Opinion 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Opinion About Increasing use of Li-Air Batteries
*Corresponding author: Juan Horacio Pacheco Sánchez, Postgraduate Studies and Research Division, Toluca Institute of Technology, Mexico.
Received: January 19, 2022; Published: January 27, 2022
DOI: 10.34297/AJBSR.2022.15.002110
Opinion
Currently, conventional lead-acid and lithium-ion batteries are inappropriate to withstand conditions and requirements of industrial applications, such as in high-range electric vehicles and electro-mobile devices such as cell phones and laptops. To meet the storage requirements of electrical energy, batteries must develop a higher energy density. The batteries that are currently dominating the field of electromobility are Li-ion batteries. To achieve a requirement of high energy density, metal-O2 mainly Zn-O2, Al-O2, Li-O2 batteries (O2 is taken from the air) are attractive as an alternative, to the fact that these have a high theoretical energy density. Metal-O2 batteries are characterized by oxygen, one of their electroactive materials. Given that oxygen is an electroactive material, in theory this simplifies the design and increases the energy density of a battery cell. For metal-O2 batteries, there are different technologies based on using different metals as an anode, for example: Ca, Al, Fe, Cd and Zn [1]. Interest has been directed more towards Zn-O2 and Li-O2 batteries, of which research into Zn-O2 batteries is considered to be a sufficiently mature technology, and most of their applications are regarded as stationary or in primary batteries, i.e., non-rechargeable batteries. Currently, there are primary batteries with Zn-O2 technology. These Zn-O2 batteries have a theoretical energy density of 1 kWh/kg, which is 5 times higher than the energy density of current Lithium batteries [2]. Li-O2 or Li-air batteries are considered as an alternative of high theoretical gravimetric energy density (11-13 kWh/kg). For Li-air batteries there are four types of cells according to the type of electrolyte used which are: aprotic, aqueous, solid, and hybrid aqueous/aprotic. The efficiency of Li-air batteries is below 70%, in addition to low charging and discharging speeds and cyclability are the main limitations of this type of battery. Therefore, a series of challenges must be overcome that focus on obtaining materials, with greater stability and greater understanding of the reaction and transport kinetics [2]. On the other hand, Zhang, et al. [3] Introduce graphene to rechargeable Li-CO2 batteries. To do this, they perform calculations of first principles to identify the discharge product. In addition, they calculate a voltage of 2.66 V for the reaction 4Li+3CO2=C+2Li2CO3. Their results exhibit good electrochemical activity in Li-CO2 batteries, including higher capacities, longer cyclabilities, and lower overpotentials. They conclude that their kinetic parameters need to be improved to achieve the efficiency of Li O2 batteries. They report high discharge capacity (up to 14774 mAhg-1) and stable cyclability for 20 cycles at a current density of 50 mAg-1 [3,4] perform the manufacture, characterization, and performance of rechargeable and long-cycle Li-O2 cells. The cells manufactured consist of a lithium metal anode, a membrane laminate made of glass-ceramic materials and polymer-ceramic, plus a solid-state cathode composed of air prepared from coal. The cells showed excellent thermal stability and rechargeability in the temperature range of 30 to 105°C. One cell underwent 40 discharge/charge cycles with excellent reversibility. Its reproducible results are brought to the manufacture of a safe, rechargeable lithium-air battery with high energy density [4]. Although there are a lot of studies that use lithium in batteries in other ways that use oxygen as the main source of electrical energy creation, this and the demonstration of the formation of lithium oxide molecules that will be seen in the following results is enough to infer limitations to the use of lithium and in general of this type of batteries and the use of metal oxides as an energy source.
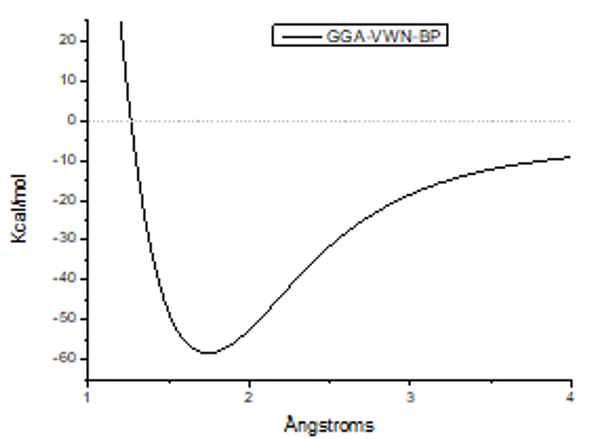
Figure 1: Potential interaction curve of the interaction between one lithium atom and one oxygen molecule.
Single-point all electron calculations of the interaction between one lithium atom and one oxygen molecule using the BIOVIA Materials Studio software with a DFT-GGA theory level by means of VWN-BP functional with a set of DND bases for unrestricted Hartree-Fock space are shown in Figure 1. It is shown that a lithium oxide molecule LiO2 is indeed formed with very strong bonding corresponding to chemisorption due to the magnitude of the potential well which is around 60Kcal/mol. This means that lithium batteries are effectively powered by molecular oxygen that is in the air. The interesting point is that in near future, the air will be shared among electronic devices and animals. Considering the exponential growth in the use of cell phones, laptops, ipads, etc. the air might be a competition problem mainly among human being and machines. Although the calculations are preliminary, the already well-known facts allow us to express the opinion that the use of metal oxides to generate electric current should be limited to reactions of lithium molecules with CO2, as an example. Extensive use of reactions among lithium atoms and oxygen molecules for energy storage does not seem pretty good.
References
- Taylor P, Bolton R, Stone D, Zhang XP, Martin Ch, et al. (2012) Pathways for Energy Storage in the UK. Center for Low Carbon Futures, Uk.
- MT Gil Austi, L Zubizarreta Saenz De Zaitegui, V Fuster Roig, A Quijano Lopez (2017) Batteries of the future: challenges and projection. Dyna 92(6): 601-605.
- Zhang Z, Qiang Zhang, Yanan Chen, Jie Bao, Xianlong Zhou, et al. (2015) The First Introduction of Graphene to Rechargeable Li-CO2. Batteries. Angewandte Chemie International Edition 127(22): 6550-6553.
- B Kumar (2010) A Solid-State, Rechargeable, Long Cycle Life Lithium-Air Battery. Journal of the Electrochemical Society 157(1): 50-54.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.