Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Merkel Cell Carcinoma of Lymph Node with an Unknown Primary Cutaneous Tumor - Immunohistochemical Analysis, Differential Diagnosis and Adjuvant Radiotherapy
*Corresponding author: Lena Marinova, Medical Oncology Clinic, Department of Radiation Oncology and Metabolic Brachytherapy, UMHAT “Queen Joanna” Sofia, Bulgaria
Received: January 03, 2022; Published: January 12, 2022
DOI: 10.34297/AJBSR.2022.15.002094
Abstract
Merkel cell carcinoma (MCC) is an aggressive neuroendocrine neoplasm with a high risk of lymph metastases to regional lymph nodes. Differential diagnosis (DD) imposes immunohistochemical (IHC) analysis to prove the neuroendocrine and epithelial immunotype of the MCC. In a known primary tumor, the primary treatment method is a wide radical excision with a clean resection lines up to 3-5 cm. Selective lymph node dissection is used to confirm the disease stage and proof of regional lymph metastases. The adjuvant post-operative local and regional radiotherapy (RT) in the primary tumor bed and metastatic lymph nodes improves disease-free survival. Against a clinical case with recurrent metastatic lymph nodes from an unknown primary MCC, pathohistological DD difficulties requiring IHC analysis, characteristic aggressive lymph metastasis and the imposed complex treatment, including surgery, chemotherapy and adjuvant RT, are discussed. In order to diagnose the primary tumor, the state of the regional lymph nodes and the presence of distant metastases, the image diagnostic consisting of CT with intravenous contrast and PET/CT, is used.
Keywords: Merkel Cell Carcinoma; Regional Lymph Node Metastases; Immunohistochemical analysis; Neuroendocrine Carcinoma; PET/CT; Adjuvant Radiotherapy
Introduction
Merkel cell carcinoma (MCC) is an unusual skin malignancy, also known as neuroendocrine or primary small cell skin carcinoma [1]. MCC is a rare aggressive neuroendocrine tumor that develops mainly in the area of head and neck or the extremities of adult patients over 75 years of age [2-11]. The incidence of the disease is 0.1-0.4 per 100,000 population [7]. Rare cases of Merkel cell carcinoma have been encountered in lymph nodes with unknown extranodal primary, which exhibit similar morphologic and immunophenotypic features to those in primary cutaneous MCC [12]. Clinical and pathohistological diagnosis is extremely difficult and requires immunohistochemical (IHC) analysis proving the squamous and neuroendocrine tumor characteristic [13-19]. The high risk of regional lymph nodes and distant metastases predetermines complex treatment, including wide radical excision, followed by adjuvant local and regional radiotherapy (RT) [8,11,20,21]. In patients after surgery and adjuvant RT, a significantly higher average disease-free survival compared to these after self-contained surgery was accounted [20,22].
Clinical case
We present a woman at the age of 63 with a painless swelling in the right submandibular area with a discharge since three months. Of the examination, indirect laryngoscopy, mesopharygoscopy and nasopharyngoscopy does not establish a pathological finding. In the palpation of the neck in the right submandibular region there is an increased lymph node with a diameter of more than 3 cm. The CT with contrast diagnosed two suspected for metastatic lymph nodes in the right cervical area (Figure 1). After surgical excision of cervical lymph nodes, the biopsy proves metastasis from small cell anaplastic /neuroendocrine carcinoma. Immunohistochemical study reports: tumor cells express synaptophysine and CD56, focally positive to EМА and pancitokeratin, but negative to TTF1, S100 protein, CК 20 and CD45 (Figure 2 & Figure 3). The patient is judged to conduct 3 courses of chemotherapy (Ch), and then restaging. In April 2021, from a PET/CT data for a metabolic active lesion located right retromandibular, most likely a metastatic lymph node. Metabolic active hyleric and mediastinal lymph nodes are reported. [Figure 4].
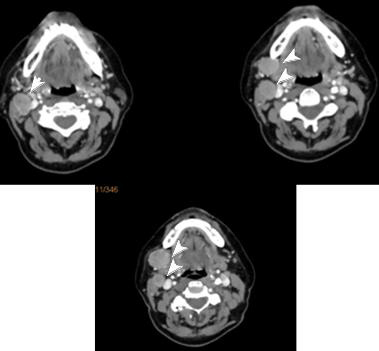
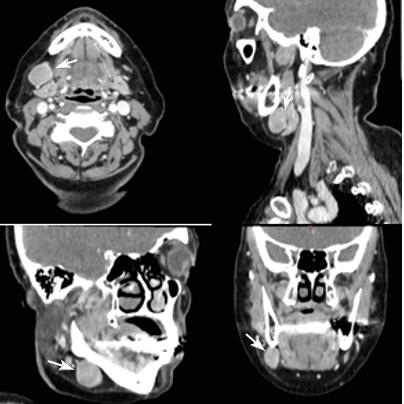
Figure 1: CT with contrast visualized two lymph nodes in the right submandibular area, which are suspected for metastatic /marked with white arrows.
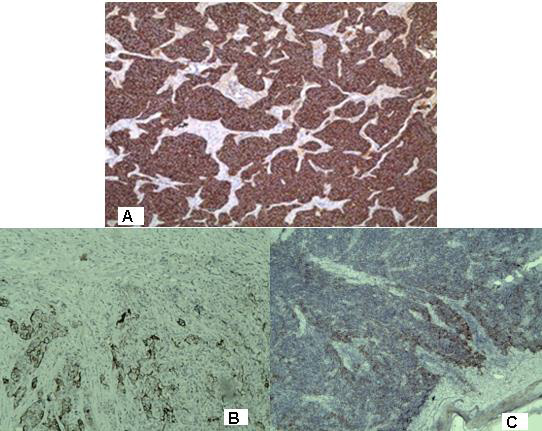
Figure 2: Photomicrography of immunohistochemistry with positive expression in tumor cells for: A / synaptophysine; Focal expression in tumor cells for: B / pancitokeratin CК AE1 / AE3; C / ЕМА/ x 20
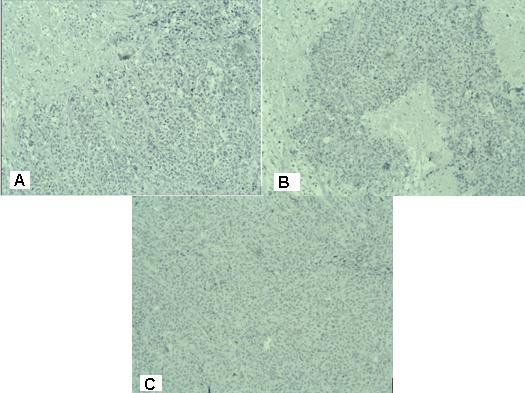
Figure 3: Photomicrography of immunohistochemistry with negative expression in tumor cells for: A / TTF1; B / S100 protein; C / CD45 / x 20
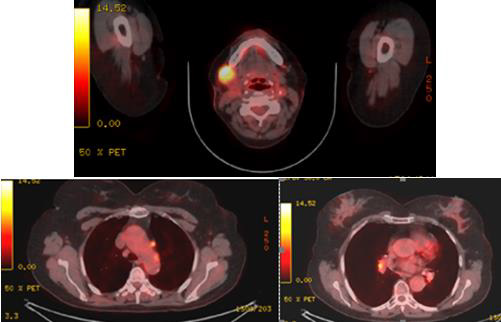
Figure 4: PET/CT (April 2021) data for a metabolic active lesion located in the right retromandibular area, most likely a metastatic lymph node. Metabolic active hyleric and mediastinal lymph nodes.
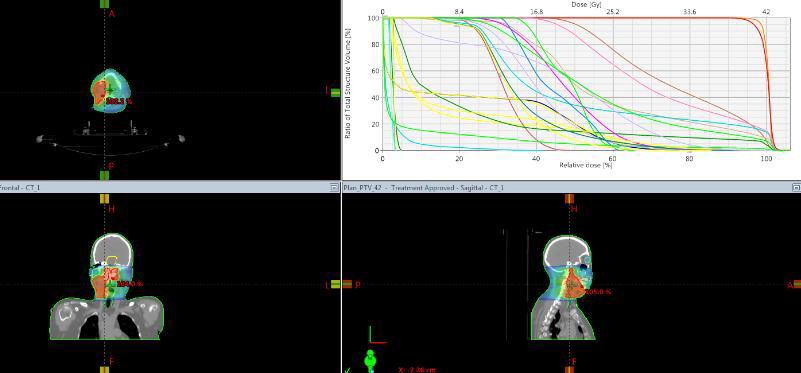
The patient continued with another 3 courses of Ch. In August 2021, the control PET/CT reported a partial metabolic and morphological response from the tracked lymph node in an IB right-cervical level. Presence of metabolic active hyleric and mediastinal lymph nodes, with slightly increased activity in part of the mediastinal nodules, against the backdrop of missing substantial morphological dynamics (Figure 5). The control restaging CT in November takes into account the increase in the right metastatic submandibular lymph node (Figure 6). A second reexcision of the metastatic lymph node was carried out with dissection of deep right cervical lymph nodes. The patient was targeted for post-operative radiotherapy (RT), which was performed with VMAT technique in the field of right-handed cervical lymph nodes from IA to IV level with daily dose (DD) 3 Gy up to total dose (TD) 42 Gy / biologically equivalent dose (BED) 50.4 Gy (Figure 7). After completing the RT, the patient is targeted for immunotherapy.
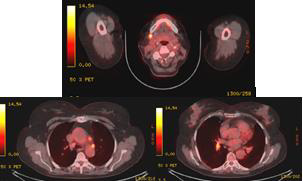
Figure 5: Control PET/CT (August 2021) - a partial metabolic and morphological response from the tracked lymph node in an IB right-cervical level. Percession of metabolic active hyleric and mediastinal lymph nodes, with slightly increased activity in part of the mediastinal nodules, against the backdrop of missing substantial morphological dynamics.
Discussion
Merkel cell carcinoma (MCC) is believed to arise from Merkel cells that are located at the dermo-epidermal junction. Majority of MCCs are intradermal, only 10% arise in the epidermis [23]. MCC is a rare primary cutaneous neuroendocrine tumor originating from the basal layer of the skin which is associated with the terminal axons [24-26]. Microscopically, MCC appears as “small blue cell tumors”, differential diagnosis includes small cell lung carcinoma, small B-cell lymphoma, Ewing sarcoma, metastatic neuroendocrine carcinoma and small cell melanoma [24,27].
Extracutaneous origin of Merkel cell carcinoma- In the presence of a nodal Merkel cell tumor, an exhaustive clinicoradiologic search for a primary tumor must be carried out. After the exclusion of any reasonable starting point of the neoplasm, a provisional diagnosis of “primary” nodal Merkel cell carcinoma may be acceptable; since a primary extracutaneous tumor is expected to follow a less aggressive course than a metastatic one, follow-up data may provide indications as to the truly extracutaneous origin of MCC [28]. In the clinical case presented, the disease begins with increased retromandibular lymph nodes on the right (Figure 1), no primary cutaneous tumor or pathological lesion from the indirect laryngoscopy, mesopharingoscopy and nasopharyngoscopy.
Pathohistology and immunohistochemical (IHC) analysis- MCC is a predominantly dermal-based tumor arranged in solid sheets and nests, composed of small, round to oval cells with vesicular nuclei, multiple small nucleoli and scant amphophilic cytoplasm [29]. The paranuclear globular coexpression of cytokeratin and neurofilaments by an undifferentiated dermal tumor is of significant help in diagnosing MCC and differentiating it from small-cell carcinoma [30]. To establish an accurate diagnosis of the nodal Merkel cell carcinoma can be challenging because of significant morphologic mimics, including lymphoblastic lymphoma and metastatic small cell carcinoma [12]. Distinction between primary cutaneous Merkel cell carcinoma and metastatic neuroendocrine carcinoma in the skin requires immunohistochemical and clinical pathologic correlation [24]. First of all, a disturbed pathochistological diagnosis is reported, assisted by IHC panel excluding lymph metastasis from another neoplasm with a small cell pathohistological characteristic. The immunohistochemical phenotype of the MCC is an epithelial tumor with neuroendocrine characteristic [11]. Definitive diagnosis is made by histological, especially immunohistochemical methods (detection of intermediate filaments such as cytokeratins and neuroendocrine markers) [31]. To demonstrate a neuroendocrine cell characteristic, the tumor cells were examined with an IHC panel comprising CD56, synaptophysin and chromogranin A [32,33]. It should be emphasized that they are expressed at 30-50% of clinical cases [34]. Positive keratin labeling (AE1/AE3, MAK-6) of filaments arranged in paranuclear aggregates, with presence of cytoplasmic synaptophysin helps to make the diagnosis [35]. MCC tumor cells express cytokeratin (CK) [9,20-22,36,37], neurofilament, synaptophysin, chromogranin and neuron specific enolase (NSE) [9,38,39]. Due to neuroendocrine differentiation, the MCC expresses NSE, which is a base neuroendocrine marker [15-17]. CD56 or neural cell adhesion molecule (NCAM) is a neuroendocrine marker which is characteristic of the pulmonary neuroendocrine cell system as well as MCC [18,19]. The thyroid transcription factor 1 (TTF-1) is a tissue specific transcription factor that is expressed in the epithelial cells of the thyroid gland and lung, as well as in certain areas of the brain [40]. TTF-1 is applied to a differential diagnosis between MCC and other small cell neoplasms [41,42]. The MCC has a negative IHC expression for TTF-1 [43]. The combined IHC panel with TTF-1 and CK-20 proves Merkel cell immunotype [5]. Diagnostic difficulties originate from the broad DD and the probability of duplication of some positive markers such as Sinaptophysine at MCC and small cell lung carcinoma. In the clinical case presented, Merkel cell immunophenotype is proven by a combination of positive neuroendocrine IHC marker synaptophysine, focal expression of epithelial antigens EMA and CK AE1 / AE3 and negative TTF-1 expression. (Figure 2, Figure 3/A). Negative expression for S100 protein excludes diagnosis of malignant melanoma (Figure 3/B), and the absence of CD45 expression rejects malignant lymphoma (Figure 3/C).
Factors relevant to staging for Merkel cell carcinoma include tumor size, depth of invasion, locoregional nodal involvement and disseminated metastases [24]. Unfavorable prognostic pathological factors with significant statistical dependence include tumor size ≥ 5 mm (p = 0.047), subcutaneous fat invasion (p = 0.005), diffuse tumor growth (p = 0.040) and abundant lymphocytic infiltration (p = 0.017) [44]. Risk factors for MCC, such as old age, immunosuppression, polyomavirus infection and exposure to UV radiation have already been identified, but the underlying mechanisms leading to carcinogenesis still need clarification [45].
Prognosis - Merkel cell carcinoma (MCC) is a rare but highly aggressive neuroendocrine carcinoma of the skin, with frequent recurrences, metastases, and a high mortality rate [46]. The prognosis is rather poor. Stage of disease at presentation predicts overall survival [47]. The typical clinical development of the MCC is progression of the primary tumor accompanied by early and frequent metastases in regional lymph nodes [5,6,21]. Local recurrences are very common, occurring in up to 44% of patients. The 5-year survival rate is between 30% and 64%. Mortality rate of MCC exceeds that of any other skin cancers [48]. In our clinical case, after two months despite the surgical selective dissection, PET/CT proves not only the metabolic active submanibular lymph node which recurs, but also hyleric and mediastinal lymph nodes (Figure 4).
Local recurrences and metastatic lymph nodes - High level of local recurrences (25-77%) and metastatic lymph nodes (50%) is a characteristic of MCC. The fatal outcome was observed in 30% of patients, despite the combined healing approach, including wide surgical excision of primary tumor with dissection of regional lymph nodes, adjuvant postoperative radiotherapy (RT) and chemotherapy (Ch) [9,49]. Like any other oncological detection, Positron emission tomography-computerized tomography (PETCT) scan helps to locate distant metastases [1]. Sentinel lymph biopsy of regional lymph nodes provides information on metastatic lymph distribution. The data from the meta-analysis of 60 patients with MCC account for 33% metastatic dissemination in sentinel lymph nodes, even in diagnosis of the primary tumor [50]. These data imposes lymph dissection of regional lymph nodes [11,51]. With the MCC in the head and neck area, the cervical lymph nodes are mostly affected where early lymph metastases are detected, which in our case are recurrent without the conduct of RT.
Complex treatment- A wide local excision with negative margins over 2,5 cm. and sentinel node (SLN) biopsy, is the recommended treatment of choice [52-54]. However, no consensus has been reached with regard to the width of the negative resection margins. There are authors who prove that the wider surgical resection line does not significantly increase the survival. [55]. When comparing pathohistologically positive resection lines with clean resection lines, the following therapeutic results are reported: local recurrences 33.3% / 9.09% (p = 0.19), regional recurrences 66.6% / 27.2% (p = 0.08) and distant metastases 66.6% /45.4% (p = 0.36) [56]. Performing a wide surgical resection is hampered in the head and neck MCC and in the elderly patients with adverse diseases incompatible with general anesthesia [57]. In tumor localization on the face, a sparing operation with a clean resection line of at least 1.5 cm, and MCC on the limbs - 3 cm, is recommended. Radiation therapy was highly effective when given as consolidation after surgery or chemotherapy [58,59]. For reduction of local recurrences, adjuvant RT is required [7,52]. In order to avoid large volume of surgery, initial RT has recommended after the primary tumor excision biopsy as well as prophylactic regional lymph nodes RT [60]. There is a significant reduction in the local (p <0.001) and regional recurrences (p <0.001) when comparing patients after surgery and adjuvant RT with those after a definitive RT. In both groups of patients reported similar results in terms of distant metastases (p = 0.31) [61]. In the clinical case presented, the adjuvant RT is only administered after metastatic lymphatic progression (Figure 5 & Figure 6) and after lymph node reoperation. The volume of post-operative RT should include regional lymph nodes [8,10]. As with the clinical case presented, the postoperative RT of regional lymph metastases is generally passed (Figure 7). The adjuvant RT improves regional tumor control and is a major factor for significantly improved disease-free survival [20,21]. Patients after surgery and adjuvant RT reported significantly higher disease-free survival compared to those after surgery - 23 months against 6 months (p <0.01). The оveral and disease-free survival of the third year reaches respectively 66% and 25% [22]. Until 2017, patients with advanced disease were typically treated with conventional chemotherapies, with a median response duration of 3 months. When used in a first-line setting, PD-1/PD-L1 inhibitors (avelumab, pembrolizumab, nivolumab) are even more promising as objective responses are observed in approximately 50-70% of patients within the first 4-8 weeks of treatment [62]. First line therapy with the PD-L1 blocking antibody avelumab is associated with a response rate of 62% [63]. Treatment with checkpoint inhibitors (CPIs) has shown a major advancement in the treatment of advanced MCC [46].
Conclusion
We present a rare clinical case with MCC of the lymph nodes with an unknown primary tumor. The MCC is an aggressive malignant tumor with a high risk of lymph metastases in regional lymph nodes. Histological and immunohistochemical profile plays a significant role in diagnosis. Like any other oncological detection, Positron emission tomography-computerized tomography (PETCT) scan helps to locate regional and distant metastases. The main primary tumor treatment is the wide radical excision with negative margins 2 to 5 cm. Selective lymph dissection optimizes early diagnosis of the disease by proof of early metastases in regional lymph nodes. The adjuvant post-operative RT in the primary tumor bed and regional lymph nodes improves disease-free survival.
References
- Priyanka Yaramada, Brian S Lim, Christopher M Flannery, Stephen S Koh, Harout Yaghsezian (2016) Merkel cell carcinoma of unknown primary with lymph node and mesenteric metastasis involving the pancreas and duodenum. J Gastrointest Oncol 7(Suppl 1): S66-70.
- Pergolizzi Jj, Sardi A, Pelczar M, Conaway Gl (1997) Merkel cell carcinoma: an aggressive malignancy. Am Surg 63(5): 450-454.
- Savage P, Constenla D, Fisher C, J M Thomas, M E Gore (1997) The natural history and management of Merkel cell carcinoma of the skin: a review of 22 patients treated at the Royal Marsden Hospital. Clin Oncol (R Coll Radiol) 9(3): 164-167.
- Jagannath Sherigar, Susim Kumar, Jawed Wali (2007) Merkel cell carcinoma of the cheek: Diagnosis in an elderly woman. Ulster Med J 76(2):116.
- Virve Koljonen (2006) Merkel cell carcinoma. World J Surg Oncol 4: 7.
- Akhtar S, Oza Kk, Wright J (2000) Merkel cell carcinoma: report of 10 cases and review of the literature. J Am Acad Dermatol 43(5 Pt 1): 755-767.
- Strub B, Weindel S, Witt P, Grünert J (2009) The interdisciplinary treatment of Merkel cell carcinoma: a retrospective case analysis and review of the current literature. Zentralbl Chir 134(5): 468-473.
- Khan Durani B, Hartschuh W (2003) Merkel cell carcinoma. Clinical and histological differential diagnosis, diagnostic approach and therapy. Hautarzt 54(12): 1171-1176.
- Helmbold P, Schröter S, Holzhausen HJ, et al. (2002) Merkel cell carcinoma. Hautarzt 53(10): 652-8.
- Weisser H, Hartschuh W, Greiner A, M Bischof, A Enk, et al. (2007) Merkel cell carcinoma-clinically often misjudged. Dtsch Med Wochenschr 132(30): 1581-1586.
- Acebo E, Vidaurrazaga N, Varas C, J J Burgos-Bretones, J L Díaz-Pérez (2005) Merkel cell carcinoma: a clinicopathological study of 11 cases. J Eur Acad Dermatol Venereol 19(5): 546-551.
- Zenggang Pan, Yuan-Yuan Chen, Xiaojun Wu, Vijay Trisal, Sharon P Wilczynski, et al. (2014) Merkel cell carcinoma of lymph node with unknown primary has a significantly lower association with Merkel cell polyomavirus than its cutaneous counterpart. Mod Pathol 27(9): 1182-1192.
- Miettinen M, Lehto Vp, Virtanen I, S Asko-Seljavaara, J Pitkänen, et al. (1983) Neuroendocrine carcinoma of the skin (Merkel cell carcinoma): ultrastructural and immunohistochemical demonstration of neurofilaments. Ultrastruct Pathol 4: 219-225.
- Moll R, Osborn M, Hartschuh W, I Moll, G Mahrle, et al. (1986) Variability of expression and arrangement of cytokeratin and neurofilaments in cutaneous neuroendocrine carcinomas (Merkel cell tumors): immunocytochemical and biochemical analysis of twelve cases. Ultrastruct Pathol 10: 473-495.
- Metz Ka, Jacob M, Schmidt U, K P Steuhl, L D Leder (1998) Merkel cell carcinoma of the eyelid: histological and immunohistochemical features with special respect to differential diagnosis. Graefes Arch Clin Exp Ophthalmol 236: 561-566.
- Leong As, Phillips Ge, Pieterse As, Milios J (1986) Criteria for the diagnosis of primary endocrine carcinoma of the skin (Merkel cell carcinoma). A histological, immunohistochemical and ultrastructural study of 13 cases. Pathology 18: 393-399.
- Sibley Rk, Dahl D (1985) Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. An immunocytochemical study of 21 cases. Am J Surg Pathol 9: 109-116.
- Motoki Kurokawa, Kazuki Nabeshima, Yutaka Akiyama, Shunichi Maeda, Takaaki Nishida, et al. (2003) CD56: a useful marker for diagnosing Merkel cell carcinoma. J Dermatol Sci 31: 219-224.
- Gallego R, Garcia-Caballero T, Fraga M, A Beiras, J Forteza (1995) Neural cell adhesion molecule immunoreactivity in Merkel cells and Merkel cell tumours. Virchows Arch 426: 317-321.
- Michael J Veness, Lakmalie Perera, Junie Mccourt, Jennifer Shannon, T Michael Hughes, et al. (2005) Merkel cell carcinoma: improved outcome with adjuvant radiotherapy. ANZ J Surg 75(5): 275-281.
- Emeline Pape, Nicolas Rezvoy, Nicolas Penel, Julia Salleron, Veronique Martinot, et al. (2011) Radiotherapy alone for Merkel cell carcinoma: a comparative and retrospective study of 25 patients. J Am Acad Dermatol 65(5): 983-90.
- Michael J Veness, Gary J Morgan, Val Gebski (2005) Adjuvant locoregional radiotherapy as best practice in patients with Merkel cell carcinoma of the head and neck. Head Neck 27(3): 208-216.
- Ratner D, Nelson Br, Brown Md, et al. (1993) Merkel cell carcinoma. J Am Acad Dermatol 29: 143-156.
- Ly TY (2021) Merkel cell carcinoma.
- Toker (1972) Trabecular carcinoma of the skin Arch Dermatol, Arch Dermatol 105: 107-110.
- Beredjiklian, Rakesh Donthineni-Rao (2004) Tumors, Review of Hand Surgery, Part:10, Saunders: 189-206.
- D F Smith, J L Messina, R Perrott, C G Berman, D S Reintgen, et al. (2000) Clinical approach to neuroendocrine carcinoma of the skin (Merkel cell carcinoma). Cancer Control 7:72-83.
- Ferrara G, Ianniello Gp, Di Vizio D, Nappi O (1997) Lymph node Merkel cell carcinoma with no evidence of cutaneous tumor-report of two cases. Tumori 83(5): 868-872.
- Weedon (2010) Neural and Neuroendocrine Tumors. Churchill Livingstone/Elsevier p. 884
- Ratner D, Nelson Br, Brown Md, Johnson Tm (1993) Merkel cell carcinoma. Journal of the American Academy of Dermatology 29(2 I): 143-160.
- Meyer-Pannwitt U, Kummerfeldt K, Boubaris P, Caselitz J (1997) Merkel cell carcinoma Langenbecks Archiv fur Chirurgie 382(6): 349-358.
- Loy Ts, Darkow Gv, Quesenberry Jt (1995) Immunostaining in the diagnosis of pulmonary neuroendocrine carcinomas. An immunohistochemical study with ultrastructural correlations. Am J Surg Pathol 19: 173-182.
- Ryan Meacham, Laura Matrka, Enver Ozer, H Gulcin Ozer, Paul Wakely, et al (2012) Neuroendocrine carcinoma of the head and neck: a 20-year case series. Ear Nose Throat J 91(3): E20-4.
- D G Guinee Jr, N F Fishback, M N Koss, S L Abbondanzo, W D Travis (1994) The spectum of immunohistochemical staining of small cell lung carcinoma in specimens from transbronchial and open-lung biopsies. Am J Clin Pathol 102: 406-414.
- S L Mount, D J Taatjes (1994) Neuroendocrine carcinoma of the skin (Merkel cell carcinoma): an immunoelectron-microscopic case study. American Journal of Dermatopathology 16(1): 60-65.
- Warner Tf, Uno H, Hafez Gr, J Burgess, C Bolles, et al. (1983) Merkel cells and Merkel cell tumors. Ultrastructure, immunocytochemistry and review of the literature. Cancer 52: 238-245.
- Chan Jk, Suster S, Wenig Bm, W Y Tsang, J B Chan, et al. (1997) Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol 21: 226-234.
- Virve Koljonen, Caj Haglund, Erkki Tukiainen, Tom Böhling (2005) Neuroendocrine differentiation in primary Merkel cell carcinoma--possible prognostic significance. Anticancer Res 25: 853-858.
- R Buffa, G Rindi, F Sessa, A Gini, C Capella, et al. (1987) Synaptophysin immunoreactivity and small clear vesicles in neuroendocrine cells and related tumours. Mol Cell Probes 1: 367-381.
- D Lazzaro, M Price, M De Felice, R Di Lauro (1991) The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 113: 1093-1104.
- A L Byrd-Gloster, A Khoor, L F Glass, J L Messina, J A Whitsett, et al. (2000) Differential expression of thyroid transcription factor 1 in small cell lung carcinoma and Merkel cell tumor. Hum Pathol 31: 58-62.
- Cheuk W, Kwan My, Suster S, Chan Jk (2001) Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med 125: 228-231.
- Ordonez NG (2000) Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol 24: 1217-1223.
- Ryan T Mott, Bruce R Smoller, Michael B Morgan (2004) Merkel cell carcinoma: a clinicopathologic study with prognostic implications. J Cutan Pathol 31(3): 217-23.
- Véronique Del Marmol, Celeste Lebbé (2019) New perspectives in Merkel cell carcinoma. Curr Opin Oncol 31(2): 72-83.
- Kotaro Nagase, Yutaka Narisawa (2018) Immunotherapy for Merkel Cell Carcinoma. Curr Treat Options Oncol 19(11): 57.
- NCCN Guidelines version 1.2014. Merkel Cell Carcinoma.
- Peter J Allen, Wilbur B Bowne, David P Jaques, Murray F Brennan, Klaus Busam, et al. (2005) Markel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol 23: 2300-2309.
- Scholz T, Rippmann V, Spilker G (2004) Merkel cell carcinoma--a case report with regard to the current treatment concepts. Handchir Mikrochir Plast Chir 36(5): 318-322.
- Khosrow Mehrany, Clark C Otley, Roger H Weenig, P Kim Phillips, Randall K Roenigk, et al. (2002) A Meta-analysis of the Prognostic Significance of Sentinel Lymph Node Status in Merkel Cell Carcinoma. Dermatol Surg 28: 113-117.
- Farshid Dayyani, Curtis A Pettaway, Ashish M Kamat, Mark F Munsell, Kanishka Sircar, et al. (2002) Market cell carcinoma. Retrospective analysis of 4 cases with special reference to diagnosis, therapy and long-term outcome. Swiss Surg 8(5): 215-219.
- Patricia Tai (2013) A practical update of surgical management of merkel cell carcinoma of the skin. ISRN Surg 2013: 850797.
- A Yiengpruksawan, D G Coit, H T Thaler, C Urmacher, W K Knapper (1991) Merkel cell carcinoma. Prognosis and management. Arch Surg 126: 1514-1519.
- O'Connor WJ, Brodland DG (1996) Merkel cell carcinoma. Dermatol Surg 22: 262-267.
- Gillenwater Am, Hessel Ac, Morrison Wh, Burgess M, E G Silva, et al. (2001) Merkel cell carcinoma of the head and neck: effect of surgical excision and radiation on recurrence and survival. Arch Otolaryngol Head Neck Surg 127: 149-154.
- Henry D Sandel, Terry Day, Mary S Richardson, Matthew Scarlett, Katharine A Gutman (2006) Market cell carcinoma: does tumor size or depth of invasion correlate with recurrence, metastasis, or patient survival? Laryngoscope 116(5): 791-795.
- Laurent Mortier, Xavier Mirabel, Charles Fournier, Frederic Piette, Eric Lartigau (2003) Radiotherapy alone for primary Merkel cell carcinoma. Arch Dermatol 139(12): 1587-1590.
- Fenig E, Lurie H, Klein B, Sulkes A (1993) The treatment of advance0d Merkel cell carcinoma: a multimodality chemotherapy and radiation therapy treatment approach. Journal of Dermatologic Surgery and Oncology 19(9): 860-864.
- Fenig E, Lurie H, Sulkes A (1993) The use of cyclophosphamide, methotrexate, and 5-fluorouracil in the treatment of Merkel cell carcinoma. American Journal of Clinical Oncology 16(1): 54-57.
- Pacella J, Ashby M, Ainslie J, Minty C (1988) The role of radiotherapy in the management of primary cutaneous neuroendocrine tumors (Merkel cell or trabecular carcinoma): experience at the Peter MacCallum Cancer Institute (Melbourne, Australia). Int J Radiat Oncol Biol Phys 14(6): 1077-1084.
- Kevan G Lewis, Martin A Weinstock, Amy L Weaver, Clark C Otley (2003) Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol 142(6): 693-700.
- Mahtab Samimi (2019) Immune Checkpoint Inhibitors and Beyond: An Overview of Immune-Based Therapies in Merkel Cell Carcinoma. Am J Clin Dermatol 20(3): 391-407.
- P Terheyden, A Mohr, E A Langan (2019) Immune checkpoint inhibition in Merkel cell carcinoma. Hautarzt 70(9): 684-690.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.