:1) in the case of treatment and then add spices, antioxidant, and stirring to homogenize the marinade solution. The amount of raw chicken fillet strips was added to previous brine after thawing it for 24h in the refrigerator and reaching a temperature of 1-4°C. Finally, the bags were closed and flipped for five minutes and placed in the refrigerator on a temperature of 1-4°C. After 24h, the bags were opened and the chicken breast strips were removed from the soaking solution and put on a stainless-steel net for 5 min to drain excess brine solution, then the increase in the weight of the chicken breast strips acquired from the marinade solution was calculated according to the following formula [14].
% Marinade uptake= marinated weight-raw weight/raw weight×100.
Deep-Frying of Marinade Chicken Breast Strips: 1.5 liters of a mixture of sunflower and soybean oil 1:1 were placed in an electric fryer and the oil temperature was raised to 186: 188°C, then the marinated and covered chicken breast slices were placed in the oil at a rate of 4 pieces each time and the weight of the piece was approximately 40 g .When the temperature of the chicken breasts reached 74-76°C, they were removed from the oil and placed on a stainless steel mesh to get rid of the excess oil from the throwing process in the control sample. In the treatment sample (without MSG), the same previous steps were repeated after getting rid of the frying oil used in the control sample and replacing it with a new oil of the same type of oil. Samples were preserved by freezing at -18°C until the completion of the tests [15].
Chemical Analysis: Moisture, ash, crude protein, and crude lipids (%) were determined according to the methods recommended by AOAC [16], while total carbohydrate content was measured by difference.
Microbiological Examination
Preparation of Samples for Microbiological Examination: 10g of each sample were homogenized with 90mL of sterile saline solution (9g NaCl/ L distilled water). The suspension was shocked by shaker for 5min to give 0.1 dilutions. Then different dilutions (1:10-1 to 1:10-6) were prepared to be used for microbiological examination.
Aerobic Plate Count (APC): The aerobic plate count (APC) was performed as described in APHA [17].
Molds and Yeasts: Potato dextrose agar was used for yeast and mold enumeration. Plates were incubated at 25°C for 5 days, according to APHA [17].
Total Coliform Bacteria Count: Violet red bile agar was used for the enumeration of coliforms. Plates were incubated at 37°C for 24h, according to APHA [17].
Staphylococcus Aureus: Staphylococcus aureus test was performed as described in ISO, 4833-1 [18].
Salmonella Spp: Salmonella spp test was performed as described in ISO, 6579 [19].
Freshness Tests
PH Value (EOS 63/11, 2006) [20]: In astomacher, approximately 10g of the examined sample were homogenized with 25mL of neutral distilled water and left to stand for 10min at room temperature with continuous shaking and filtered. The PH was determined by using electrical PH meter (ACTWA-AD1200-1034678) calibration of PH meter by using two buffer solutions of exactly known PH (alkaline PH 7.01, acidic PH 4.01). Therefore, PH electrode was washed with neutralized water and then introduced into the homogenate.
Determination of Total Volatile Basic Nitrogen (TVB/ N)”mg” % (EOS 63/10, 2006) [21]: Teng of sample were minced in a stomacher for 1-2 min until homogenization. Then in a distillation flask add 2g of magnesium oxide and 300mL distilled water to the minced sample. Make distillation and receive 100mL distillate within 30min in a beaker contain 25mL of 2% boric acid. Then titrate against H2SO4 0.1M until faint pink color.
TVN mg/100g = R× 14
Where R is the volume of H2SO4 exhausted in titration.
Determination of Thiobarbituric Acid (TBA)”mg/Kg” (EOS 63/9, 2006) [22]: TBA number is expressed as milligrams of malondialdehyde equivalents per kilogram of sample. Ten g of sample were blended with 48ML of distilled water, to which 2mL of 4% of ammonium chloride (to brings the PH to 1.5) were added in astomacher for 2min and left at room temperature for 10 min. The mixture was quantitatively transferred into Kjeldahl flasks by washing with additional 50 mL distilled water, followed by an antifoaming preparation and few glass beads. The Kjeldahl distillation apparatus were assembled, and the flask was heated to 50°C. 50mL distillate was collected in 10 min from the time of boiling commences. The distillate was mixed, and then 5mL was pipette into a glass- Stoppard tube. 5mL of TBA reagent (0.2883g/100mL of 90% glacial acetic acid) were added. The tube was stoppered, shacked, and immersed in boiling water bath for 35 min. A blank was similarly prepared using 5mL distilled water with 5ml TBA reagent and treated like the sample. After heating, the tube was cooled under tape water for 10 min. A portion was transferred to a curette and the optical density (D) of the sample was read against the blank by means of spectrophotometer (Perkin Elmer, 2380, USA) at a wavelength of 538nm.
TBA value (mg malondialdehyde /kg of sample) = D×7.8
D: the read of sample against blank.
Physical Tests
Water Holding Capacity (WHC) and Plasticity: Water Holding Capacity (WHC) and plasticity were measured according to the method described [19]. A weight of 0.3g of ground meat was placed under ash less filter paper (Whatman, No. 41) between tow glass plates (20x20cm) and pressed for 10min., using 1Kg weight. Two zones were measured using the planimeter, the water holding capacity was calculating by subtracting, the area of the internal zone from that of the outer zone. The internal zone represented the plasticity. Results were presented in cm2 per 0.3g of raw sample.
Cooking Loss: Samples weighing 25-30g (W1) were packed in plastic tubes. The tubes were then heated at 95°C, until the internal temperature of the samples reaches 75°C. The temperature was checked using thermocouples inserted in the center of the sample. The samples were considered cooked when the internal temperature reached 75°C after cooking, the meat was weighed again (W2) to determine the loss in weight during cooking as described [23].
Cooking loss (%) = (W1- W2/ W1) x 100
Sensory Evaluation: Ten experienced panelists made a sensory evaluation of full fried chicken strips. For the following attributes, each panelist was invited to give a numerical value from 0 to 10. Scores extended from 1 to 10 which illustrate dislike extremely to the like extremely. The sensory characteristics assessed were texture, color, odor, and crispness [24].
Statistical Analysis: All data of the present study were subjected to analyses of variance (AVOVA) using software (SAS institute, 1998). Differences between means were collected by the least significant differences (LSD) at p<0.05. All measurements were carried out in triplicate.
Results and Discussion
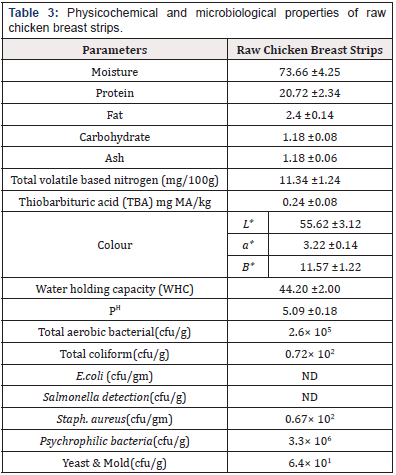
Physicochemical and Microbiological Properties of Raw Chicken Breast Strips: The chemical composition of raw chicken breast strips is presented in Table 3. Moisture, protein, fat, carbohydrate and ash contents of raw chicken breast strips were (73.66, 20.72, 2.4, 1.18 and 1.18g/100g respectively. These results agree with the data obtained [25] who found that moisture, protein, fat, and ash contents of raw chicken breast meat were 75.10, 22.90, 0. 78, and 1.30g/100g respectively. Total volatile based nitrogen (TVBV) (mg/100g) and thiobarbituric acid milligrams of malonaldehyde (TBA) mg / kg of raw chicken breast strips were (11.34 mg/100g and 0.24 (MA) / kg), respectively. These results agree with the data obtained [26]. Data presented in Table 3 showed that the color values (L*, a*, and b*) of raw chicken strips were 55.6, 3.2 and 11.5 respectively. While water holding capacity (WHC) and pH values of raw chicken strips were 44.2 and 5.09 respectively. These results agree with the data obtained [14,27]. Total aerobic bacterial, coliform, E. coli, salmonella, staph. positive coagulase, psychrophilic bacteria and yeast and mold counts of raw chicken breast strips were presented in Table 3. Total aerobic bacterial, coliform, salmonella, staph. aureus, psychrophilic bacteria and yeast and mold counts were 2.6× 105, 0.72× 102, ND, ND, 0.67× 102, 3.3× 106 and 6.4× 101 respectively. These results agree with the data obtained [28,30].
Chemical Composition of Deep Fat Fried Chicken Breast Strips During Frozen Storage (-18±1°c): Chicken breast strips samples werechemically analyzed to determine the gross chemical composition and physical properties. The obtained data are shown in Table 4. It could be noticed that moisture loss of deep fat fried chicken breast stripssignificantly decreased as a function of storage time for both samples. The control samples had statistically higher moisture contents than the treated fried samples. This could be due to water loss during frying. All coatings provided a beneficial barrier for moisture and preserved samples from moisture loss during storage. The lower water loss for the coated deep fat fried chicken breast stripsmight be due to controlling the loss of water and reducing dehydration. Similar results were observed [31,32].
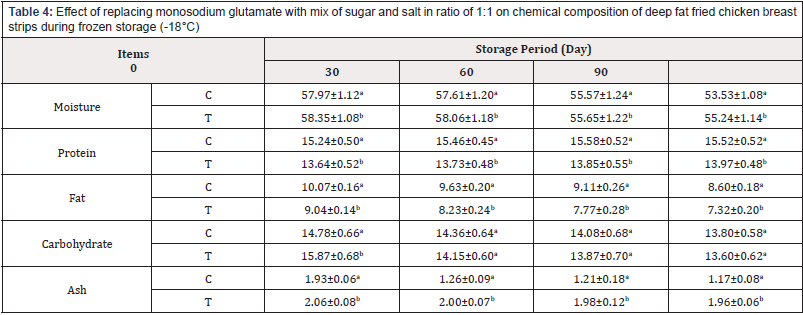
Table 4: Effect of replacing monosodium glutamate with mix of sugar and salt in ratio of 1:1 on chemical composition of deep fat fried chicken breast strips during frozen storage (-18°C)
The crude protein content of deep fat fried chicken breast stripsdecreased by replacing MSG with a mixture of 1;1 sugar and salt this may be due to containing of MSG on amino acids. The crude protein content of all treatments slightly increased as storage period progresses. Freezing storage has been shown to induce protein carboxylation, and the formation of Schiff bases in chicken meat [33]. Freezing storage has impacts on the activities of endogenous proteolytic enzymes responsible for the degradation of meat protein as well as the relaxation of meat tissue structures [34]. Study conducted [35] revealed increased content of both total and soluble protein in breast meat after 6 weeks of freezing storage. Similar results were observed [31,32].
With regard to fat content of chicken breast strips died not affecting by replacing. Control samples had the highest fat content than treated samples. The fat content of all treatments slightly decreased as storage period progressed. This decrease of fat content may be explained by the autolysis of lipid [36]. Carbohydrate and ash content were higher in sample containing sugar and salt mixture as alternative for MSG. The observed reduction in ash content was probably due to increased meat leakage during the fried process, hence the subsequent increased loss of mineral salts [37]. also demonstrated the impact of thawing (in atmospheric air and microwave) methods on the ash content of pork meat.
Physiochemical Quality of Deep Fat Fried Chicken Breast Strips During Frozen Storage (18±1°c)
(Table 5) It could be noticed that the pH value of deep fat fried chicken breast strips during frozen storage of both treatments increased as storage period progressed. Treatment contains mix of sugar and salt in ratio of 1:1 as MSD alternative had the higher pH values than treatment containing MSG (control sample). These results agree with those obtained [31,32]. The slight increase in pH during storage may be due to inhibition of bacterial activity during frozen storage [38]. The TVBN of both treatments increased as storage period progressed. Treatment containing mix of sugar and salt in ratio of 1:1 as MSD alternative had the lower TVBN values than treatment containing MSG (control sample). The increasing in TVBN value due to the breakdown of nitrogenous substances by microbial activity as reported [32].
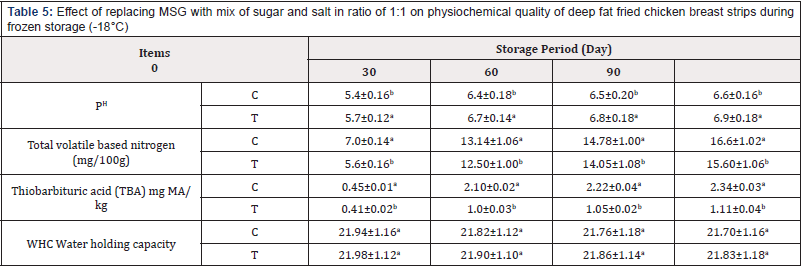
Table 5: Effect of replacing MSG with mix of sugar and salt in ratio of 1:1 on physiochemical quality of deep fat fried chicken breast strips during frozen storage (-18°C)
On the other hand, the TBA values of both treatments increased as storage period progressed. Treatment contains mix of sugar and salt in ratio of 1:1 as MSD alternative had the lower TBA values than treatment containing MSG (control sample. These results agree with those obtained [31,32]. The increasing of TBA value taken place due to lipid oxidation as reported [39]. However, a high degree of poly unsaturation accelerates oxidative processes leading to deterioration in meat flavor, color, texture, and nutritional value [40]. Water holding capacity (WHC) of deep fat fried chicken breast strips during frozen storage of both treatments decreased as storage period progressed.
Treatment contains mix of sugar and salt in ratio of 1:1 as MSD alternative had the higher WHC values than treatment containing MSG (control sample). These results agree with those obtained [41,42]. The cooking loss of deep fat fried chicken breast strips increased significantly as storage period progressed for all samples. Treatments containing MSG had the higher cooking loss percentage values than control sample. These results agree with those obtained [41-43].
Microbiological Examinations of Deep Fat Fried Chicken Breast Strips During Frozen Storage (-18°C): The microbiology examinations of deep fat fried chicken breast strips during frozen storage (-18°C) were examined to determine some microbiological quality and shelf-life validity throughout frozen storage. Microbial growth in meat and meat products can result in slime formation, structural components degradation, decrease in water holding capacity, off odors, and texture and appearance changes which reduce their quality, nutritional value and reduce the shelf life [44].
Total Bacterial Count: (Table 6) shows that there were significant differences in viable bacterial count between the control chicken breast strips and other chicken breast strips sample. The results indicated that total bacterial count decreased gradually throughout the storage period until the end of storage period. The obtained results also showed that control chicken breast strips had the highest counts of total bacterial count than other treatment. This might be due to the antimicrobial activity of salt or sugar [45]. Similar results were reported [31,32,38,41,46,]
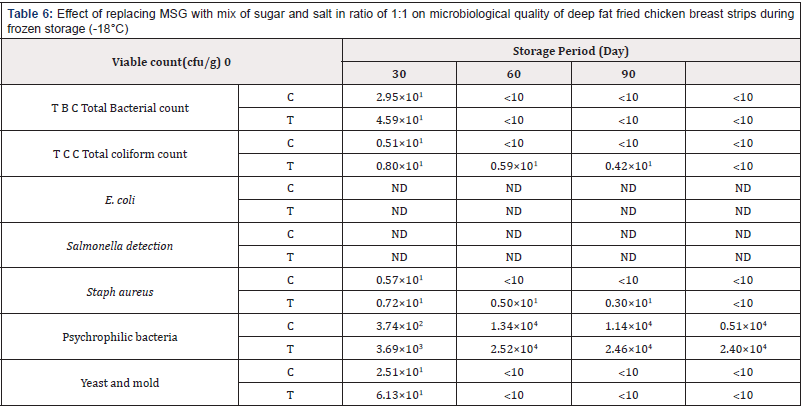
Table 6: Effect of replacing MSG with mix of sugar and salt in ratio of 1:1 on microbiological quality of deep fat fried chicken breast strips during frozen storage (-18°C)
Total Coliform Count: (Table 6) shows the differences in coliform counts. The results indicated that total coliform count decreased gradually throughout the storage period until the end of storage period. The obtained results also showed that control chicken breast strips had the lowest counts of total coliform count than other treatment. Similar results were reported [31,32,35,38].
E. Coli Count
The results presented in Table 6 indicated that total E. coli count did not detect in both treatments until the end of storage period. Similar results were reported [31,32,35,38].
Salmonella Count
The results presented in (Table 6) indicated that Salmonella did not detect in both treatments until the end of storage period. Similar results were reported [31,32,35,38].
Staph. Aureus Count: (Table 6) shows the differences in Staph coagulase counts. The results indicated that total Staph aureus count decreased gradually throughout the storage period until the end of storage period. The obtained results also showed that control chicken breast strips had the lowest counts of total Staph coagulase count than other treatments. Similar results were reported [31,32,35,38].
Psychrophilic Bacteria Count: (Table 6) shows the differences in psychrophilic bacteria counts. The results indicated that total psychrophilic bacteria count increased gradually throughout the storage period until the end of storage period. The obtained results also showed that control chicken breast strips had the lowest counts of total psychrophilic bacteria than other treatment. Similar results were reported [31,32,35,38].
Yeast and Mold Count: The differences in yeast and mold counts of deep fat fried chicken breast strips during frozen storage are shown in Table 6. The results indicated that total yeast and mold count decreased gradually as the storage period progressed until the end of storage period. The obtained results also showed that control chicken breast strips had the lowest counts of total yeast and mold than other treatment. Similar results were reported [31,32,35,38].
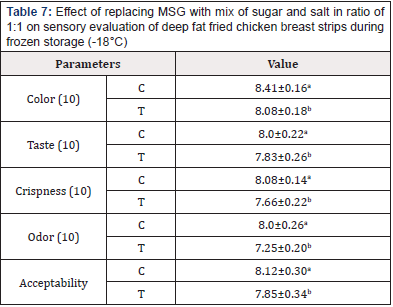
Table 7: Effect of replacing MSG with mix of sugar and salt in ratio of 1:1 on sensory evaluation of deep fat fried chicken breast strips during frozen storage (-18°C)
Sensory Evaluation of Deep Fat Fried Chicken Breast Strips During Frozen Storage (-18°C): Poultry meat is a nutritious food, and it is consumed all over the world because of its relatively low cost and low-fat content. However, it is highly perishable with a relatively short shelf life even when it is kept under refrigeration. Thus, developing more appropriate technologies for its preservation could be highly useful, to increase the shelf life of meat products [47]. Statistical analysis appears a significant difference in sensory evaluation between both samples. Treatment containing MSG (control) had the higher sensory proprieties (color, taste, crispness, odor, and acceptability) than that treatment containing mix of sugar and salt as MSG replacer (Table 7). The overall acceptability of deep fat fried chicken breast strips during frozen storage (-18±1°c) were significantly higher in (C), while it was significantly lower in the sample treated with mix of sugar and salt as MSG replacer. Statistical analysis appears a significant difference in overall acceptability between both samples. These results agree with those obtained [31,32,38,43,48-52].
References
- Nguyen Thuy, Liana C Salanță, Maria Tofană, Sonia A Socaci, Anca C Fărcaș, et al. (2020) A Mini Review About Monosodium Glutamate. Bulletin UASVM Food Science and Technology 77(1):1-12.
- Baryłko Pikielna N, Kostyra E (2007) Sensory interaction of umami substances with model food matrices and its hedonic effect. Food Qual Prefer 18(5): 751-758.
- Miyaki T, Retiveau Krogmann A, Byrnes E, Takehana S (2016) Umami increases consumer acceptability and perception of sensory and emotional benefits without compromising health benefit perception. J Food Sci 81(2): 483-493.
- Wang S, Zhang S, Adhikari K (2019) Influence of Monosodium Glutamate and Its Substitutes on Sensory Characteristics and Consumer Perceptions of Chicken Soup. Foods, 8(2): 71.
- Wang S, Adhikari K (2018) Consumer perceptions and other influencing factors about monosodium glutamate in the United States. J Sens Stud 33(4): e12437.
- Hui YH, Feng C, Leo M L N, Raquel PF Guiné, Olga Martín-Belloso, et al. (2010) Handbook of Fruit and Vegetable Flavors 935-943.
- Bethke PC, Jansky S (2008) The effects of boiling and leaching on the content of potassium and other minerals in potatoes. J Food Sci 73(5): H80-H85.
- Wijayasekara K, Wansapala J (2017) Uses, effects and properties of monosodium glutamate (MSG) on food & nutrition. Int J Food Sci Nutri 2(3): 132-143.
- Chun JY, Byong Soo Kim, Jung Gyu Lee, Hyung Yong Cho, Sang Gi Min, et al. (2014) Effect of NaCl/Monosodium Glutamate (MSG) Mixture on the Sensorial Properties and Quality Characteristics of Model Meat Products. Korean J Food Sci An 34(5): 576-581.
- Katrine Chwastowska I, Kondratowicz J (2005) Technological properties of a frozen pork meat depending on the storage time and a thawing method. J Food Sci Technology 3(44): 11-20.
- Maluly HDB, Arisseto Bragotto AP, Reyes F G R (2017) Monosodium glutamate as a tool to reduce sodium in foodstuffs: Technological and safety aspects. Food Sci Nutr 5(6): 1039-1048.
- Campagnol PCB, dos Santos B A, Wagner R, Terra NN, Pollonio MAR (2011) The effect of yeast extract addition on quality of fermented sausages at low NaCl content. Meat Sci 87(3): 290-298.
- Myrdal Miller A, Mills K, Wong T, Drescher G, Lee SM, et al. (2014) Flavor-enhancing properties of mushrooms in meat-based dishes in which sodium has been reduced and meat has been partially substituted with mushrooms. J Food Sci 79(9): 1795-1804.
- Qiao M, Fletcher D L, Smith D P, Northcutt J K (2001) The Effect of Broiler Breast Meat Color on PH, Moisture, Water Holding Capacity, and Emulsification Capacity. Poul Sci 80(5): 676-680.
- Park J M, Kim J M (2016) Monitoring of Used Frying Oils and Frying Times for Frying Chicken Nuggets Using Peroxide Value and Acid Value. Korean J Food Sci An 36(5): 612-616.
- (2007) AOAC Official methods for Analysis of the Association of Official Analytical Chemists A.O.A.C, 12.
- (1992) APHA American Public Health Association, Compendium of Methods for the Microbiological Examination of Foods, (3rd Edn)., Washington, DC.
- (2013) International Organization for Standardization (ISO): NO. 4833 1. Microbiology of the food chain horizontal method for the enumeration of microorganisms.
- (2004) International Organization for Standardization (ISO): No.11291-1. Microbiology of food and animal feeding stuffs – Horizontal methods for detection and enumeration of Enterobacteriaceae part2: colony count. Method.
- (2006) Egyptian Organization for Standardization ‘E.O.S, 63/11’’: Egyptian Organization for Standardization and quality control. Egyptian Standards for meat products treated with heat.
- (2006) Egyptian Organization for Standardization ‘E.O.S, 63/10’’: Egyptian Organization for Standardization and quality control. Egyptian Standards for meat products treated with heat.
- (2006) Egyptian Organization for Standardization ‘E.O.S, 63/9’’: Egyptian Organization for Standardization and quality control. Egyptian Standards for meat products treated with heat.
- Mamaghani VM (2010) Physiochemical and Rheological Properties of Alkaline Isolated Poultry Proteins. M.Sc. Thesis Fac Agric University of Alberta, Canada.
- Ramadhan K, Huda N, Ahmad R (2011) Physicochemical characteristics and sensory properties of selected Malaysian commercial chicken burgers. Int Food Res J 18(4): 1349-1357.
- Petracci M, Mudalal S, Babini E, Cavani C (2018) Effect of White Striping on Chemical Composition and Nutritional Value of Chicken Breast Meat. Italian J Ani Sci 13(3138): 179-183.
- Kim J H, Hong G E, Lim K W, Park W, Lee C H (2015) Influence of citric acid on the pink color and characteristics of sous vide processed chicken breasts during chill storage. Korean J. Food Sci Anim Resource 35(5): 585-596.
- Kaewthong P, Waiyagan K, Wattanachant S (2017) Imaging Analysis by Digital Camera for Separating Broiler Breast Meat with Low Water Holding Capacity. J Poult Sci 54(3): 253-261.
- Eglezos S, Dykes G A, Huang B, Fegan N, Stuttard, E (2008) Bacteriological Profile of Raw, Frozen Chicken Nuggets. J Food Prot 71(3): 613-615.
- Al Nehlawi A, Saldo J, Vega L F, Guri S (2013) Effect of high carbon dioxide atmosphere packaging and soluble gas stabilization pre-treatment on the shelf-life and quality of chicken drumsticks. Meat Sci. 94(1): 1-8.
- Rouger A, Tresse O, Zagorec M (2017) Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 5(3): 50.
- Hwang K E, Choi Y S, H Y Kim, Kim C J, Mi Ai Lee, et al. (2011) Effect of ganghwayakssuk (artemisiaprincepspamp.) on oxidative stability of deep-fried chicken nuggets. Food Sci Biotechnol 20(5): 1381-1388.
- Prejsnar A A, Ormian M, Sokołowicz Z (2018) Physicochemical and sensory properties of broiler chicken breast meat stored frozen and thawed using various methods. J Food Quality 3: 1-9.
- Utrera M Estevez M (2013) Oxidative damage to poultry, pork, and beef during frozen storage through the analysis of novel protein oxidation markers, J Agri Food Chem 61(33): 7987-7993.
- Farouk M M, Wieliczko K J, Merts I (2003) Ultra-fast freezing and low storage temperature as are not necessary to maintain the functional properties of manufacturing beef. Meat Science 66(1): 171-179.
- Smiecinska K, Hnatyk N, Daszkiewicz T, Kubiak D, Matusevicius P (2015) Effect of frozen storage on the quality of vacuum packaged turkey meat. Veterinarijair Zootechnika, 71(93): 61-66.
- El Nashi H B, Fattah A F A K A, Rahman N R A, El-Razik M A (2015) Quality characteristics of beef sausage containing pomegranate peels during refrigerated storage. Ann Agri Sci 60(2): 403-412.
- Katrine Chwastowska I, Kondratowicz J (2005) Technological properties of a frozen pork meat depending on the storage time and a thawing method. J Food Sci Technology 3(44): 11-20.
- Bouacidaa S, Snoussia A, Arouac M, Jemmalie B, Nabiha Bouzouitaa, et al. (2020) Effect of marination with Erucavesicarialongirostris leaves on Turkey meat properties during storage and consumer acceptance. J new sci Sustainable Livestock Management 12(1): 253-264.
- El Gharably AMA, Ashoush IS (2011) Utilization impact of adding pomegranate rind powder and red beet powder as natural antioxidant on quality characteristics of beef sausage. World J Dairy Food Sci 6(1): 86-97.
- Mielnick MB, Olsen E Vogt G, Adeline D, Skrede G (2006) Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT Food Science and Technology 39(3): 191-198.
- Aksu M Y, Alp E (2012) Effects of Sodium Tripolyphosphate and Modified Atmosphere Packaging on the Quality Characteristics and Storage Stability of Ground Beef. Food Technol Biotechnol 50(1): 81 87.
- Sampaio G R, Saldanha T, Soares R A M, Torres E A F S (2012) Effect of natural antioxidant combinations on lipid oxidation in cooked chicken meat during refrigerated storage. Food Chemistry 135(3): 1383-1390.
- Kruk Z A, Kim H J, Kim Y J, Rutley D L, Jung S, et al. (2014) Combined effects of high-pressure processing and addition of soy sauce and olive oil on safety and quality characteristics of chicken breast meat. Asian Australas J Ani Sci 27(2): 256-265.
- Doulgeraki A, Ercolini D, Villani F, Nychas G E (2012) Spoilage microbiota associated to the storage of raw meat in different conditions. International Journal of Food Microbiology 157(2): 130-141.
- Shee A K, Raja R B, Sethi D, Kunhambu A, Arunachalam K D (2010) Studies on the antibacterial activity potential of commonly used food preservatives. Int J Eng Sci Techno 2(3): 264-269.
- Malay OP, Sharma B D, Talukder S, Kumar R R, Mendiratta S K (2013) Shelf life evaluation of restructured chicken meat blocks extended with sorghum flour and potato at refrigerated storage (4±1°C). Int Food Res J 20(1): 105-110.
- Mantilla SPS, Santos ÉB, Vital HD, Mano SB, Freitas MQ, et al. (2011) Microbiology, Sensory Evaluation and Shelf Life of Irradiated Chicken Breast Fillets Stored in Air or Vacuum. Brazilian Archives Bio Techno 54(3): 569-576.
- AL Dughaym, A.M., and Altabari, G.F. (2010) Safety and quality of some chicken meat products in Al Ahsa markets Saudi Arabia. Saudi J of Bio Sci 17: 37-42.
- Soloviev VE (1966) Aging of meat. Food Industry Pub, Mosc tissues and liver enzymes in mice. World J Neurosci 5: 339-349.
- Shee AK, Raja RB, Sethi D, Kunhambu A, Arunachalam KD (2010) Studies on the antibacterial activity potential of commonly used food preservatives. International Journal of Engineering Science and Technology 2(3): 264-269.
- SAS (1998) User's guide.6.12 end Statistical Analysis System Institute Inc. Cary NC 27511-8000, USA.
- Prejsnar, AA, Ormian M, Sokołowicz Z (2018) Physicochemical and sensory properties of broiler chicken breast meat stored frozen and thawed using various methods. Journal of Food Quality 1-9.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.