Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Management and Treatment for Tuberculosis Laten
*Corresponding author: Nanda Rachmad Putra Gofur, Department of Health, Faculty of Vocational Studies, Universitas Airlangga, Surabaya, Indonesia.
Received: December 15, 2021; Published: January 12, 2022
DOI: 10.34297/AJBSR.2022.15.002095
Abstract
Introduction: Pulmonary tuberculosis is an infectious disease caused by the bacterium Mycobacterium tuberculosis. This bacterium is in the form of a bacillus and is acid-fast, so it is also known as an acid-fast bacillus (BTA). Pulmonary TB mainly attacks the lungs as the site of primary infection, in addition, tuberculosis can also attack the skin, lymph nodes, bones, and the lining of the brain. Pulmonary TB is transmitted through infectious droplets inhaled by healthy people. Aims of this study is to review management of Tuberculosis.
Discussion: The first experimental evidence of the potential efficacy of new antituberculosis drugs was obtained when a dapsone-derivative compound. Individuals diagnosed with a pulmonary form of tuberculosis, not exposed to antituberculosis drugs for >1 mo, must be treated for 6 mo. According to the 2015 WHO report, Indonesia has succeeded in reducing morbidity and mortality due to TB in 2015 compared to 1990. The TB prevalence rate, which in 1990 was > 900 per 100,000 population, in 2015 became 647 per 100,000 population. TB treatment is divided into 2 stages, namely the initial stage to reduce the number of germs and the advanced stage to kill the remaining germs and prevent recurrence. Drugs for latent TB infection as Isoniazid for 6 months, or Isoniazid for 9 months, or Isoniazid and Rifampetine (RPT) once a week for 3 months, or3-4 months of Isoniazid and Rifampicin, or 3-4 months Rifampicin.
Conclusion: Pulmonary TB treatment is divided into 2 phases, namely the intensive phase (2-3 months) and the continuation phase of 4 or 7 months. The combination of drugs used is a combination of the main drug and additional drugs. The main types of drugs (line I) are INH, rifampin, pyrazinamide, streptomycin, ethambutol, while other additional drugs are: kanamycin, amikacin, quinolones.
Keywords: SDGs, Tuberculosis, Management, Treatment, Drug choice
Introduction
Pulmonary tuberculosis is an infectious disease caused by the bacterium Mycobacterium tuberculosis. This bacterium is in the form of a bacillus and is acid-fast, so it is also known as an acid-fast bacillus (BTA). This bacterium was first discovered by Robert Koch on March 24, 1882, so that in memory of his services the bacteria was given the name Koch’s bacillus. Pulmonary TB mainly attacks the lungs as the site of primary infection, in addition, tuberculosis can also attack the skin, lymph nodes, bones, and the lining of the brain. Pulmonary TB is transmitted through infectious droplets inhaled by healthy people [1].
Tuberculosis germs that enter through the respiratory tract will lodge in the lung tissue, where it will form a pneumonic nest, which is called the primary nest or primary affect. These primary nests may arise anywhere in the lung, in contrast to reactivation nests. From the primary nest will be seen inflammation of the lymph channels to the hilus (local lymphangitis). The inflammation is followed by enlargement of the hilar lymph nodes (regional lymphadenitis). The primary affect together with regional lymphangitis is known as the primary complex [1,2].
According to the 2015 WHO report, at the global level it was estimated that 9.6 million new TB cases were present. With 1.5 million deaths due to TB of which 480,000 cases are women. Of these TB cases, 1.1 million (12%) were found to be HIV positive with 320,000 deaths and 480,000 Drug Resistant TB (TB-RO) with 190,000 deaths. Of the 9.6 million new TB cases, it is estimated that 1 million TB cases in children (under 15 years of age) and 140,000 deaths/year [1,3].
The first experimental evidence of the potential efficacy of new antituberculosis drugs was obtained when a dapsone-derivative compound [1,2]. Individuals diagnosed with a pulmonary form of tuberculosis, not exposed to antituberculosis drugs for >1 mo, must be treated for 6 mo. Aims of this study is to review management of Tuberculosis.
Discussion
According to the 2015 WHO report, Indonesia has succeeded in reducing morbidity and mortality due to TB in 2015 compared to 1990. The TB prevalence rate, which in 1990 was > 900 per 100,000 population, in 2015 became 647 per 100,000 population. Of all the MDG’s indicators for TB in Indonesia, only the target for reducing the incidence rate has been achieved. For this reason, larger and more integrated efforts are needed so that Indonesia can achieve the SDG’s target by 2030 [4].
Pulmonary tuberculosis is an infectious disease caused by the bacillus Mycobacterium tuberculosis which has special properties that is resistant to acid on staining (Acid Resistant Bacillus) because TB bacilli have lipoid cells. TB bacilli are so sensitive to sunlight that they will die in a few minutes. TB bacilli will also be killed within minutes if exposed to 70% alcohol and 50% lysol. TB bacilli take 12- 24 hours to do mitosis, this allows intermittent drug administration (2-3 days) [4,5].
According to the 2015 WHO report, the number of TB cases in Indonesia is estimated to be 1 million new TB cases per year (399 per 100,000 population) with 100,000 deaths per year (41 per 100,000 population). An estimated 63,000 TB cases are HIV positive (25 per 100,000 population). The Case Notification Rate (CNR) of all cases was reported as 129 per 100,000 population. The total number of cases is 324,539 cases, of which 314,965 are new cases. Nationally, the estimated prevalence of HIV among TB patients is estimated at 6.2%. The number of cases of Drug Resistant TB (TB-RO) is estimated at 6700 cases originating from 1.9% of TB-RO cases from new TB cases and 12% of RO-TB cases from TB with re-treatment [4,6].
Classification of TB based on how to diagnose [7]:
1. Bacteriologically confirmed TB patient
Patients who prove positive for sputum or tissue results by
direct microscopy, TB TCM, or culture.
a) smear positive pulmonary TB patient
b) Pulmonary TB patients with positive M.tb cultures
c) Patients with pulmonary TB rapid test results for M.tb are
positive
d) Bacteriologically confirmed extrapulmonary TB patient
e) TB in children diagnosed by bacteriological examination
2. Clinically diagnosed TB patients [7]
a) AFB smear negative pulmonary TB patients with chest results
supporting TB
b) smear negative pulmonary TB patients with no clinical
improvement after non-antibiotic therapy, and have TB risk
factors
c) Extrapulmonary TB patients diagnosed clinically and
laboratory and histopathologically without bacteriological
confirmation
d) TB in children diagnosed by the scoring system
Pulmonary Tuberculosis is TB located in the lung parenchyma. Miliary TB is considered as pulmonary TB because of the presence of lesions in the lung tissue. Patients suffering from pulmonary TB as well as extrapulmonary TB are classified as pulmonary TB. Extrapulmonary tuberculosis isTB that occurs in organs other than the lungs, such as the pleura, lymph nodes, abdomen, urinary tract, skin, joints, lining of the brain and bones [7,8].
Tuberculous lymphadenitis in the chest cavity (hilar and/or mediastinal) or pleural effusion without radiological features that support TB in the lungs, is declared extrapulmonary TB.
The diagnosis of extrapulmonary TB can be established based on the results of bacteriological or clinical examination. The diagnosis of extrapulmonary TB should be pursued bacteriologically with the discovery of Mycobacterium tuberculosis. If the TB process is found in several organs, the name according to the organ affected by the TB process is the heaviest [7].
3. Classification based on previous medical history [7,8]:
1) New TB patients: patients who have never been treated for TB
before or have taken OAT but less than 1 month (<28 doses).
2) Patients who have been treated for TB: patients who have
previously taken OAT for 1 month (≥ 28 doses). Classification
based on the results of the last treatment:
a) Relapse patients: TB patients who have been declared cured
or have completed treatment and are currently diagnosed
with TB based on the results of bacteriological or clinical
examinations (either due to real relapse or due to reinfection)
b) Patients who are re-treated after failure: the patient has been
treated for TB and is declared a failure
c) Patients who are re-treated after dropping out of treatment
(loss to follow-up)
d) Others: the patient has been treated for TB but the results of
previous treatment are not known.
3) Patients whose previous medication history is unknown:
neither 1) or 2)
4. Classification based on the results of drug susceptibility tests
1) Mono-resistant TB (MR TB): resistant to one of the first-line
OATs
2) Poly-resistant (PR TB): resistant to more than one firstline
drug other than Isoniazid (H) and Rifampicin (R)
simultaneously
3) Multi drug resistant (MDR TB): Isoniazid (H) and Rifampicin
(R) resistant concurrently, with or without other first-line
drugs
4) Extensive drug resistant (XDR TB): MDR TB that is resistant
is also common h one fluoroquinolone OAT and at least one
of the second-line injections (Kanamycin, Capreomycin, and
Amikacin)
5) Rifampicin resistance (RR TB): Rifampicin resistance with
or without resistance to other drugs detected by genotyping
method (molecular rapid test) or phenotyping method
(conventional)
5. Classification of TB patients based on HIV status
1) TB patient with HIV positive
2) TB patients who are HIV negative
3) TB patient with unknown HIV status
Extrapulmonary tuberculosis is TB that attacks organs other
than the lungs, such as the pleura, lining of the brain, lining of the
heart, lymph nodes, bones, joints, skin, intestines, kidneys, urinary
tract, genitals, and others. Diagnosis based on positive specimen
culture, or histology, or strong clinical evidence consistent with
active extrapulmonary TB [8].
Extrapulmonary TB is divided according to the severity of the disease, namely, Mild extra pulmonary TB. For example: lymph node TB, unilateral exudative pleurisy, bone (except spine), joints and adrenal glands. Severe extra pulmonary TB as example meningitis, miliary, pericarditis, peritonitis, bilateral pleurisy exudativa, spinal TB, intestinal TB, urinary tract TB and genitalia. Latent TB infection is a person infected with Mycobacterium tuberculosis without any signs and symptoms or radiological or bacteriological results, but positive on TST or IGRA examination [4].
Management and Treatment of Tuberculosis
TB treatment is divided into 2 stages, namely the initial stage to reduce the number of germs and the advanced stage to kill the remaining germs and prevent recurrence. Line drug guidelines in Indonesia, namely3,10:
1) Category 1: 2(HRZE)/ 4(HR)3 or 2(HRZE)/4(HR)
2) Category 2: 2(HRZE)S/(HRZE)/5(HR)3E3 or 2(HRZE)S/
(HRZE)/5(HR)E
3) Child category: 2(HRZ)/4(HR) or 2HRZE(S)/4-10HR
4) Guidelines for drug-resistant TB patients: 2nd-line drugs,
namely Kanamycin, Capreomycin, Levofloxacin, Etionamide,
Cycloserine, Moxifloxacin, PAS, Bedaquiln, Clofazimine,
Linezolid, Delamanid, and other new TB drugs as well as firstline
anti-TB drugs, namely Pyrazinamide and ethambutol [9].
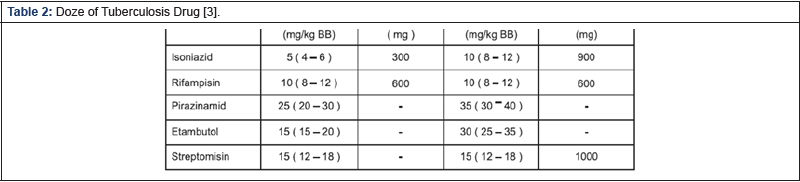
There are OATs in the form of fixed dose combination drugs
(OAT-KDT) which in 1 tablet contains a combination of 2 and 4
types of drugs whose dosages are adjusted to body weight, in
addition there is a kombipak package, namely a loose drug form
consisting of Isoniazid (H), Rifampicin (R). ), Pyrazinamide (Z),
and Ethambutol (E) which are packaged in blister form [3,9&10]
[Table1&2].
Treatment of Latent Tuberculosis [10,11]
Drugs for latent TB infection:
1. Isoniazid for 6 months, or
2. Isoniazid for 9 months, or
3. Isoniazid and Rifampetine (RPT) once a week for 3 months, or
4. 3-4 months of Isoniazid and Rifampicin, or
5. 3-4 months Rifampicin
Conclusion
Pulmonary TB treatment is divided into 2 phases, namely the intensive phase (2-3 months) and the continuation phase of 4 or 7 months. The combination of drugs used is a combination of the main drug and additional drugs. The main types of drugs (line I) are INH, rifampin, pyrazinamide, streptomycin, ethambutol, while other additional drugs are: kanamycin, amikacin, quinolones.
References
- Black JM, Hawk JH (2005) Medical Surgical Nursing Clinical Management for Positive Outcomes. (7th Edn.), St Louis, Missouri: Elsevier Saunders.
- Smeltzer SC, Bare BG, Hinkle JL, Cheever KH (2008) Brunner & Suddarth’s Textbook of Medical-Surgical Nursing. (11th Edn.), Philadelphia: Lippincott Williams & Wilkins.
- Aditama TY, Soedarsono, Thabrani, Z, et al. (2006) Guidelines for Diagnosis and Management in Indonesia. Indonesian Lung Doctors Association, Jakarta.
- Arentz M, Pavlinac P, Kimerling ME, Horne DJ, Falzon D, et al. (2012) Use of anti-retroviral therapy in tuberculosis patients on second-line anti-TB regimens: A systematic review. PLoS ONE 7(11): e47370.
- De Lorenzo S, Alffenaar JW, Sotgiu G, Centis R, D’Ambrosio L, et al. (2013) Efficacy and safety of meropenem-clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J 41(6): 1386-1392.
- Skripconoka V, Danilovits M, Pehme L, Tomson T, Skenders G, et al. (2013) Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J 41(6): 1393-1400.
- Sotgiu G, Lange C, Richardson MD, Matteelli A, Centis R, et al. (2009) Comment on: Daily 300 mg dose of linezolid for the treatment of intractable multidrug-resistant and extensively drug-resistant tuberculosis. J Antimicrob Chemother 64(5): 879-883.
- Dheda K, Migliori GB (2012) The global rise of extensively drug-resistant tuberculosis: Is the time to bring back sanatoria now overdue? Lancet 379(9817): 773-775.
- Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, et al. (2009) The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 360(23): 2397-2405.
- Shaw KJ, Barbachyn MR (2011) The oxazolidinones: Past, present, and future. Ann N Y Acad Sci 1241: 48-70.
- Sotgiu G, Centis R, D’Ambrosio L, Alffenaar JW, Anger HA, et al. (2012) Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: Systematic review and meta-analysis. Eur Respir J 40(6): 1430-1442.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.