Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Effects of Turbulent Mixing and Orbitally Shaking on Cell Growth and Biomass Production in Active Fluids
*Corresponding author: Hassan Peerhossaini, Department of Civil and Environmental Engineering, Western University, Canada.
Received: February 14, 2022; Published: February 23, 2022
DOI: 10.34297/AJBSR.2022.15.002129
Abstract
We have previously reported the effects of mixing on bacterial growth. Mixing can be achieved with different methods and in diverse devices. However, the nature of the intimate contact (the main purpose of mixing) made between different substances in a mixing vessel is a strong function of the hydrodynamic that underlies the specific mixing method. This study aimed to compare the growth and biomass yield of cyanobacterium Synechosystis sp. CPCC 534 (as a microorganism model) obtained by three different mixing methods commonly used in life science labs and in the industry, i.e., turbulent stirring (TS), orbital shaking (OS), and simple molecular diffusion (MD). The results revealed that imposing mixing on the culture significantly improved the specific growth rate as well as biomass yield production in comparison with simple molecular diffusion. Mixing obtained by turbulent stirring proved to be more efficient than the one achieved by orbitally shaking, in the production of Chlorophylla (Chla) and phycocyanin (PC). The results of this study can help choosing the appropriate mixing method in life science research.
Keywords: Photobioreactor (PBR); Photosynthetic microorganisms; Incubator; Incubator shaker; Magnetic stirrer; Molecular diffusion; Synechocystis; Yield production; Chlorophylla (Chla); Phycocyanin (PC)
Abbreviations: PBR: Photobioreactors; TS: Turbulent Stirring; MD: Molecular Diffusion; OS: Orbitally Shaking; r: Specific Growth Rate; k: Doubling Per Day; OD: Optical Density; PC: Phycocyanin; Chla: Chlorophylla
Introduction
Cyanobacteria, also recognized as “blue-green algae”, are the oldest oxygenic photosynthesizers on the planet earth [1] which have a major role in the carbon and oxygen cycles using CO2 and solar light energy [2,3]. Cyanobacteria may range from unicellular to complicated multicellular aggregates or filamentous strains and are found in almost any ecological environment on the earth, including soil, freshwater, polar latitudes, and oceans [2,4]. Cyanobacteria are mass cultivated and used in a wide variety of industries including food, pharmaceutical, and cosmetics [5].
The major focus in the mass cultivation of cyanobacteria is the development of high-efficiency, well-controlled, and low-cost systems [6]. Therefore, various types of photobioreactors (PBRs) have been employed in the laboratory and at industrial scale to cultivate photosynthetic microorganisms. Efficiency, design, and optimization of bioreactors are being impacted by several essential factors including availability of light and nutrients, pH, mixing, and temperature distribution [7]. Among these parameters, mixing is the core factor in creating a homogenous environment for microorganisms’ growth and improving the cellular contact with chemical nutrients and light. Mixing has two significant effects on the productivity of microorganisms: first, it enhances the penetration of the light in the culture environment, and second, breaks the boundary layers, which improves mass transfer between the microorganisms and the media [8]. However, excessive mixing can enhance the amount of shear stress imposed on the microorganism which may damage the microorganism cells and reduce growth rate [9].
In laboratory or industrial photobioreactors, mixing can be achieved by various methods including mechanical agitation, bubbling, aeration, and pumping or a combination of these modes [10]. Turbulent stirring (TS) and orbitally shaking (OS) are two major mixing modes commonly used in life science laboratories and industry. Most photobioreactors, and open ponds, work in turbulent regime where microorganisms confront turbulent flow in bodies of water [11] or similar liquids [12]. While orbitally shaking is a more suitable mixing technique for shear-sensitive cells [13]. On the laboratory scale, turbulent stirring (TS) can be generated by using a magnetic bar spinning under the effect of an external rotating magnetic field (Figure 1(a)). By increasing the rotation speed of the magnetic field, a laminar vortex or an almost uniform turbulent micro-mixing field can be generated. To maintain optimal conditions including, temperature and light, turbulent stirrers can be placed in controllable incubators.
The “orbital shaking”, is another type of mixing created by the orbital displacement of a vessel, providing a permanent direction regarding an inertial force, along with a rotary path at a fixed angular velocity. This mixing mode is being used in a wide range of industries such as marine, drug production, and in biological technology [14]. As a piece of equipment, an orbital shaker provides a uniform environment for the cultivation of microorganisms, in the form of a circular shaking motion (Figure 1(b)). Orbital shaking is recommended for providing a low-shear stress environment, and to prevent cellular damage in active fluid suspension [15]. Recently, some models of orbital shakers are integrated into an incubator to maintain the optimal conditions for the growth of microorganism cells. The velocity field and shear stress distribution in an orbital shaker are less uniform than in a turbulent stirrer. This difference can be expected to provide different cellular growth in these two systems.
In a recent study by Mehdizadeh Allaf, et al. [5], the response of physical and biological characteristics of suspensions of cyanobacterium Synechocystis sp. CPCC 534 was investigated in turbulent stirrers under various stirring rates (450, 900, 1500 rpm). Their results showed that adding mixing, in the form of turbulent stirring, to the culture had a significant impact on the growth, biomass production, yield, and total pigments production compared to the motionless bioreactors. Most previous work had focused on a single mixing method in the laboratory or industrial devices, few studies had compared the efficiency of the two most used mixing modes; turbulent stirring and orbitally shaken, in life science applications. In one study, Fadlallah, et al. [16] carried out experiments with two photobioreactors using two different mixing methods; agitated photobioreactors (APBR) and draft tube airlift photobioreactors (DPBR). The aim was to investigate the performance of each photobioreactor on the Synechocystis sp. PCC 6803 specific growth rate and pigments production under different hydrodynamic shear stress rates (0 to 400 mPa). Their results indicated that limited shear stress rates (less than 30 mPa in APBR and between 80 mPa and 180 mPa in DPBR) can break the groups of cell colonies, and therefore, improve the growth rate and pigments production. However, higher hydrodynamic shear stress did not show a more significant impact on the growth rate of cyanobacterium Synechocystis sp. PCC 6803.
In this study, the efficiency of turbulent stirring (TS) and orbitally shaking (OS) mixings versus a molecular diffusion (MD, no mixing) mode on the growth of cyanobacterium Synechocystis sp. CPCC 534 was investigated. Cell growth was used as a proxy to compare different mixing modes. To perform this study, Synechocystis sp. CPCC 534 was used in the working suspensions.
Among different strains of cyanobacteria, Synechocystisis, a unicellular, freshwater cyanobacterium, is utilized as a model microorganism for investigating photosynthesis, energy metabolism, molecular biology, biofilm formation, and environmental stress [17-19]. The entire genome of Synechocystis sp. strain PCC 6803 was sequenced, for the first time, in 1996 by Kaneko and co-workers [20]. The cellular surface of the Synechocystis is covered with non-flagellar appendages, known as pili or fimbriae, which assist in adhesion, motility, biofilm structure, and DNA uptake [21]. Cyanobacterium Synechocystis sp. can navigate toward or away from favorable or unfavorable stimuli. For performing this movement, the cells convert nutrient chemical energy into mechanical work for driving the flow [5,22]. The characteristics of “active fluids” (bacterial suspensions) are different from those of conventional or “passive” fluids. In passive fluids, pressure, velocity, or temperature gradients are driving forces for the flow, while in active fluid, cells as the microstructural elements of the fluid, use chemical nutrients or light to stimulate microorganism cells for running the metabolic functions, and creating the directed motions called chemotaxis and phototaxis respectively [16,19,23,24].
Materials and Methods
Strain and Culture Conditions
Synechocystis sp. CPCC 534 wild type was purchased from the Canadian Phycological Culture Center (Waterloo, ON, Canada), and was cultured in chemically defined liquid BG11 medium at 20±1°C. The initial cell concentration was 5×105 cell.ml-1 and the culture were carried out under the light cycle of 12/12 h and photon flux of 50±5 μmole.m-2. s-1.
Mixing Modes
Cells were cultivated under controlled conditions in three different mixing modes. In the turbulent stirring (TS) mode, digitally controlled magnetic stirrers (VWR Canada) were used to create turbulent mixing inside the lab photobioreactors placed in a fully controlled PHCbi incubator. Cylindrical magnetic stirring bars with a diameter of 7.9 mm and a length of 19.8 mm were employed to mix the sample. In the Orbitally shaking (OS) mode, the photobioreactors were placed in a fully controlled Eppendorf Innova S44i incubator shaker. In the Molecular Diffusion (MD) mode, the photobioreactors were placed standstill inside compartments in well-controlled conditions. The light intensity and temperature were set at 50±5 μmole.m-2. s-1 and 20±1°C, respectively for all three modes. The turbulent stirring and orbitally shaking mixing devices are schematically shown in Figure 1.
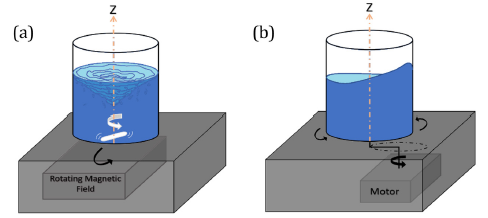
Figure 1: Schematic illustration of (a) a turbulent stirring (ST) mixer, and (b) an orbital shaker (OS) mixer.
Experimental Protocol
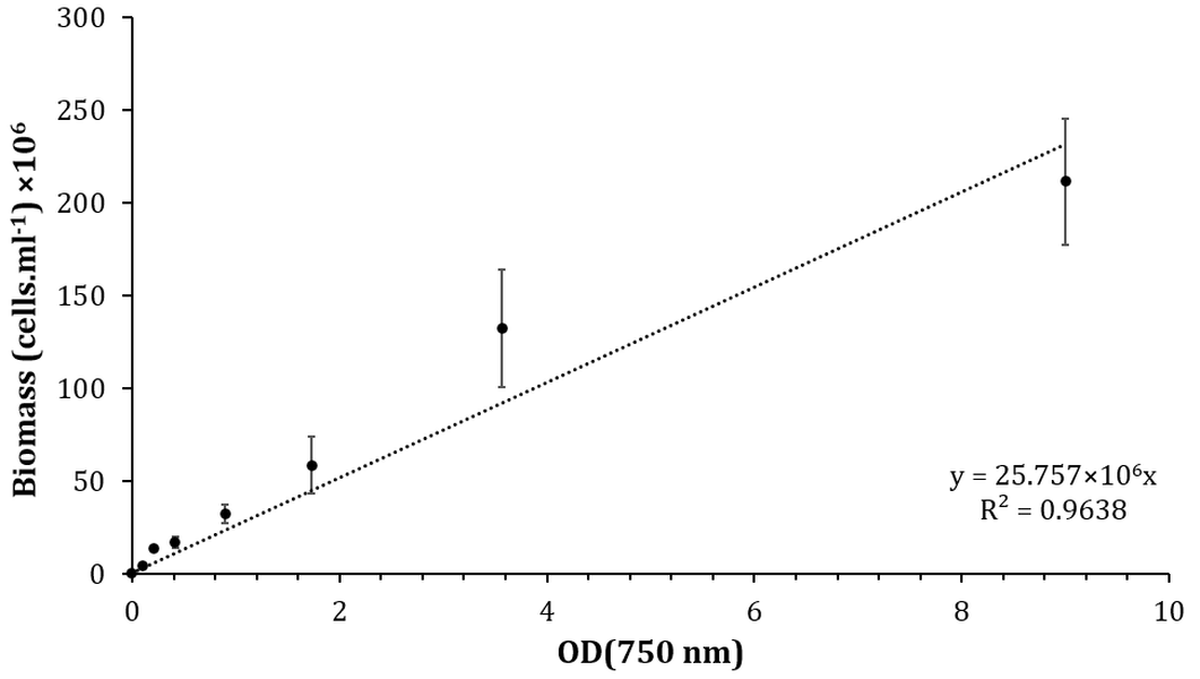
Each mixing mode experiment was performed in triplicates and was carried out in 125 ml Pyrex flask photobioreactors. The optical density (OD) of Synechocystis sp. CPCC 534, as a proxy of growth, was measured daily at 750 nm wavelength using a Spectronic 200E spectrophotometer. A calibration curve was prepared to estimate the number of cyanobacteria cells per cubic milliliter. For this purpose, a dilution series of Synechocystis sp. CPCC 534, all grown at the same temperature and light intensity, were prepared and the optical density and the number of cells.ml-1 were measured using the Spectronic 200E spectrophotometer and a Hausser Scientific hemocytometer, respectively. The calibration curve (also known as standard curves) was generated to calculate the number of cyanobacteria cells in the sample solution from the OD measurements.
Calculation of Cynobactrium Specific Growth Rate (r) and Doubling Time (k)
The specific growth rate (r) of the microorganism population was calculated for the exponential growth phase to assess the rate of rising in cells number per time by using equation (1) [25].
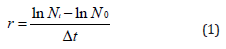
where N0 is the number of microorganism cells at the starting of a time period, Nt is the microorganism cells number at the end of the time period, and Δt is the duration of the time period (tt - t0).
The specific growth rate (r) is used to estimate the number of cell divisions per day known as doubling per day (k), by equation (2) [25].
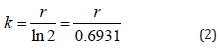
The cell yield of Synechocystis sp. CPCC 534 was computed by integrating the area under the growth curve from the beginning of the exponential phase up to the end of the stationary phase. A photometric scan of Synechocystis sp. CPCC 534 was conducted using a Spectronic 200E spectrophotometer and the absorbance peaks for Chlorophylla (Chla) and Phycocyanin (PC) were detected at 683 nm and 630 nm, respectively. The Chlorophylla (Chla) and Phycocyanin (PC) production were evaluated by the mean value of all data points obtained during the exponential growth phase.
Statistical Analysis
The significant differences in growth, doubling per day, yield, Chla, and PC production were investigated by a one-way ANOVA followed by the Tukey multiple comparison tests, and p<0.05 was evaluated as significant. The relationship between the aforementioned variables was calculated by Pearson correlation (R). The correlation coefficients of 90-100 and 70-90 were considered very high and high, respectively. The statistical analyses were calculated by OriginPro 2017 (OriginLab Corporation, Northampton, MA, USA).
Results
Standard Curve
In the first step, a serial dilution of Synechocystis sp. CPCC 534 was prepared. Then, the OD of each sample was measured by the spectrophotometer and the number of Synechocystis sp. cells in each sample was counted by hemocytometer under Nikon microscope (Nikon ECLIPSE Ti2 inverted microscope). Then, the calibration curve was plotted, as is shown in Figure 2. By using the obtained equation from the calibration curve, Synechocystis sp. CPCC 534 concentration was calculated (Figure 2).
Comparison of Cell Growth Under the Three Mixing Modes
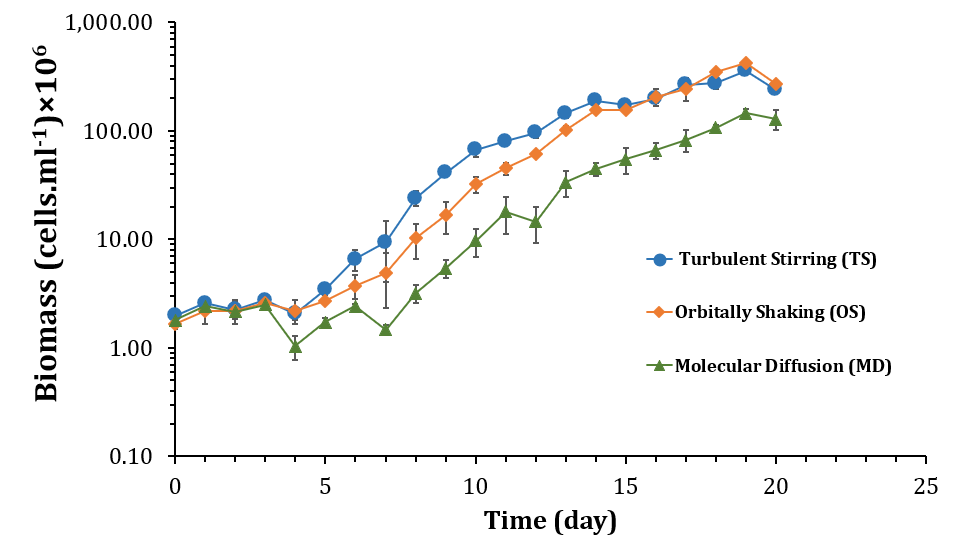
A growth curve, shown in Figure 3, was constructed from the experimental data to monitor the increase in the number of Synechocystis sp. CPCC 534 cells during a time period of 20 days. In the bacterial culture, once the cells adapted themselves to the new environment, they moved from the lag phase into a logarithmic growth phase, which continued up to reaching a linear growth phase, then followed by the stationary phase. The self-shading of cells impedes the accessibility of microorganism cells to the light source. Figure 3 shows the Synechocystis cells that were grown under TS and OS mixing modes entered the logarithmic growth phase around day 4, while the motionless culture entered the logarithmic phase around day 7. The faster start of the exponential phase in mixed cultures can be attributed to the abundance of nutrients and light available to cells due to their spatial displacement.
In the same manner, the cultures grown under TS and OS mixing modes reached stationary phase later than the cultures grown in motionless PBRs; day 15 for the formers and around days 12 or 13 for the latter. Here again, efficient movement of cells inside the batches, caused by mixing, allowed the bacteria to uptake nutrients from unexplored regions and to be exposed to light more efficiently compared to the motionless cultures. A similar pattern was obtained by Fadlallah, et al. [26]. Their results showed that the biomass of Synechocystis sp. PCC6803 was increased considerably under turbulent stirring conditions (360 rpm) compared to motionless samples. Monteila, et al. [27] also observed cell densities improvement in the orbitally shaken bioreactor. They reported increasing the shaking rotation speed from 180 rpm to 220 rpm could improve the cell densities from 5×105 to 7×106 cell.ml-1.
Cultures grown under TS and OS mixing modes showed also a better specific growth rate (r) than cultures grown under motionless conditions (Figure 4(a)). Nevertheless, the specific growth rate for TS was slightly higher than that of OS, suggesting that turbulent stirring was more efficient in mixing the active fluid than orbitally shaking. A closer analysis of the fluid mechanics of TS and OS modes (not shown here) confirmed the superiority of TS mixing above and over that of OS mixing. However, the OS growth curve overtook the TS growth curve at the beginning of the linear phase and overlapped with it for the whole period of stationary growth. The later start of the logarithmic phase, smaller specific growth rate, and earlier start of the linear phase in motionless culture resulted in a significant lag in biomass yield, as is shown in Figure 5 (Figure 3).
The specific growth rate and doubling per day for the three mixing modes were estimated during the exponential phase, and the results are presented in Figure 4. The results show that under mixing conditions, there is a significant change (p<0.05) in a specific growth rate and doubling per day of Synechocystis sp. in comparison to non-mixing (motionless) conditions. Regarding the efficiency of the two mixing modes, there was not a major difference between the turbulent stirring and orbitally shaking modes as observed by the specific growth rate and doubling per day (Figure 4). However, the difference is more apparent in Chla and PC production (Figure 6). Mehdizadeh Allaf, et al. [5] showed that the use of TS mixing increased the specific growth rate (p<0.05) and doubling per day (p<0.05) of Synechocyctis sp. cells significantly compared to stationary conditions, which is in the agreement with the present results. Fadlallah, et al. [16] also concluded that applying gentle turbulent stirring (TS) intensity (shear stress intensity < 30 mPa) enhanced the exponential growth rate, around 50%, compared to non-turbulent stirring cases (Figure 4).
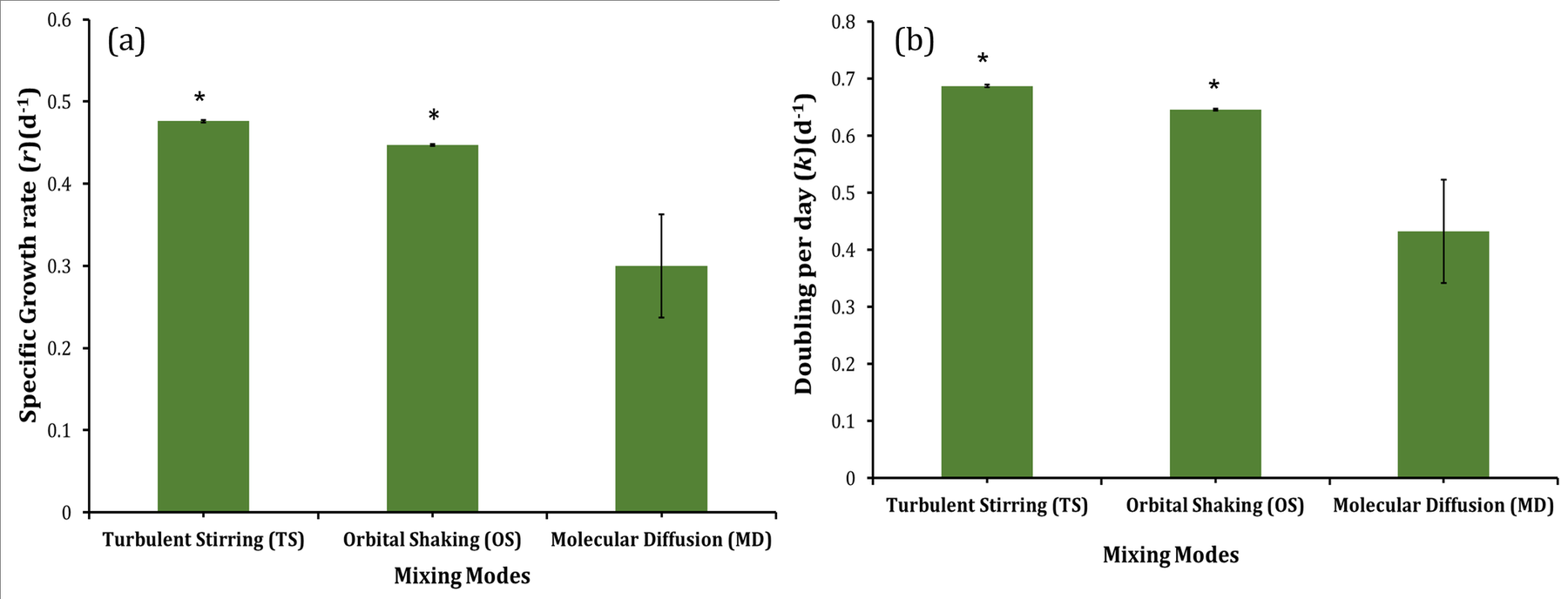
Figure 4: (a) Specific growth rate (r) and (b)Doubling per day (k) of Synechocystis sp. CPCC 534 under various mixing conditions. * indicates the significant effect at the level of 0.05 among different mixing modes.
The Synechocystis sp. CPCC 534 yield, under different mixing conditions, is plotted in Figure 5. The biomass yield production was significantly (p<0.05) higher when cells were grown under mixing conditions. It showed an increase of over 213% for TS mode and 197% for OS mode compared to motionless cultures, in agreement with the findings in [5]. Mixing contributes to microorganism growth in several ways. It obviates the self-shading of microorganisms, breaks the boundary layer in the culture system, and facilitates access to CO2, nutrients, and light [28] (Figure 5).
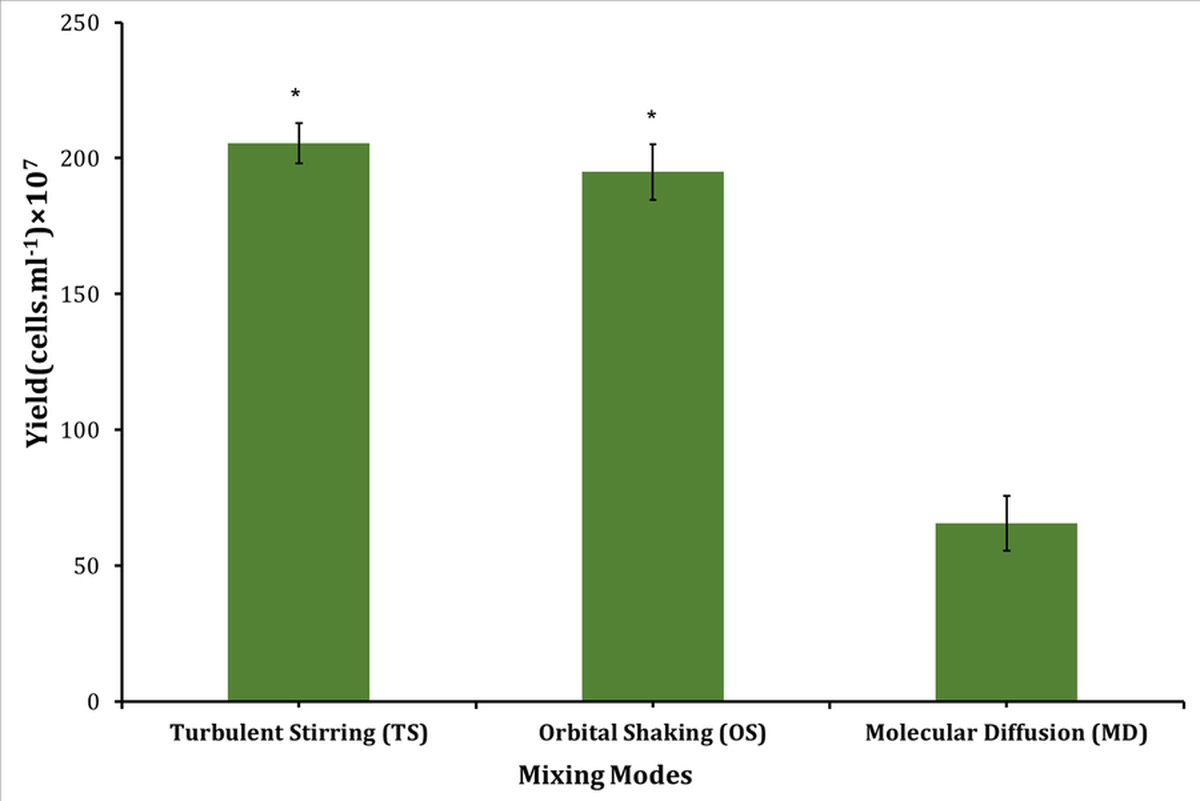
Figure 5: Profiles of yield for Synechocystis sp. CPCC 534 under different mixing modes. * indicates the significant effect at the level of 0.05 among different mixing modes.
Cyanobacteria also produce various pigments like Chlorophylla (Chla) and Phycocyanin (PC) [29]. The mixing effect was investigated on the production of Chla and PC and the results were plotted in Figure 6. Experiments under three different mixing modes showed that turbulent stirring enhanced significantly (p<0.05) the production of Chla in comparison to other modes. Under the defined mixing conditions, the production of PC was measured at the wavelength of 630 nm, and Chla at 683 nm. Phycocyanin (PC) is recognized as a photosynthetic pigment-protein complex from the phycobiliprotein groups, which is found in most cyanobacteria and red algae [30]. From the measurement of the Synechocystis sp. CPCC 534 OD in the exponential phase, the average PC production was estimated and shown in Figure 6(b). Production of PC under turbulent stirring mode was increased significantly (p<0.05) in comparison to other modes, similar to Chla production. The effect of turbulent mixing on Chla and PC production was more evident in comparison to orbitally shaking mode (Figure 6).
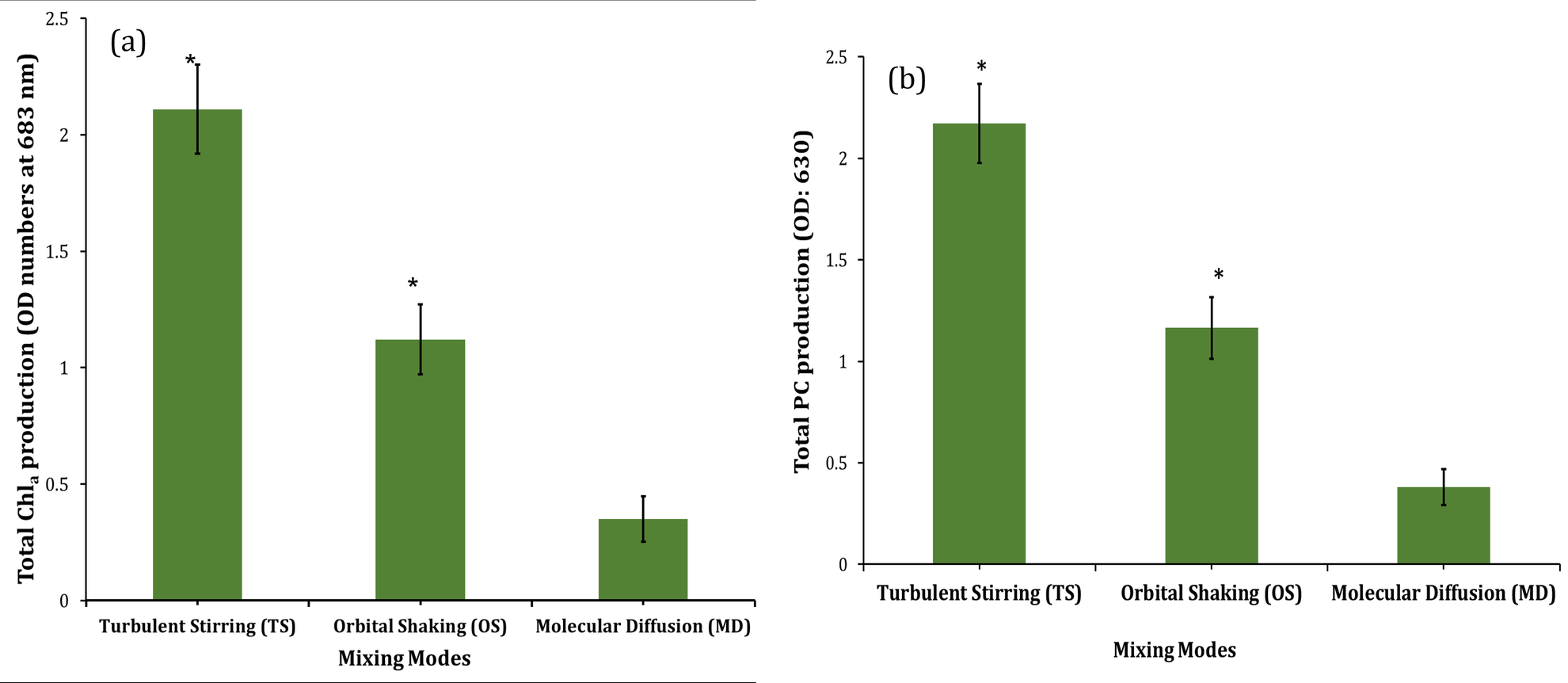
Figure 6: (a) Profiles of Chla production and (b) PC production of Synechocystis sp. CPCC 534 under various mixing conditions. * indicates the significant effect at the level of 0.05 among different mixing modes.
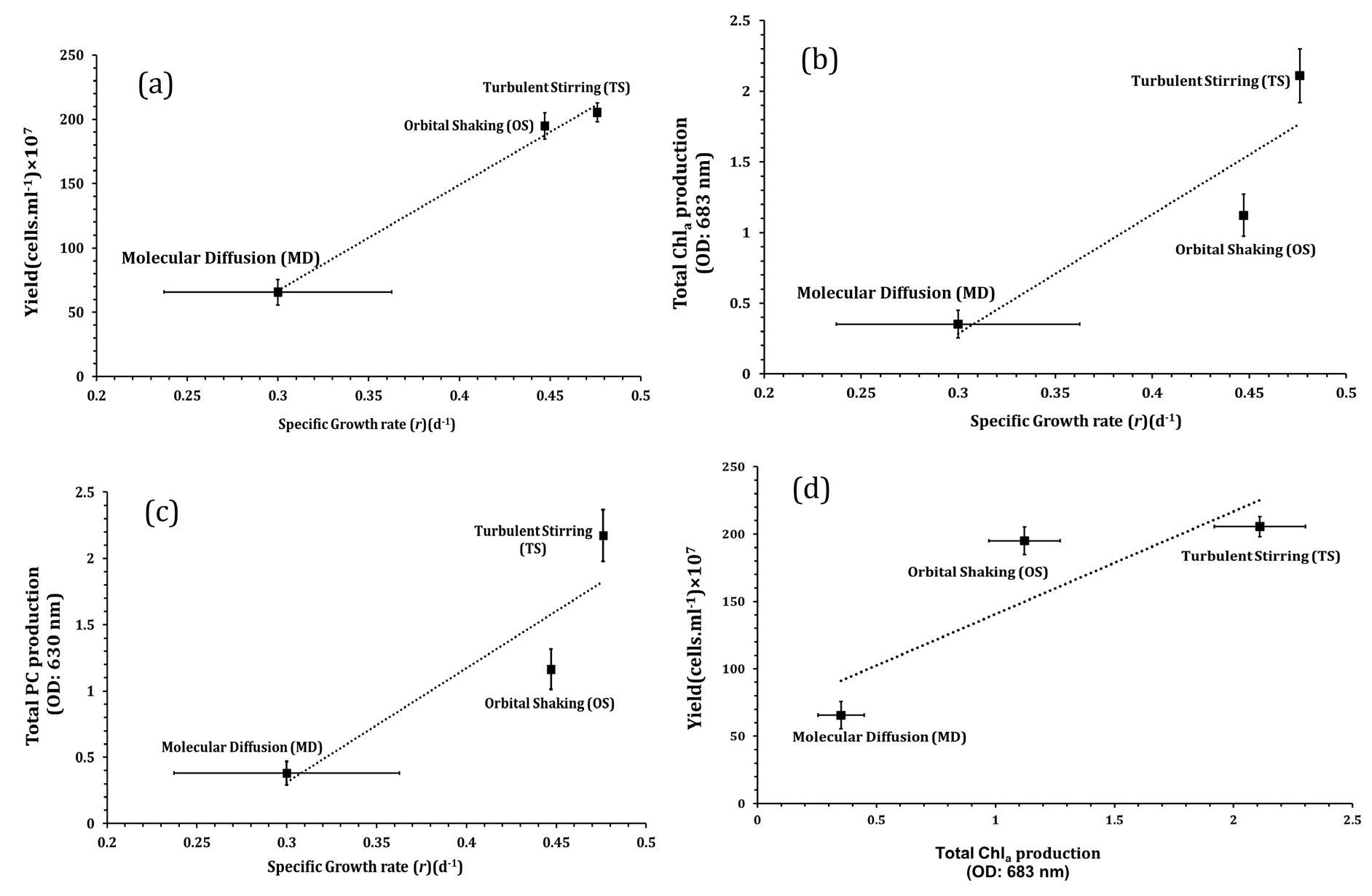
The correlations between yield production, Chla, PC production, and specific growth rate (r), were also calculated and the Pearson correlation coefficient (R) was computed; the results are plotted in Figure 7. Strong positive linear correlations were found between the couples of correlated parameters; including the specific growth rate and yield production (Pearson’s r: 0.996), specific growth rate and Chla production (Pearson’s r: 0.928), specific growth rate, and PC production (Pearson’s r: 0.932), and Chla and yield production (Pearson’s r: 0.887). Overall, these findings are in agreement with findings reported in [31] and. [5]. Ajala, et al. [31] reported that the maximum biomass concentration and productivity were achieved under, turbulent stirring (TS) mode at 1400 rpm. The data presented by Mehdizadeh Allaf, et al. [5] showed a similar trend in the selection of mixing methods for the production of Chla, PC, and yield (Figure 7).
Discussion
The results of this study suggest that mixing by turbulent stirring is more efficient than mixing by orbitally shaking, as appears in most cell growth metrics, this difference can be attributed to the dissimilarity in the fluid motion and wall shear stress distribution between the two mixing modes. In orbitally shaking, the fluid undergoes a solid body rotation about the central axis of the cylindrical vessel, and therefore, most of the fluid and suspended cell particles’ movement is concentrated in the peripheral zone of the reactor. In solid body rotation, the fluid body is not sheared thus, the relative distance between fluid particles remains constant. Fluid particles trapped in the solid body rotation zone have a very limited chance (except by molecular diffusion) to visit other places and other fluid particles in the culture medium for nutrient uptake, and especially getting close to the free surface and wall vicinity where they can be exposed to light. While this difference can affect the logarithmic growth period (shorter in TS than in OS) however, the OS growth curve catches up to this delay and reaches the TS growth curve in the stationary phase.
Comparable types of orbiting reactors, such as orbiting dishes, are used in endothelial cell studies. In this configuration, the height of the culture medium is smaller than the one considered in this study. In the endothelial cell application, one is interested in the effects of wall shear stress on the response of anchored cells exposed to culture medium flow. Nevertheless, the hydrodynamic of the problem remains like the one studied here.
It is expected that the rotating speed of the orbital shaker has a chief influence on the mixing efficiency with the OS method, the problem that was not addressed here. Ojo, et al. [32] evaluated the performance of microalgae cultivation (Chlorella sorokiniana) under orbitally shaken photobioreactors at three shaking frequencies 70, 90, and 180 rpm. They concluded that more efficient biomass production was achieved at 180 rpm. However, this study did not show whether there is a limit to this efficiency increase with rotation frequency. Ajala, et al. [31] also studied the effect of orbitally shaking mode at different frequencies on the Chla production from Dunaliella salina, Nannochloropsis oculata, Oocystis minuta, and Neochloris conjuncta. In all microalgae strains, their results showed that the highest Chla was achieved at 200 rpm.
Conclusions
In this study, the impact of two methods of mixing (turbulent stirring and orbitally shaking), on various growth metrics of Synechocystis sp. CPCC 534 was investigated and compared with stationary (motionless) culture. The results revealed that mixing of Synechocystis cultures can improve growth rate, doubling per day, yield, and Chla production in comparison with the cultures where no mixing was imposed. Mixing by turbulent stirring seems to be more efficient than mixing by orbitally shaking, as appears in most of the cell growth metrics. This difference can be attributed to the dissimilarity in the fluid motion and wall shear stress distribution between the two mixing modes. Mixing enhances the nutrient uptake as well as homogeneity of the light and temperature distribution in the culture.
Acknowledgments
The authors would like to thank Dr. Charles G. Trick for providing Synechocystis strain used in this research. This research was funded by the HP’s Western Research Chair in Urban Sustainability & Resilience chair fund. HP also acknowledges the financial support of NSERC (RGPIN-2019-05156) which partially funded MMA, ZS, and RS. CD acknowledges the financial funding of NSERC (RGPIN-2017-04078) which partially supported ZS.
Conflict of Interest
There is no conflict of interest by researchers.
References
- Govindjee, Shevela Dmitriy (2011) Adventures with cyanobacteria: a personal perspective. Front Plant Sci 2: 28.
- Stucken Karina, Robin Koch, Tal Dagan (2013) Cyanobacterial defense mechanisms against foreign DNA transfer and their impact on genetic engineering. Biol res 46(4): 373-382.
- Sebesta Jacob, Allison Werner, Christie Ann Marie Peebles (2019) Genetic engineering of cyanobacteria: Design, implementation, and characterization of recombinant Synechocystis PCC 6803. Methods Mol Biol 1927: 139-154.
- Conradi Fabian D, Conrad W Mullineaux, Annegret Wilde (2020) The role of the cyanobacterial type IV Pilus Machinery in finding and maintaining a favorable environment. Life 10(11): 252.
- Mehdizadeh Allaf Malihe, Habib Zahra, de Bruyn John R, DeGroot Christopher T, Peerhossaini Hassan (2022) Rheological and Biophysical Properties of Living Fluids Under Shear: Active Suspensions of Synechocystis CPCC 534. Journal of Fluids Engineering 144(2): 021208.
- Wolf J, E Stephens, S Steinbusch, J Yarnold, IL Ross, et al. (2016) Multifactorial comparison of photobioreactor geometries in parallel microalgae cultivations. Algal Research 15: 187-201.
- Samadi Zahra, Johlin Eric, DeGroot Christopher, Peerhossaini Hassan (2021) Modelling Optical Properties of Algae Using the Finite-Difference Time-Domain Method. In Fluids Engineering Division Summer Meeting (Vol. 85307, p. V003T05A016). American Society of Mechanical Engineers, USA.
- Grobbelaar Johan U (1991) The influence of light/dark cycles in mixed algal cultures on their productivity. Bioresource technology 38(2-3): 189-194.
- Wang Chinchin, Christopher Q Lan (2018) Effects of shear stress on microalgae-A review. Biotechnol Adv 36(4): 986-1002.
- Wang Bei, Christopher Q Lan, Mark Horsman (2012) Closed photobioreactors for production of microalgal biomasses. Biotechnol Adv 30(4): 904-912.
- Rusconi Roberto, Melissa Garren, Roman Stocker (2014) Microfluidics expanding the frontiers of microbial ecology. Annu Rev Biophys 43: 65-91.
- Wang Chinchin, Christopher Q Lan (2018) Effects of shear stress on microalgae-A review. Biotechnol Adv 36(4): 986-1002.
- Lehmann Nicolai, Heiko Rischer, Dieter Eibl, Regine Eibl (2013) Wave‐mixed and orbitally shaken single‐use photobioreactors for diatom algae propagation. Chemie Ingenieur Technik 85(1‐2): 197-201.
- Reclari Martino, M Dreyer, S Tissot, D Obreschkow, F M Wurm, et al. (2014) Surface wave dynamics in orbital shaken cylindrical containers. Physics of Fluids 26(5): 052104.
- Alpresa Paola, Spencer Sherwin, Peter Weinberg, Maarten van Reeuwijk (2018) Orbitally shaken shallow fluid layers. I. Regime classification. Physics of Fluids 30(3): 032107.
- Fadlallah H, M Jarrahi, Herbert, Ferrari, Méjean, et al. (2019). Active Fluids: effects of hydrodynamic stress on growth of self-propelled fluid particles. Journal of Applied Fluid Mechanics 13(2): 561-570.
- Heidorn Thorsten, Daniel Camsund, Hsin Ho Huang, Pia Lindberg, Paulo Oliveira, et al. (2011) Synthetic biology in cyanobacteria: engineering and analyzing novel functions. Methods enzymol 437: 539-579.
- Xu Chen, Bing Wang, Lin Yang, Lucas Zhongming Hu, Lanxing Yi, et al. (2021). Global landscape of native protein complexes in Synechocystis PCC 6803. Genomics, proteomics & bioinformatics.
- Vourc'h Thomas, H Peerhossaini, J Léopoldès, A Méjean, F Chauvat et al. (2018) Slowdown of surface diffusion during early stages of bacterial colonization. Phys Rev E 97(3): 032407.
- Kaneko Takakazu, A Tanaka, S Sato, H Kotani, T Sazuka, et al. (1995) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis strain PCC6803. I. Sequence features in the 1 Mb region from map positions 64% to 92% of the genome. DNA Res 2(4): 153-166.
- Schuergers Nils, Annegret Wilde (2015) Appendages of the cyanobacterial cell. Life 5(1): 700-715.
- Saintillan David (2018) Rheology of active fluids. Annual Review of Fluid Mechanics 50: 563-592.
- Alexandre Gladys (2015) Chemotaxis control of transient cell aggregation. J Bacteriol 197(20): 3230-3237.
- Varuni P, Shakti N Menon, Gautam I Menon (2017) Phototaxis as a collective phenomenon in cyanobacterial colonies. Scientific reports 7(1): 1-10.
- Wood A Michelle, R C Everroad, LM Wingard (2005) Measuring growth rates in microalgal cultures. Algal culturing techniques 18: 269-288.
- Fadlallah Hadi, Peerhossaini Hassan, DeGroot Christopher, Jarrahi, Mojtaba (2021) Motility response to hydrodynamic stress during the growth cycle in active fluid suspensions. Journal of Fluids Engineering 143: 7.
- Monteil Dominique T, et al. (2013). Disposable 600-mL orbitally shaken bioreactor for mammalian cell cultivation in suspension. Biochemical engineering journal 76: 6-12.
- Mehdizadeh Allaf Malihe, Zahra Habibi, Zahra Samadi, Christopher T DeGroot, Lars Rehmann, et al. (2020) Physical and Rheological Properties of Active Fluids Under Shear Stress: Suspensions of Synechocystis. Fluids Engineering Division Summer Meeting 83723.
- Saini Dinesh Kumar, Sunil Pabbi, Pratyoosh Shukla (2018) Cyanobacterial pigments: perspectives and biotechnological approaches. Food Chem Toxicol 120: 616-624.
- Safari Reza, Zeynab Raftani Amiri, Reza Esmaeilzadeh Kenari (2020) Antioxidant and antibacterial activities of C-phycocyanin from common name Spirulina platensis. Iranian journal of fisheries sciences 19(4): 1911-1927.
- Ajala Sheriff Olalekan, Matthew L Alexander (2020) Evaluating the effects of agitation by shaking, stirring and air sparging on growth and accumulation of biochemical compounds in microalgae cells. Biofuels 1-11.
- Ojo E O, Hadiza Auta, Frank Baganz, G J Lye (2014). Engineering characterization of a shaken, single-use photobioreactor for early-stage microalgae cultivation using Chlorella sorokiniana. Bioresour Technol 173: 367-375.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.