Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Stroke in Young Patients: Epidemiology, Manifestations, Diagnosis and Treatment
*Corresponding author: Christian Pérez Calvo, Residente de Medicina interna, Colombia.
Received: December 02, 2021; Published: December 16, 2021
DOI: 10.34297/AJBSR.2021.15.002076
Abstract
Stroke ranks fifth among the leading causes of death and is considered one of the main causes of morbidity and mortality. However, its incidence has been decreasing in the general population in recent decades. Nevertheless, in the young adult population (18-44 years) the incidence and global burden of stroke has increased due also to the increase of traditional risk factors in this population. Its etiology is varied and may differ from the most frequent causes in older adults (>65 years), in addition to the fact that in its clinical presentation there may be atypical manifestations, making its diagnosis delayed compared to older adults. Regarding its management, medical and surgical treatment is similar to that performed in the elderly, although the evidence shows better results in terms of treatment complications. However, the prognosis of stroke in the young adult is not as favorable as previously thought, as well as in mortality and physical/psychosocial consequences.
Keywords: Young Adult; Ischemic Stroke; Hemorrhagic Stroke; Embolic Stroke; Diagnostic, Management
Introduction
In the United States (USA), the prevalence of all types of cardiovascular disease is 10.6%, affecting more men than women [1]. Among these, cerebrovascular accident (CVA) is the fifth leading cause of death in the U.S.A., being considered one of the main causes of morbidity and mortality [1,2]. Stroke is defined as brain injury secondary to vascular injury, which can be of two types, ischemic or hemorrhagic [3]. Generally, stroke affects people older than 65 years of age more frequently than patients between 45 and 64 years. However, a decrease in the mortality rate has been observed in patients >65 years of age due to efforts to control risk factors such as arterial hypertension, dyslipidemia, and especially atrial fibrillation (AF), the latter with the use of anticoagulants [4]. Consequently, in recent years there has been evidence of an increase in the incidence of stroke in young adults (18 - 45 years), with numbers of 1 in 10 strokes [5,6]. In the U.S.A., the prevalence of stroke in young adults is around 10-15% in relation to the general population [7]. This increase in the rates of stroke in young people has been parallel to the increase in acute myocardial infarction (AMI) in this population, causing an increase in the number of years of useful life, creating disability and impacting productivity, societies, the economy and the sustainability of health systems [6]. It should be noted that the initial and current knowledge about stroke in young adults comes from data obtained in the population >65 years of age [2]. Despite increasing rates, little is known about the patterns of both ischemic and hemorrhagic stroke in young adults, as well as its clinical characteristics and epidemiological behavior. For this reason, the aim of this narrative review is to describe the current evidence on the prevalence, etiology, clinical presentation, among others, of stroke in young adults.
Epidemiology of Stroke in Young Adults
The increase in the rate of stroke in young adults has been evidenced in several studies. However, the rates of stroke in this population differ considerably throughout the world, mainly affecting developing countries [8]. This difference could be explained by the characteristics of each population, such as differences in access to health services, identification and timely mitigation of risk factors [8]. In 2012, the rate of stroke hospitalization in young adults in the U.S. between 2003 to 2004 and 2011 to 2012 increased considerably from 11.2 to 18.0 and from 3.8 to 5.8 per 10,000 hospitalizations/ year, respectively, in ages between 18 and 34 years. On the other hand, in the 35 to 44 years age group, it increased substantially in men (37.7 to 68.2 per 10,000 hospitalization-years) and in women by 44% (from 24.8 to 35.8 per 10,000 hospitalization-years) [9]. By 2013, a high global prevalence of stroke was estimated in young adults between the ages of 20 and 64 years, reaching 11 million people, with a mortality of 1.5 million, noting an increase between 1990 and 2013 of both ischemic and hemorrhagic strokes [5]. In 2015, an increase in the incidence of stroke in young people aged 20 to 44 years was reported, from 17 to 28 per 100,000 populationyears, with a higher incidence in men than in women (31 and 26 per 100,000 population-years, respectively). While in the 45 to 65 years age group the incidence was much higher (171 per 100,000 population-years: men 368 per 100,000 population-years and women 284 per 100,000 population-years) [10]. However, Leppert, et al. [10,11] found in their retrospective cohort study in the U.S. in a court of patients between 2001 and 2014 demonstrated a higher incidence in women than in men in the age range of 25 to 44 years, showing that there may be specific risk factors for women [11-13]. This provides us with useful information for the development of sex-specific strategies in the evaluation and prevention of stroke in young adults [14]. Furthermore, the incidence in European studies varies from 5 to 15 per 100,000 population and up to 40 per 100,000 population-years in African countries [8]. In the USA, the prevalence of stroke in young adults is 10-15% of all cardiovascular diseases [2].
Moreover, the reported frequency of stroke in young adults varies from 57-65% for ischemic stroke, 17-20% for intracerebral hemorrhage (ICH) and 16-22% for subarachnoid hemorrhage (SAH), all in patients between 20 and 44 years of age [2,15]. Simultaneously, variations in the incidence of stroke in young adults have been reported with respect to racial and ethnic conditions. In the USA, African American and Hispanic patients (both 11 per 100,000 population-years) have a higher incidence compared to white patients (7 per 100,000 population-years) [8]. The difference in incidence between African Americans and white people is greater between the third and fourth decade of life. However, in both groups there has been an increasing impact of stroke in young adults. Meanwhile, Hispanics have a longer hospital stay compared to African American and white patients; the latter even have less impact on hospitalization days, probably due to a lower frequency of medical complications such as pneumonia, deep vein thrombosis and urinary tract infection. [8]. Finally, African Americans have higher mortality than Hispanics and Caucasians, with whom they have lower early mortality rates [8]. An international, multicenter, retrospective and observational study conducted in North America, Europe and Asia that evaluated early mortality in 1134 young adults showed a higher 30-day mortality rate in African American patients (10.0%) compared to white people (6.0%) and Asian (1.9%) patients [16]. The epidemiological data on stroke in young adults change according to the study methodology and the characteristics of the patients, creating discordance in the results, making them not comparable, mainly from the point of view of age classification for the definition of stroke in young adults.
Cardiovascular Risk Factors
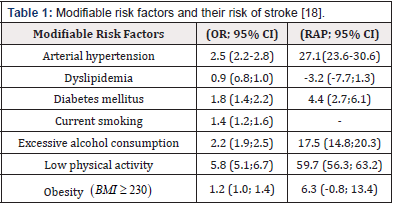
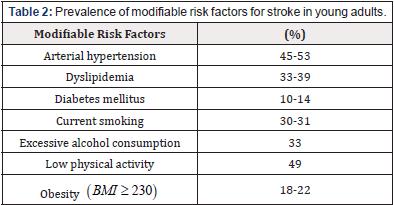
Many cardiovascular risk factors have been decreasing in the general population in recent years; however, their impact on young adults has shown a tendency to increase [8,17]. This leaves a higher risk of future vascular events in this population, with a markedly high prevalence in the population aged 35 years and older [8]. Despite this, few specific studies in the young adult population have evaluated the association between risk factors and the development of stroke [7]. A German case-control study including 2125 patients aged 18-55 years from the SIFAP1 and GEDA studies found that hypertension (HT), excessive alcohol consumption and low physical activity are the most associated risk factors for all strokes (Table 1) [18]. When evaluating the risk factors for each type of stroke, it was found that diabetes mellitus (DM), smoking and obesity are more associated with the development of ischemic stroke, while hypertension, excessive alcohol consumption and sedentary lifestyle are more associated with the development of hemorrhagic stroke [18]. In the USA, it was reported that the modifiable risk factors most associated with the development of hemorrhagic stroke between 1994 and 1999 were HT (OR 5.71; 95% CI 3.61- 9.05), DM (OR 2.40; 95% CI 1.15-5.01) and excessive alcohol consumption (OR 2.23; 95% CI 1.16-4.32) [19]. The prevalence of risk factors varies according to each study, with the most prevalent being HT, physical inactivity and dyslipidemia (Table 2) [13,18,20].
Arterial Hypertension: It is described that 45-53% of young adults who present with stroke are diagnosed with hypertension [8]. Southeast Asia has the highest rates of young adult HT as a risk factor with a population attributable risk (PAR) of 54.8% while Central and Eastern Europe as well as the Middle East had a lower AR (40.7%) [21]. Furthermore, HT is the risk factor that impacts the development of stroke, which is more associated with hemorrhagic stroke (AR of 55.8%: 95% CI: 44.7 - 67.0 and OR 11.1; 95% CI: 5.8 - 21.4) compared to ischemic stroke (AR of 25.5%: 95% CI: 22.1 - 28.2 and OR: 2.3; 95% CI: 2.0 - 2.6) [18,22].
Diabetes Mellitus: Diabetes mellitus is present in up to 14% of strokes in young patients [8]. This disease in young adults has a PAR of 4.8% (95% CI 2.9 to 6.7) and is associated with an increased risk of ischemic stroke (OR = 1.9; 95% CI 1.5 to 2.3) [18]. In addition, the prevalence of DM in young adults is increasing, with India, China, and the USA being the main countries with the highest prevalence [8]. The RAP for DM was highest in Southeast Asia (28.6%) and lowest in Western Europe, North America, and Australia (3.5%) [21]. In the USA, a higher incidence of DM is observed in African Americans and Hispanics compared with whites [23]. Although the prevalence of type 2 DM does not differ between sexes, it is associated with a higher risk of stroke in young adults in women (HR 2.8; 95% CI 2.4 to 3.4) than in men (HR 2.2; 95% CI 1.8 to 2.5) [24].
Dyslipidemia: Dyslipidemia is the second most prevalent factor in young adults, being more common in men than in women [8]. Furthermore, dyslipidemia is frequently found in patients with large or small vessel disease [9,20]. However, dyslipidemia is not associated with the development of ischemic stroke in adults as shown in Table 1 [18]. It is believed that dyslipidemia is not a risk factor for most causes of stroke. Another explanation for why it does not contribute significantly to the outcome of ischemic stroke in young adults is that most studies defined dyslipidemia as elevated low-density cholesterol (LDL) or decreased high-density cholesterol (HDL) and did not associate other lipid variables with stroke [8]. Fewstudies have evaluated the association between different lipid variables, Sabino et al. found that increased values of Apolipoprotein B (ApoB) and the ApoB/Apolipoprotein A-I (ApoA-I) ratio were associated with the development of ischemic stroke in young patients [25].
Smoking: Smoking is one of the most prevalent risk factors in the young adult population, increasing in the last decade reaching a high proportion of up to 50% in some studies [9,20,26]. Smoking is associated with the development of ischemic stroke in young adults mainly with a PAR of 19.9%; 95% CI 14.8 to 23.9 and OR 1.78; 95% CI 1.50 to 2.11 [22]. In addition, dose-response association between the number of cigarette days and the development of stroke in the young population have been found, those who smoked less than 11 cigarettes had an OR 1.46 (95%; CI 1.04 - 2.06) for the development of stroke, while those who smoked more than 40 cigarettes days obtained an OR of 5.66 (95%; CI 2.14-14.95) [27]. There are currently no worldwide records of smoking prevalence in young adults, however prevalence at all ages is higher in Europe (28.7%) and Southeast Asia (24.8%) compared to Africa (13.9%) [28]. On the other hand, daily cigarette consumption is higher in men (RAP 25%; 95% CI: 24.2 to 25.7) compared to women (RAP 5.4%; 95% CI: 5.1 to 5.7) and a higher prevalence of smoking has been found in white people than in blacks (30.6% vs. 18.5%) [8,28].
Obesity and Sedentary Lifestyle: Obesity and sedentary lifestyle are two interrelated risk factors that contribute to the development of vascular pathologies [8]. Obesity, understood as body mass index or BMI ≥30, is observed in up to 22-29.6% of young adults with stroke [18,29]. It is associated with increased risk of developing ischemic stroke in young adults (PAR 6.9%; 95% CI 0.0 to 13.8 and OR 1.2; 95% CI 1.5 to 2.3) [18]. Obesity and overweight, along with the increase in young adults with stroke, has been increasing in both developed and developing countries in recent years [30,31]. Currently there are no data on the worldwide prevalence of obesity in young adults with stroke, but in the general population the highest prevalence is found in the USA (61.1%), Europe (61.1%) and the USA (61.1%). (61.1%), Europe (54.8%) and the Eastern Mediterranean (46%) and the lowest prevalence in Africa (26.9%), Western Pacific (25.4%) and Southeast Asia (13.7%) [32].
Finally, obesity occurs more frequently in women than in men [8]. Other parameters used to assess vascular risk are waist circumference and waist-to-hip ratio, and it has even been found that these parameters may be more associated with the development of stroke than BMI (2.46, 95% CI 2.09 to 2.90 and 3.50, 95% CI 2.87 to 4.27 vs. 1.45, 95% CI 1.26 to 1.67) [33]. This is described in a multicenter cohort study conducted in Europe of patients between 18 and 55 years of age with stroke which found that abdominal obesity is more prevalent in women (73%) than in men (64%) and was even much more frequent in both sexes than general obesity [34]. In addition, the use of waist circumference helps to predict the risk of developing metabolic syndrome, which in turn is related to an increased risk of cardiovascular disease and DM2 [8]. Sedentary lifestyles and low physical activity are associated with obesity, thus increasing the risk of stroke in patients (PAR 59.8; 95% CI: 56.2 to 63.4 and OR 5.9; 95% CI: 5.1 to 6.7) [18].
Other
Frequent heavy alcohol consumption is associated with an increased risk of hemorrhagic stroke in young adults (PAR 17.3; 95% CI 14.2 to 20.5 and OR 2.2; 95% CI 1.9 to 2.5) [18]. This was observed in an international case-control INTERSTROKE study conducted in 32 countries in Asia, America, Europe, Australia, the Middle East, and Africa, which found that heavy alcohol consumption was associated with the development of both ischemic and hemorrhagic stroke (OR 2-09; 99% CI 1-64 to 2-67 and RAP 5.8; 99% CI 3.4 to 9.7) (OR 2.14; 99% CI 1.62 to 2-82 and OR 2.44; 99% CI 1.64 to 3.63) [35]. In addition, the association of stroke risk was greater in men than women, but it is worth noting that in this study only 11.8% of the population were ≤45 years old [35]. The use of illicit drugs or psychoactive substances is another risk factor for stroke in young adults that has been described and investigated in recent decades [36]. Cocaine and amphetamine have been the most related and have been notified in case reports and case series of young people with stroke who abused these drugs. Thus, in the USA, amphetamines were found to be more associated with the development of hemorrhagic stroke (OR 7.35; 95% CI 4.97 - 10.87), while cocaine was more associated with the development of ischemic stroke. In addition, amphetamine use was associated with death from both hemorrhagic and ischemic stroke (OR 2.63; 95% CI 1.07 - 6.50 and OR 6.35; 95% CI 0.56 - 72.20) [36]. Marihuana consumption has been associated with a significantly increased likelihood of stroke in young adults compared with those who do not use it [37,38].
Etiology
Ischemic Stroke
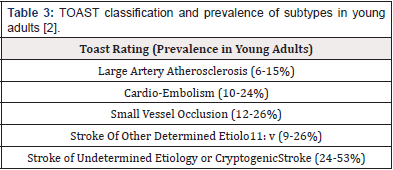
Approximately 20-25% of all ischemic strokes occur in young adults [39]. It is caused by diverse etiologies that vary according to age, sex and geographic location [2]. For this reason, causal classification systems for ischemic stroke have been developed to optimize choices and prognostic evaluation for patients [40]. Currently, the most widely used classification is according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) study which classifies ischemic stroke into 5 subtypes or categories: [1] large artery atherosclerosis (LAA), [2] small vessel occlusion (SVO), [3] cardio-embolism (CE), [4] stroke of other determined etiology, and 5) stroke of undetermined etiology (Table 3) [40,41]. However, despite improvements in the diagnosis and treatment of ischemic stroke in young adults, the main cause remains undetermined etiology (45%) [39,42,43]. It is believed that this is due to the TOAST system because it classifies a large proportion of patients with undetermined etiology although advances in diagnosis allow the identification of multiple mechanisms that may contribute to the development of ischemic stroke (the literature describes a proportion between 13.3% and 62.4%) [40,44]. To reduce this problem, the updated Stop Stroke Study TOAST (SSS-TOAST) system was developed that subdivides each TOAST category into evident, probable, and possible. Then, an automated SSSTOAST system called Causative Classification System (CCS) was developed to improve the accuracy of subtyping each type of ischemic stroke through a computerized algorithm [2]. In addition, ASCO (Atherosclerosis, Small vessel disease, Cardiac source, other cause) created another classification system for ischemic stroke in 2019 [45]. These latter classification systems have proven useful for the classification of young patients with ischemic stroke and have decreased the proportion of patients in the category of undetermined etiology. However, that proportion remains high [40]. LAA and SVO or small vessel disease are common causes of ischemic stroke in young adults after 35-40 years of age [2]. It is important to indicate that the increase of the mentioned risk factors in the young adult population generates an increase, as well as atherosclerosis as a cause of ischemic stroke compared to other causes [46]. LAA occurs mainly in the middle cerebral artery, internal carotid artery and vertebrobasilar artery [2]. One of the important causes of ischemic stroke in young people is CE which is commonly caused by patent foramen ovale (PFO), dilated cardiomyopathy and atrial fibrillation. For the diagnosis of this cause, electrocardiogram or EKG monitoring is necessary to identify the presence of Paroxysmal Atrial Fibrillation in the young adult population [2]. In addition, transesophageal and transthoracic echocardiography are important complementary tools for the diagnosis of cardioembolic stroke [2]. It should be noted that the presence of PFO is found in up to 45% of young patients with cryptogenic stroke [47]. PFO is estimated to be 15-25% prevalent in the general population and approximately 40-50% of young adults with cryptogenic stroke [48]. A retrospective study involving 215 patients aged 18-45 years in the period between 2005 and 2010 in the USA found that 47% of cases had cardioembolic stroke as etiology and of these 17% had asymptomatic PFO [49]. Ischemic stroke related to PFO is explained by three mechanisms: paradoxical embolism, thrombosis in situ in the foramen ovale, and arrhythmias related to PFO [50]. In addition, patients <45 years of age with no risk factors have a higher risk of CE in the presence of PFO [46]. Less common causes of CE are infective endocarditis, congenital cardiac malformation, mechanical aortic valve, left ventricular thrombus, hypokinetic left ventricular segment, akinetic left ventricular segment, atrial myxoma and nonbacterial thrombotic endocarditis [2].
Arterial dissection is another important cause of ischemic stroke in young adults [41]. Cervical artery dissection constitutes 10-25% of strokes in the young adult, thus being a common cause reaching up to 2% of stroke types [46]. However, dissection of the internal carotid artery is more frequent than the cervical artery [46]. Dissection of the arterial wall generates turbulent blood flow with the consequent generation of thrombus-emboli and thus causes ischemic stroke. This condition occurs spontaneously; however it can be associated with trauma and genetic and connective tissue diseases such as Ehlers-Danlos syndrome, Marfan syndrome and fibromuscular dysplasia [46]. Other conditions such as migraine, prothrombotic states due to hyperhomocysteinemia, use of oral contraceptive pills, alpha-1 anti-trypsin deficiency and neck manipulation are also associated with increased risk of dissection [2,46].
Hemorrhagic Stroke
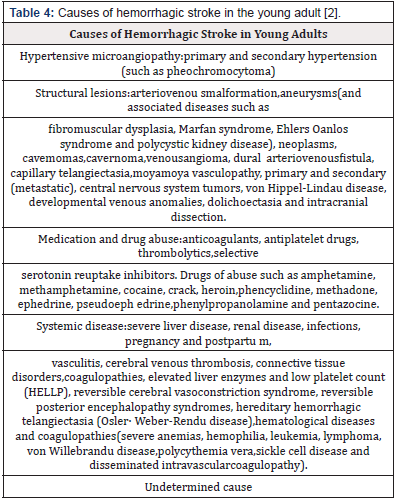
Hemorrhagic stroke accounts for about 10 to 27% of all strokes [15]. This type of stroke is divided into two groups: SAH and ICH (2). Although these two conditions are seen more frequently in the young population (40-50%) compared with the general population (15-20%), hemorrhagic stroke is less common than ischemic stroke [46]. Currently, there is only one classification system for hemorrhagic stroke established in 2012. This classification system is based on the causes of hemorrhagic stroke and contains six categories: structural causes, medication, amyloid angiopathy, systemic disease, hypertension, and undetermined etiology (SMASH-U). Although this classification system helps in predicting prognosis based on cause, SMASH-U was not validated for use in patients with hemorrhagic stroke [51]. Among the main causes of hemorrhagic stroke are cerebral aneurysms, cerebrovascular malformations, which mainly affect the population aged 20-29 years, and arterial hypertension, since this disease generates hypertensive microangiopathy as the patient gets older [2,52]. Few studies have evaluated the causes of hemorrhagic stroke in young adults; however, different causes are suggested and grouped according to the SMASH-U system (Table 4) [2,15].
Cryptogenic Stroke
This type of stroke is considered the most frequent etiological cause of stroke in young adults. However, its incidence varies considerably (25-70%) [39]. Cryptogenic or indeterminate stroke was born in 2014 when the clinical construct of embolic stroke of undetermined source (ESUS; Embolic Stroke of Undetermined Source) was established in order to identify those patients with no lacunar cryptogenic stroke in which embolism was the likely mechanism of stroke [53,54]. The criteria for establishing cryptogenic stroke as the cause of cerebral ischemia are: (a) establish ischemic stroke by CT or MRI that is not lacunar, (b) rule out the presence of extra- or intracranial atherosclerosis causing stenosis in at least 50% of the lumen of the arteries supplying the ischemic area, (c) cardioembolic cause is not likely, and (d) not identify another specific cause of stroke (For example, arteritis, dissection, migraine/vasospasm, and drug abuse) [55]. Although the origin of the embolism is undetermined, many studies have identified the presence of PFO in up to 58-69% of cases [56,57]. In turn, factors with high prevalence have been identified such as migraine (50%) and atrial septal aneurysm, the latter being associated with a higher risk of stroke and recurrences (OR 4.96; 95% CI, 2.37 - 10.39 and OR 23.93; 95% CI, 3.09 - 185.42) [57,58]. The diagnosis of FOT is mainly established with right heart catheterization with demonstration of the guidewire crossing the atrial septum. However, transesophageal echocardiography with bubble study is the most widely used and accepted noninvasive standard test for the diagnosis of PFO. In addition, it allows us to identify particularities of PFO such as the size of the shunt, anatomical characteristics and PFO differences from atrial septal defect or pulmonary shunt [59].
Other Causes
Hereditary and acquired thrombophilia can be a cause of cryptogenic stroke in the young adult population. Common causes include antithrombin III deficiency, prothrombin gene mutation, factor V Leiden mutation, protein C and S deficiency, antiphospholipid antibody syndrome, methylenetetrahydrofolate reductase (MTHFR) mutation, hyperhomocysteinemia [46]. Another cause would be inflammatory and non-inflammatory vasculopathy in which we find vasculitis caused by human immunodeficiency virus (HIV), varicella zoster virus (VZV), syphilis and among inflammatory vasculopathies such as Cogan syndrome, Susac syndrome, Sneddon syndrome or Eales disease are included in this category [46]. Other conditions included would be isolated primary vasculitis of the central nervous system and vasculopathies such as moyamoya syndrome or disease and reversible cerebral vasoconstriction syndrome. Infections play an important role in the pathogenesis of stroke due to the affectation generated in the cerebral parenchyma and meninges, in addition to the systemic inflammation that generates alterations in coagulation and endothelial dysfunction [46]. Finally, genetic causes, the use of illegal drugs and cancer are involved in the pathogenesis of stroke in young adults. Among the genetic causes, rare diseases are recognized as possible etiologies such as CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarct and leukoencephalopathy), CARASIL (cerebral autosomal recessive arteriopathy with subcortical infarct and leukoencephalopathy), Fabry disease, Marfan syndrome, MELAS (mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes), sickle cell anemia, and vascular Ehlers-Danlos syndrome [46]. However, a case-control study found no significant association in the development of stroke in young adults [60,61]. Regarding illicit drug use, it is estimated that 4.8% of the population aged 15-64 years use drugs worldwide, and the 18-year age group is considered the worst group among chronic users [62]. Cocaine and methamphetamine are the drugs most associated with the development of stroke, and it is believed that cocaine induces vascular complications due to cerebral vasospasm, arrhythmia, increased platelet activation and vasculitis, which can lead to hemorrhage [46]. On the other hand, the association between cancer and stroke is increasingly known. This was demonstrated by a cohort study on the development of cerebrovascular events in cancer survivors aged 12-39 years, which found that cancer survivors had a 40% higher risk of any cerebrovascular event [63]. This relationship may be due to secondary hypercoagulable states, direct tumor invasion, radiation vasculopathy or toxic chemotherapy effect [46].
Clinical Manifestations
In contrast to older adult patients, in the young adult population it has been described that they may come to the emergency department late after the onset of stroke, due to the fact that they present symptoms that are so variable and even mild in terms of neurological deficit or even extremely severe, which initially raises doubts about the diagnosis, in addition to their age [64,65]. Semiological variability also refers to the delay and fault in diagnosis, in addition to the fact that they may not present any risk factor [66]. There are situations that can mimic a stroke or the socalled “stroke mimics” such as convulsive crises, acute vestibular syndrome, migraine, infections, brain tumors, toxic-metabolic encephalopathy (for example, hypoglycemia), hypertensive encephalopathy, confusional syndrome and even states of altered consciousness [67]. For this reason, a high level of suspicion is important when a young adult patient presents with symptoms suggestive of stroke [64].
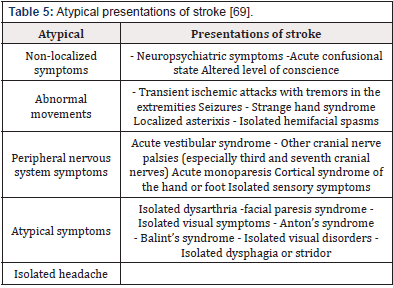
In the emergency department, the characterization of neurological symptoms is usually focal, which in most cases identifies the affected neurovascular area. In the case of occlusion of the anterior cerebral artery, middle cerebral artery and internal carotid artery, symptoms may manifest with variable contralateral hemiparesis, contralateral hemisensory loss, language disturbances and aphasia (when the dominant hemisphere is affected) [64]. On the other hand, syndromes related to the posterior cerebral circulation manifest themselves in a variable ways, from visual acuity impairment to headache, neck or jaw pain, nausea and incoercible emesis, ataxia, alteration in cranial pairs and in the state of consciousness due to involvement of the encephalic stalk [64,68]. Atypical presentations of stroke in young adults are frequent, resulting in a challenging etiologic diagnosis based on their semiology (Table 5) [69]. Neuropsychiatric symptoms, acute confusional states, and decreased alertness may be unique features of stroke presentation [66]. Finally, the association between migraine and stroke has been described mainly in women >45, smokers or users of hormonal contraceptives [70]. Patients with migraine may present with prolonged focal symptoms, mainly visual and language disturbances, which may lead to confusion and difficult diagnosis of stroke in these patients [66]. In young patients with hemorrhagic stroke, mainly ICH, the clinical presentation generally reflects the underlying cause, location and even the volume of the hemorrhage, which could be directly proportional to the neurological involvement in a similar way to the ischemic cerebral event [71]. However, the younger the age of the patient, the less severe the neurological symptoms. This may be due to the fact that most of the causes are structural and this allows greater viability of cerebrovascular compensatory mechanisms in young adults [71].
The etiological approach to these patients should be based on clinical manifestations as well as risk factors; manifestations such as acute or subacute headache of severe intensity with or without persistence or meeting migraine criteria with aura and motor symptoms located in the anatomical region where the hemorrhage occurred is feasible [15]. Risk factors to consider are drug abuse, sickle cell anemia, history of hemorrhage at the same site of presentation, family history of hemorrhagic stroke, hemophilia, or use of oral anticoagulants. All of these features are important in young adults because unusual causes of hemorrhagic stroke in the elderly are much more common in this population [15]. In the case of SAH, the typical presentation consists of severe, suddenonset headache (expressed as “the worst headache ever”, which occurs in up to 80% of cases) [72], associated with nausea, emesis, photophobia, neck pain, and loss of consciousness. The latter are due to increased intracranial pressure (ICP) which in severe cases will lead to coma or brain death [73]. In addition, a 40% prevalence of Terson’s syndrome (intraocular hemorrhage secondary to an acute increase in ICP) has been found in patients with SAH [74]. Regarding atypical presentations of SAH we found that some patients may present with seizures, acute encephalopathy and the presence of subdural hematoma making the diagnosis of SAH difficult. A small group of patients may present isolated headache days before aneurysm rupture, believed to be due to small leaks in the aneurysm [73].
Diagnosis
The etiologic diagnosis of stroke is mainly important to determine the clinical and functional prognosis and to initiate secondary prevention, thus decreasing the risk of recurrence [75]. However, the lack of diagnostic algorithms and the wide range of etiologies in young adult patients with stroke, these should be thoroughly evaluated by using different tests usual for any stroke patient and specialized for the most frequent causes in the young adult patient [66]. Strategies focus on the evaluation of common etiologic causes. Once the patient has been evaluated within the first few minutes of admission to the ED and the airway and circulation have been stabilized, the cause of the stroke should be identified immediately; the cause of the acute cerebral event must be identified immediately, and for this, the neurological assessment is started with the NIHSS scale (National Institutes of Healt Stroke Scale), the performance of a CT scan of the brain without contrast and the evaluation of risk factors; thanks to its availability and the speed of the study, it helps us to identify such complex cerebral events as ischemic or hemorrhagic stroke [3,66]. Most stroke protocols are classified with CT, however, complementary studies are implemented in multiple specialized centers and units dedicated to the care of the acute neurological patient are performed with angiography by cerebral tomography and neck vessels, simple cerebral magnetic resonance imaging (MRI), magnetic resonance angiography of the brain and neck vessels [66]. In general, MR images illustrate in a detailed way the ischemic brain areas by means of sequences such as diffusion weights (RMD), its complement with the separate diffusion coefficient (ADC) and the sequence in attenuated T2 or FLAIR, thus being able to determine in most cases a classification regarding the type of cerebrovascular event such as complex stroke etiologies like MELAS (mitochondrial encephalopathy, lactic acidosis and stroke) which in MRI manifests with multifocal infarcts that do not respect the vascular territories, potentially with cortical involvement and preserving the deepest structures of the white matter [66]. Structural evaluation of the heart is then performed. Evaluation of this organ to establish etiology can be performed using EKG and echocardiogram. However, unlike older adults whose main cause of ischemic stroke is cardioembolic due to atrial fibrillation, in young adults arrhythmias are infrequent (66). However, due to the availability and rapidity of EKG, it is used as a first line test to detect arrhythmias such as atrial fibrillation, atrial flutter and sick sinus syndrome [66]. However, the test indicated in young adults and which has demonstrated a diagnostic yield of up to 51% in this population is the transthoracic echocardiogram which allows us to identify multiple cardioembolic etiologies such as thrombi in the left atrium or left ventricle, cardiac myxomas, infectious and non-bacterial endocarditis, rheumatic heart disease, degenerative valvular disease, cardiomyopathy and PFO [49,66]. Of these, cardiomyopathies are an important risk factor for ischemic stroke in young adults and children [76].
Furthermore, the identification of PFO can be performed using transthoracic echocardiography if the images are of good quality. However, the Gold standard for the diagnosis of PFO is the transesophageal echocardiogram because it allows establishing the morphology and size, as well as determining the presence associated with an aneurysm of the atrial septum [50,77]. It is also important to evaluate the vasculature of the head and neck because the second most common cause of stroke in young adults is cervico-cerebral arterial dissection (10 25%) [66]. The arteries most affected are the vertebral arteries and most of the dissection occurs spontaneously [78]. On this occasion, the use of Doppler is of limited value for the evolution of stroke in young adults. While the use of CT offers us greater diagnostic possibilities [66]. In addition, the evolution of the cervicocerebral vasculature allows us to evaluate various arthropathies that predispose to the development of stroke [66]. Among these we find fibromuscular dysplasia which generates a non-inflammatory arteriopathy that commonly affects women and predisposes to stroke, mainly affecting renal and cerebral arteries [79]. Angiography is a useful tool which would allow us to observe the pathognomonic “chain of beads” pattern. Another non-inflammatory arteriopathy is Moyamoya disease which mainly affects children and causes 6-15% of nonatherosclerotic vasculopathies [80]. Angiography demonstrates the presence of the well-known smoke puff appearance resulting from bilateral stenosis or occlusion of the intracranial carotid artery with associated dilatation of the lenticulostriated arteries [66]. If the presence of hemorrhage in the young patient is confirmed by non-contrast CT. The next step is the use of further imaging studies in order to determine the etiology or underlying mechanism of the hemorrhagic stroke [15]. The choice of imaging study will depend on the anatomic location of the hemorrhage and any symptoms that indicate the possible cause. In the case of young adult patients presenting with lobar hemorrhage and those without a history of hypertension or coagulopathy are more likely to present a vascular cause such as arteriovenous alterations [15,81]. Sometimes the CT scan used to identify hemorrhagic stroke can be used to establish the cause or rely on CT angiography. Alternatively, MRI or MR angiography can help us to more accurately identify abnormalities and even other MR sequences can be used. In addition, contrastenhanced images should be obtained [15]. CT and MRI are tools that offer good sensitivity (≈95%) for the identification of arteriovenous malformations compared to the gold standard, angiography [82]. Another important sign is the Spot sign which consists of contrast extravasation in the image suggesting the presence of active hemorrhage with consequent expansion of the hematoma and is a predictor of in-hospital mortality in patients [83].
Laboratory Tests
As for laboratory tests, routine tests should be considered such as complete blood count (to detect anemia, infection, thrombocytopenia, and thrombocytosis), blood electrolytes, renal function tests (uremic acid and creatinine) and liver function tests (ALT, AST, total and direct bilirubin and LDH), measurement of C-reactive protein, troponins, evaluation of coagulation time (to determine coagulation disorders or effects of anticoagulant treatment) and glycemia [3,15]. The latter is important because states of hyperglycemia or hypoglycemia may simulate a stroke, which is important to clarify before initiating specific treatment [84]. In addition, toxicological tests can be performed in case of suspected drug abuse [15].
Management Of Stroke in the Young Adult: Current Evidence
The therapeutic approach is the same as in the older adult; however, it may vary according to etiology and risk factors.
Ischemic Stroke and Thrombolysis
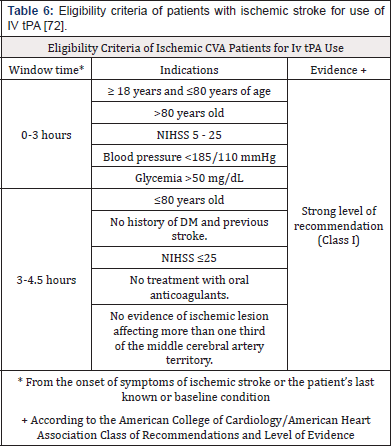
Initial management consists of stabilization of vital signs, use of supplemental oxygen to maintain O2 saturation above 94%, ventilatory support in patients with ischemic stroke and decreased consciousness and/or loss of airway protection reflexes [84]. Maintain adequate tissue perfusion, sustaining lower blood pressure (BP) levels <185 mmHg systolic and <110 mmHg diastolic before starting intravenous thrombolytic therapy [84], adequate temperature and metabolic control, especially optimal blood glucose levels [84-86]. The specific treatment for ischemic stroke consists of intravenous infusion of Alteplase or tissue plasminogen activator, a drug approved in 1996 by the FDA in the USA [87]. The greatest benefit with respect to risks of IV tPA is time-dependent, for this reason it should be indicated as soon as possible in those patients diagnosed with ischemic stroke and who meet the eligibility criteria (Table 6). The dose of IV tPA is 0.9 mg/ kg dose, maximum dose of 90 mg over 60 minutes with the initial 10% of the dose administered as a bolus over 1 minute [84]. The American Heart Association/American Stroke Association in 2019 recommends IV tPA is for those patients who can be treated within 3 hours of the onset of ischemic stroke symptoms. In addition, there is safety with the use of IV tPA between 3 to 4.5 hours following the onset of ischemic stroke symptoms, NIHSS between 5 and 25 points, no history of diabetes and stroke price [84]. Furthermore, with the use of IV tPA between 3 to 4.5 hours is safe and may be as effective in young patients [84]. Although young adults are less likely to have any contraindications to IV tPA and may be treated more frequently than young adults. Dodds, et al. [88] found from a cohort of 173,946 ischemic stroke patients between 2009 and 2015 in the U.S. that young adults are more likely to have delays in the evaluation and treatment of ischemic stroke compared to the older adult. Which young patients were less likely to have a brain imaging study within 25 (62.5%) and 60 (37.0%) minutes of arrival to the emergency department compared with older adult (71.5% and 42.8%) [88].
However, young adult patients had a lower proportion of IV tPA complications such as ICH 8 (1.7% vs. 4.5%) and systemic hemorrhage (0.3% vs. 1.0%). In addition, the proportion of inhospital mortality in young adults was lower (2.0% vs. 4.3%) [88]. Likewise, a study conducted in China in 2017 compared the safety and efficacy of IV tPA in young adults with ischemic stroke, found that the incidence of cerebral hemorrhage and mortality was low for young adults compared with older patients, but there was no statistically significant difference between the two groups [89]. Similarly, Toni et al. in 2012 analyzed the Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR) study database which obtained a total of 3,246 (11.7%) of patients aged 18-50 years; finding that in young patients bleeding occurred in 0.6% vs 1.9% of patients aged 51-80 (OR 0.53; 95% CI 0.31-0.90, p = 0.02). In addition, 3-month mortality of young patients was 4.9% and 14.4%, respectively (OR 0.49; 95% CI 0.40-0.60, p = 0.001) and functional independence was 72.1% vs. 54.5%, respectively (OR 1.61; 95%; CI 1.43-1.80, p = 0.0001) [90]. All these studies demonstrate the safety of IV tPA use in young adult patients with ischemic stroke. However, these patients tend to present delays in the use of diagnostic imaging due to the high percentage of atypical presentations as well as in the initiation of treatment.
Hemorrhagic stroke, Medical and Surgical Management
Medical management in hemorrhagic stroke patients is similar to that performed in the older adult. However, treatment trials in stroke populations with spontaneous ICH have included few young individuals, with the result that these trials may not be generalized to the young adult population [15]. Initial management consists of supportive care and aggressive therapy, e.g., treatment for increased ICP and BP [91]. The latter is common in acute ICH and is associated with further hematoma expansion, neurological deterioration, and death. For this reason, the American Heart Association/American Stroke Association recommends intensive BP reduction in patients with systolic BP between 150 and 220 mmHg until values below 140 mmHg are reached, and that this rapid reduction is safe [91]. However, the different clinical studies have been controversial. The Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage 2 (INTERACT2) study evaluated the efficacy of intensive BP reduction (systolic <140 mmHg) in 2839 patients with SBP between 150- and 220-mm Hg within 6 hours after the event; the results demonstrated a small benefit in patients who received intensive therapy. In addition, finding significantly better functional recovery (OR 0.87; 95% CI, 0.77 to 1.00; P = 0.04) and better physical and mental health-related quality of life from intensive treatment [92,93]. While the second Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH-II) trial that evaluated intensive therapy in 1000 with hypertensive ICH found that the results demonstrated no benefit in the intensive therapy patients (systolic BP 110-139 mmHg) compared with the standard therapy group (systolic BP 140-179 mmHg) [85]. However, Moullaali et al. performed an analysis of the patient acquired INTERACT2 and ATACH-II, which indicate that achieving an early and stable systolic BP appears to be safe and associated with favorable outcomes in patients with acute ICH of predominantly mild to moderate severity [94]. In the management of increased ICP, osmotic agents and hyperventilation are actions that can be used temporarily in patients awaiting definitive treatment, either surgical evacuation of a hematoma or the use of a ventricular drainage. Corticosteroids in these patients are ineffective and their use is not recommended [15,91]. As for the presence of seizures, those should be treated with drugs mainly those seizures that occur early since they represent cortical involvement by ICH. For this reason, patients with clinical or electroencephalographic seizures should be treated [91].
Regarding surgical management, there are no European or American guidelines on the surgical management of ICH in young adults [84,95]. Two randomized studies that evaluated the role of surgical evacuation of the hematoma in patients with ICH showed no benefit of this intervention [96,97]. In the subgroup analysis comparing patients >65 years vs patients of this age or older, no clear benefit of surgery was observed in either age group. It should be clarified that the number of young patients included was small [96,97]. However, despite the lack of evidence from randomized trials, young patients with ICH, mainly those with large hematomas or impaired consciousness, are usually treated surgically [15]. The recommendations regarding the management of ruptured aneurysms, the main cause of hemorrhage in young adults, both American and European guidelines agree that clip or microvascular intervention should be performed as early as possible, and the patient should be evaluated by a multidisciplinary group for decision-making [72,95]. However, between clipping or coiling of the aneurysm, the latter is mostly recommended, depending on factors such as age, comorbidity, presence of ICH, degree of SAH, aneurysm size, location and configuration, as well as the state of the collaterals [72,95,98].
For patients with SAH the initial management is similar to ICH, which consists of securing the airway, stabilizing breathing and circulation. In case of coma, hydrocephalus, seizures or the need for sedation, the recommendation is to perform endotracheal intubation [73]. Then, we proceed with medical management which is aimed at preventing rebleeding once the aneurysm has been controlled, because this occurs mainly within the first 2 to 12 hours and with an occurrence rate between 4% and 13.6% within the first 24 hours [72,73]. Risk factors for rebleeding are a delay in the initiation of treatment for aneurysm, worse neurological status on admission, initial loss of consciousness, previous sentinel headaches, larger aneurysm and systolic blood pressure >160 mmHg [72]. For the latter, there are no data from controlled clinical trials and different organizations differ; the American Heart Association/ American Stroke Association and the Neurocritical Care Society recommend systolic BP values below 160 mmHg to maintain adequate perfusion, whereas the European Stroke Organization recommends maintaining systolic BP<180mmHm [72,95,98]. It is recommended to maintain mean arterial pressure between 90 and 110 mmHg (95,98). For BP control IV administration of Labetalol (5mg to 20mg), Hydralazine (5mg to 20mg), Nicardipine (5mg/h to 15mg/h) are the drugs of choice [72]. Other aspects should be evaluated and treated such as: glucose control (hyperglycemia should be treated when it is above 180 mg/dL), temperature (fever is an indicator of poor outcome, therefore it should be treated with physical means and drugs), treat seizures (these occur in 7% of patients and should be treated, there is no evidence in favor of prophylaxis with drugs) [72,95,99].
Cryptogenic stroke, Medical Management vs Surgical PFO Closure
Many randomized trials have compared the safety in terms of prevention of subsequent events between PFO closure and medical management with anticoagulants. The CLOSE (Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence) study was a randomized, multicenter, open-label trial involving 663 patients aged 16 to 60 years who had recent strokes attributed to PFO [100]. With a follow-up of 5.3 ± 2 years, percutaneous PFO closure had the lowest rate of recurrent stroke compared with medical therapy (0% vs. 6.0%; HR: 0.03; 95% CI: 0 to 0.26; p<0.001). PFO closure was successful in 93% of patients and only 5.9% had procedural complications [100]. Likewise, the Gore REDUCIR study (Gore Helex Septal Occluder/Gore Cardioform Septal Occluder and Antiplatelet Medical Management for Reduction of Recurrent Stroke or Imaging-Confirmed TIA in Patients with Patent Foramen Ovale) was a randomized, multicenter, open-label, multicenter study that included patients with patent foramen ovale, multicenter, open-label study that included 664 patients, aged 18 to 59 years, with recent cryptogenic stroke who had a PFO documented by transesophageal echocardiogram bubble study [101]. Patients were randomized in a 2:1 ratio to undergo percutaneous PFO closure with Gore Helex or Cardioform septal occluders plus antiplatelet therapy or antiplatelet therapy alone. With a 3.2-year follow-up, recurrent stroke occurred at a lower rate in those patients with percutaneous PFO closure vs. the medical treatment group (1.4% vs. 5.4%; HR: 0.23; 95% CI: 0.09 to 0.62; p = 0.002) [101].Finally, a meta-analysis evaluating 5 randomized clinical trials with a total of 3440 patients concluded that percutaneous PFO closure reduces the risk of recurrent stroke compared to medical therapy (2.0% vs. 4.5%; RR: 0.42; 95% CI: 0.20 0.91; p = 0.027). However, an increased risk of atrial fibrillation was found in patients whose PFO was closed with devices (4.0% vs. 0.7%; RR: 4.55; 95% CI: 2.16 to 9.60; p <0.01) [102].
Prognosis
The prognosis of stroke in young adults is not as favorable as previously thought, as well as in mortality and physical/ psychosocial consequences [103]. Although overall stroke mortality has declined in the general population in recent years, this decline has been slow in young adults compared with older adults [5]. This was demonstrated by a population-based study evaluated cumulative all-cause mortality in 30-day survivors at the end of follow-up, stratified by age, sex, and stroke subtype, and compared with cumulative all-cause mortality in the general population in 15 257 young adults [104]. At the end of follow-up a total of 3540 patients (23.2%) died, and in 30-day survivors, cumulative mortality after any stroke increased from 3.1% (95% CI, 2.8% -3.4%) after 1 year to 7.0% (95% CI, 6.6% -7.4%) at 5 years, 11.5% (95% CI, 11.0% -12.1%) at 10 years, and 17.0% (95% CI, 16.2% -17.9%) after 15 years [104]. Regarding the annual risk of death by sex and stroke subtype. After ischemic stroke, the risk of death after 1 year was 2.0% (95% CI, 1.8% -2.3%) for men and 1.6% (95% CI, 1.4% -1.8%) for women. Whereas for ICH, the annual risk of death was 4.8% (95% CI, 4.4% -5.1%) for men and 3.9% (95% CI, 3.6% -4.3%) for women after 1 year [104]. Finally, long-term mortality in 30-day stroke survivors compared with the general population was 5.6 (95% CI, 5.3-5.9). The observed mortality rate was 13.3 per 1000 person-years (95% CI, 12.6-14.0) versus an expected rate of 2.4 per 1000 person-years [104].
Prevention2
Worldwide, 90% of the burden of stroke is attributable to modifiable risk factors. For this reason, it has been proposed that control of modifiable risk factors and lifestyle improvements could prevent about three-quarters of the global stroke burden [105]. Furthermore, with the increasing increase in traditional cardiovascular risk factors in young adults and the rise in AMI, preventive measures in young adults are becoming increasingly important [2]. Among these strategies are healthy lifestyle changes, such as smoking cessation, body weight control, regular physical activity, and a healthy diet, which reduce stroke morbidity and mortality by mitigating the development of key risk factors such as HT, DM, dyslipidemia, obesity, and exposure to tobacco products [106].
Conclusion
Despite the decrease in the proportion of stroke in the general population, stroke in young adults has gained importance in recent years due to the increase in cardiovascular risk factors in the population between 18 and 44 years of age. This has led to the search for epidemiological, diagnostic and etiological characteristics in order to identify the causes that most affect this population and thus establish guidelines for diagnosis and treatment. However, the major associations do not include sufficient recommendations on the approach to this population due to the limited inclusion of young adult stroke patients in clinical trials. In short, international guidelines need to be updated and more studies of young adult stroke patients need to be included or, failing that, more studies need to be conducted.
References
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, et al. (2020) Heart disease and stroke statisticsupdate-2020 Update: A report from the American Heart Association. Circulation 141(9): 139-596.
- Yahya T, Jilani MH, Khan SU, Mszar R, Hassan SZ, et al. (2020) Stroke in young adults: Current trends, opportunities for prevention and pathways forward. Am J Prev Cardiol 3: 100085.
- Stack CA, Cole JW (2017) A Diagnostic Approach to Stroke in Young Adults. Curr Treat Options Cardiovasc Med 19(11): 84.
- Goeggel Simonetti B, Mono ML, Huynh Do U, Michel P, Odier C, et al. (2015) Risk factors, aetiology and outcome of ischaemic stroke in young adults: the Swiss Young Stroke Study (SYSS). J Neurol 262(9): 2025-2032.
- Krishnamurthi RV, Moran AE, Feigin VL, Barker Collo S, Norrving B, et al. (2015) Stroke Prevalence, Mortality and Disability-Adjusted Life Years in Adults Aged 20-64 Years in 1990-2013: Data from the Global Burden of Disease 2013 Study. Neuroepidemiology 45(3): 190-202.
- Ekker MS, Boot EM, Singhal AB, Tan KS, Debette S, et al. (2018) Epidemiology, aetiology and management of ischaemic stroke in young adults. Lancet Neurol 17(9): 790–801.
- Ekker MS, Verhoeven JI, Vaartjes I, van Nieuwenhuizen KM, Klijn CJM, et al. (2019) Stroke incidence in young adults according to age, subtype, sex and time trends. Neurology 92(21): 2444-2454.
- Boot E, Ekker MS, Putaala J, Kittner S, De Leeuw F E, et al. (2020) Ischaemic stroke in young adults: a global perspective. J Neurol Neurosurg Psychiatry 91(4): 411-417.
- George MG, Tong X, Bowman BA (2017) Prevalence of Cardiovascular Risk Factors and Strokes in Younger Adults. JAMA Neurol 74(6): 695-703.
- Madsen Tracy E, Khoury Jane C, Leppert Michelle, Alwell Kathleen, Moomaw Charles J, et al. (2020) Temporal Trends in Stroke Incidence Over Time by Sex and Age in the GCNKSS. Stroke 51(4): 1070-1076.
- Leppert Michelle H, Ho P Michael, Burke James, Madsen Tracy E, Kleindorfer Dawn, et al. (2020) Young Women Had More Strokes Than Young Men in a Large, United States Claims Sample. Stroke 51(11): 3352-3355.
- Towfighi A, Skolarus LE (2020) Inequities in Stroke Preparedness in Young Adults: What do we Know and Where Should we Go? Stroke 51(12): 3479-3481.
- Lasek Bal A, Kopyta I, Warsz Wianecka A, Puz P, Łabuz Roszak B, et al. (2018) Risk factor profile in patients with stroke at a young age: Stroke risk factors in young patients. Neurol Res 40(7): 595-601.
- Tang M, Yao M, Zhu Y, Peng B, Zhou L, et al. (2020) Sex differences of ischemic stroke in young adults-A single-center Chinese cohort study. J Stroke Cerebrovasc Dis 29(9): 105087.
- Tatlisumak T, Cucchiara B, Kuroda S, Kasner SE, Putaala J (2018) Nontraumatic intracerebral haemorrhage in young adults. Nat Rev Neurol 14(4): 237-250.
- Tsivgoulis G, Putaala J, Sharma VK, Balucani C, Martin Schild S, et al. (2014) Racial disparities in early mortality in 1,134 young patients with acute stroke. Neurol Sci 35(7): 1041-1049.
- Béjot Yannick, Delpont Benoit, Giroud Maurice (2016) Rising Stroke Incidence in Young Adults: More Epidemiological Evidence, More Questions to Be Answered. J Am Heart Assoc 5(5): 003661.
- Aigner Annette, Grittner Ulrike, Rolfs Arndt, Norrving Bo, Siegerink Bob (2017) Contribution of Established Stroke Risk Factors to the Burden of Stroke in Young Adults. Stroke 48(7): 1744-1751.
- Feldmann Edward, Broderick Joseph P, Kernan Walter N, Viscoli Catherine M, Brass Lawrence M, et al. (2005) Major Risk Factors for Intracerebral Hemorrhage in the Young Are Modifiable. Stroke 36(9): 1881-1885.
- Hauer Allard J, Ruigrok Ynte M, Algra Ale, van Dijk Ewoud J, Koudstaal Peter J, et al. (2017) Age‐Specific Vascular Risk Factor Profiles According to Stroke Subtype. J Am Heart Assoc 6(5): 005090.
- Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, et al. (2016) Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol 15(9): 913-924.
- Kivioja Reetta, Pietilä Arto, Martinez Majander Nicolas, Gordin Daniel, Havulinna Aki S, et al. (2018) Risk Factors for Early‐Onset Ischemic Stroke: A Case‐Control Study. J Am Heart Assoc 7(21): 009774.
- Bhupathiraju Shilpa N, Hu Frank B (2016) Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ Res 118(11): 1723-1735.
- Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ (2012) Diabetes, hyperglycaemia and acute ischaemic stroke. Lancet Neurol 11(3): 261-271.
- Adriano Paula Sabino, Marinez De Oliveira Sousa, Luciana Moreira Lima, Daniel Dias Ribeiro, Maria Das Graças (2008) ApoB/ApoA-I ratio in young patients with ischemic cerebral stroke or peripheral arterial disease. Transl Res 152(3): 113-118.
- Van Alebeek ME, Arntz RM, Ekker MS, Synhaeve NE, Maaijwee NA, et al. (2018) Risk factors and mechanisms of stroke in young adults: The FUTURE study. J Cereb Blood Flow Metab 38(9): 1631-1641.
- Markidan J, Cole JW, Cronin CA, Merino JG, Phipps MS, et al. (2018) Smoking and Risk of Ischemic Stroke in Young Men. Stroke 49(5): 1276-1278.
- Reitsma MB, Fullman N, Ng M, Salama JS, Abajobir A (2017) Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. The Lancet 389(10082): 1885-1906.
- Darke S, Duflou J, Kaye S, Farrell M, Lappin J (2019) Body mass index and fatal stroke in young adults: A national study. J Forensic Leg Med 63: 1-6.
- Gjærde LK, Gamborg M, Ängquist L, Truelsen TC, Sørensen TIA, et al. (2017) Association of Childhood Body Mass Index and Change in Body Mass Index With First Adult Ischemic Stroke. JAMA Neurol 74(11): 1312-1318.
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. (2014) Global, regional and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384(9945): 766-781.
- Yatsuya H, Li Y, Hilawe EH, Ota A, Wang C, et al. (2014) Global Trend in Overweight and Obesity and its Association With Cardiovascular Disease Incidence. Circ J 78(12): 2807-2018.
- Winter Yaroslav, Rohrmann Sabine, Linseisen Jakob, Lanczik Oliver, Ringleb Peter A, et al. (2008) Contribution of Obesity and Abdominal Fat Mass to Risk of Stroke and Transient Ischemic Attacks. Stroke 39(12): 3145-3151.
- Von Sarnowski Bettina, Putaala Jukka, Grittner Ulrike, Gaertner Beate, Schminke Ulf, et al. (2013) Lifestyle Risk Factors for Ischemic Stroke and Transient Ischemic Attack in Young Adults in the Stroke in Young Fabry Patients Study. Stroke 44(1): 119-125.
- O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, et al. (2016) Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 388(10046): 761-775.
- Westover AN, McBride S, Haley RW (2007) Stroke in Young Adults Who Abuse Amphetamines or Cocaine: A Population-Based Study of Hospitalized Patients. Arch Gen Psychiatry 64(4): 495.
- Parekh T, Pemmasani S, Desai R (2020) Marijuana Use Among Young Adults (18-44 Years of Age) and Risk of Stroke: A Behavioral Risk Factor Surveillance System Survey Analysis. Stroke 51(1): 308-310.
- Wolff V, Jouanjus E (2017) Strokes are possible complications of cannabinoids use. Epilepsy Behav 70: 355–363.
- Divišová P, Šaňák D, Král M, Bártková A, Hutyra M, et al. (2020) Young cryptogenic ischemic stroke: A descriptive analysis of clinical and laboratory characteristics, outcomes and stroke recurrence. J Stroke Cerebrovasc Dis 29(9):105046.
- Gökçal E, Niftaliyev E, Asil T (2017) Etiological classification of ischemic stroke in young patients: a comparative study of TOAST, CCS and ASCO. Acta Neurol Belg 117(3): 643-648.
- Chung Jong Won, Park Su Hyun, Kim Nayoung, Kim Wook Joo, Park Jung Hyun, et al. (2014) Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Classification and Vascular Territory of Ischemic Stroke Lesions Diagnosed by Diffusion‐Weighted Imaging. J Am Heart Assoc 3(4): 001119.
- Huggins HE, Brady M, Emma J P, Thaler DE, Leung LY (2020) Differences in presenting symptoms of acute stroke among young and older adults. J Stroke Cerebrovasc Dis 29(8): 104871.
- Shaban A, Molian V, Garg A, Limaye K, Leira EC, (2020) Secular Trends for Etiologies of Acute Ischemic Stroke in Young Adults. J Stroke Cerebrovasc Dis 29(12): 105270.
- Martín F, Tarducci ME, Tabares SM, Martín JJ, Sembaj A (2019) Application of the TOAST and CCS systems in the diagnosis of ischemic stroke. Neurol Neurocir Psiquiatr 47(1): 22-28.
- Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG (2009) New Approach to Stroke Subtyping: The A-S-C-O (Phenotypic) Classification of Stroke. Cerebrovasc Dis 27(5): 502-508.
- Hathidara MY, Saini V, Malik AM (2019) Stroke in the Young: A Global Update. Curr Neurol Neurosci Rep 19(11): 91.
- Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A (2007) Patent Foramen Ovale and Cryptogenic Stroke in Older Patients. N Engl J Med 357(22): 2262-2268.
- Favilla CG, Messé SR (2020) Patent foramen ovale and stroke: current evidence and treatment options. Curr Opin Neurol 33(1): 10-16.
- Ji R, Schwamm LH, Pervez MA, Singhal AB (2013) Ischemic Stroke and Transient Ischemic Attack in Young Adults: Risk Factors, Diagnostic Yield, Neuroimaging and Thrombolysis. JAMA Neurol 70(1): 51-57.
- Miranda B, Fonseca AC, Ferro JM (2018) Patent foramen ovale and stroke. J Neurol 265(8): 1943-1949.
- Meretoja Atte, Strbian Daniel, Putaala Jukka, Curtze Sami, Haapaniemi Elena, et al. (2012) Smash-u. Stroke 43(10): 2592-2597.
- W L, Q S, X D, F Y, Y Z, et al. (2018) Etiologies and risk factors for young people with intracerebral hemorrhage. Zhong Nan Da Xue Xue Bao Yi Xue Ban 43(11): 1246-1250.
- Hart RG, Diener H C, Coutts SB, Easton JD, Granger CB, et al. (2014) Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 13(4): 429-438.
- Calvet D (2016) Infarctus cérébral du sujet jeune. Rev Médecine Interne 37(1): 19-24.
- Hart Robert G, Catanese Luciana, Perera Kanjana S, Ntaios George, Connolly Stuart J (2017) Embolic Stroke of Undetermined Source. Stroke 48(4): 867-872.
- Ueno Yuji, Yamashiro Kazuo, Tanaka Ryota, Kuroki Takuma, Hira Kenichiro, et al. (2016) Emerging Risk Factors for Recurrent Vascular Events in Patients With Embolic Stroke of Undetermined Source. Stroke 47(11): 2714-2721.
- West Brian H, Noureddin Nabil, Mamzhi Yakov, Low Christopher G, Coluzzi Alexandra C, et al. (2018) Frequency of Patent Foramen Ovale and Migraine in Patients with Cryptogenic Stroke. Stroke 49(5): 1123-1128.
- Mojadidi MK, O Zaman M, Y Elgendy I, N Mahmoud A, K Patel N, et al. (2018) Cryptogenic Stroke and Patent Foramen Ovale. J Am Coll Cardiol 71(9): 1035-1043.
- Mojadidi MK, Mahmoud AN, Elgendy IY, Agarwal N, Tobis JM (2017) Transesophageal Echocardiography for the Detection of Patent Foramen Ovale. J Am Soc Echocardiogr 30(9): 933-934.
- Zhang RV, Ryan KA, Lopez H, Wozniak MA, Phipps MS, et al. (2021) Sickle Cell Trait and Risk of Ischemic Stroke in Young Adults. Stroke 3: 51(9).
- Terni E, Giannini N, Brondi M, Montano V, Bonuccelli U, et al. (2015) Genetics of ischaemic stroke in young adults. BBA Clin 3: 96-106.
- Fonseca AC, Ferro JM (2013) Drug Abuse and Stroke. Curr Neurol Neurosci Rep 13(2): 1-9.
- Bright Chloe J, Hawkins Mike M, Guha Joyeeta, Henson Katherine E, Winter David L, et al. (2017) Risk of Cerebrovascular Events in 178 962 Five-Year Survivors of Cancer Diagnosed at 15 to 39 Years of Age. Circulation 135(13): 1194-1210.
- McCarty JL, Leung LY, Peterson RB, Sitton CW, Sarraj A, et al. (2019) Ischemic Infarction in Young Adults: A Review for Radiologists. RadioGraphics 39(6): 1629-1648.
- Leung LY, Caplan LR (2016) Factors Associated with Delay in Presentation to the Hospital for Young Adults with Ischemic Stroke. Cerebrovasc Dis 42(1-2): 10-14.
- Singhal AB, Biller J, Elkind MS, Fullerton HJ, Jauch EC, et al. (2013) Recognition and management of stroke in young adults and adolescents. Neurology 81(12): 1089-1097.
- Tsivgoulis G, Alexandrov AV, Chang J, Sharma VK, Hoover SL, et al. (2011) Safety and outcomes of intravenous thrombolysis in stroke mimics: a 6-year, single-care center study and a pooled analysis of reported series. Stroke 42(6): 1771-1774.
- Balami JS, Chen RL, Buchan AM (2013) Stroke syndromes and clinical management. QJM Int J Med 106(7): 607-615.
- Edlow JA, Selim MH (2011) Atypical presentations of acute cerebrovascular syndromes. Lancet Neurol 10(6): 550-560.
- Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, et al. (2009) Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 27;339: 3914.
- Koivunen RJ, Satopää J, Meretoja A, Strbian D, Haapaniemi E, et al. (2015) Incidence, risk factors, etiology, severity and short-term outcome of non-traumatic intracerebral hemorrhage in young adults. Eur J Neurol 22(1): 123-132.
- Connolly E Sander, Rabinstein Alejandro A, Carhuapoma J Ricardo, Derdeyn Colin P, Dion Jacques, et al. (2012) Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage. Stroke 43(6): 1711-1737.
- Suarez JI (2015) Diagnosis and Management of Subarachnoid Hemorrhage. Contin Minneap Minn 1263-1287.
- Kang HM, Cho JM, Kim SY, Choi JH (2020) Clinical characteristics of asymptomatic Terson syndrome in the patients with aneurysmal subarachnoid hemorrhage. Int J Ophthalmol 13(2): 292-300.
- Arauz A, Merlos Benítez M, Roa LF, Hernández Curiel B, Cantú C, et al. (2011) Cryptogenic cerebral infarction in young patients. Long-term prognosis and recurrence. Neurology 26(5): 279-284.
- Vázquez López M, Castro de Castro P, Barredo Valderrama E, Miranda Herrero MC, Gil Villanueva N, et al. (2017) Ischaemic stroke in children with cardiopathy: An epidemiological study. Neurol Engl Ed 32(9): 602-609.
- Sun Y P, Homma S (2016) Patent Foramen Ovale and Stroke. Circ J 80(8): 1665-1673.
- Caicedo J, Ortiz López A, Cardozo A, Cohen Cajiao JI (2016) Spontaneous dissection of the vertebral artery. CES Med 30(1): 93-98.
- Kirton A, Crone M, Benseler S, Mineyko A, Armstrong D, et al. (2013) Fibromuscular dysplasia and childhood stroke. Brain J Neurol 136( 6): 1846-1856.
- Smajlovic D (2015) Strokes in young adults: epidemiology and prevention. Vasc Health Risk Manag 11: 157-164.
- Olavarría VV, Bustamante G, López MJ, Lavados PM (2014) Diagnostic accuracy of a simple clinical score to screen for vascular abnormalities in patients with intracerebral hemorrhage. J Stroke Cerebrovasc Dis 23(8): 2069-2074.
- Josephson CB, White PM, Krishan A, Al Shahi Salman R (2014) Computed tomography angiography or magnetic resonance angiography for detection of intracranial vascular malformations in patients with intracerebral haemorrhage. Cochrane Database Syst Rev 9: CD009372.
- Almandoz JED, Kelly HR, Schaefer PW, Brouwers HB, Yoo AJ, et al. (2012) CT angiography spot sign predicts in-hospital mortality in patients with secondary intracerebral hemorrhage. J NeuroInterventional Surg 4(6): 442-427.
- Powers William J, Rabinstein Alejandro A, Ackerson Teri, Adeoye Opeolu M, Bambakidis Nicholas C, et al. (2019) Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 50(12): 344-418.
- Qureshi AI, Palesch YY (2011) Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: Design, Methods and Rationale. Neurocrit Care 15(3): 559-576.
- Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, et al. (2016) Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N Engl J Med 375(11): 1033-1043.
- Cheng NT, Kim AS (2015) Intravenous Thrombolysis for Acute Ischemic Stroke Within 3 Hours Versus Between 3 and 4.5 Hours of Symptom Onset. Neurohospitalist 5(3): 101-109.
- Dodds JA, Xian Y, Sheng S, Fonarow GC, Bhatt DL, et al. (2019) Thrombolysis in young adults with stroke: Findings from Get with The Guidelines–Stroke. Neurology 92(24): 2784-2792.
- Shi J, Cao Y, You S, Huang Z, Zhang X, et al. (2017) Young Stroke Patients Treated with Intravenous Thrombolysis have a More Favorable Outcome and Mortality Compared with Older Patients. Curr Neurovasc Res 14(2): 141-148.
- Toni D, Ahmed N, Anzini A, Lorenzano S, Brozman M, et al. (2012) Intravenous thrombolysis in young stroke patients: Results from the SITS-ISTR. Neurology 78(12): 880-807.
- Hemphill J Claude, Greenberg Steven M, Anderson Craig S, Becker Kyra, Bendok Bernard R, et al. (2015) Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Stroke 46(7): 2032-2060.
- Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, et al. (2013) Rapid Blood-Pressure Lowering in Patients with Acute Intracerebral Hemorrhage. N Engl J Med 368(25): 2355-2365.
- Delcourt C, Huang Y, Wang J, Heeley E, Lindley R, et al. (2010) The Second (Main) Phase of an Open, Randomised, Multicentre Study to Investigate the Effectiveness of an Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (Interact2). Int J Stroke 5(2): 110-116.
- Moullaali TJ, Wang X, Martin RH, Shipes VB, Robinson TG, et al. (2019) Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: a preplanned pooled analysis of individual participant data. Lancet Neurol 18(9): 857-864.
- Steiner T, Salman RA S, Beer R, Christensen H, Cordonnier C, et al. (2014) European Stroke Organisation (ESO) Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Int J Stroke 9(7): 840-8 55.
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, et al. (2005) Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 365(9457): 387-397.
- Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, et al. (2013) Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 382(9890): 397-408.
- Diringer MN, Bleck TP, Claude Hemphill J, Menon D, Shutter L, et al. (2011) Critical Care Management of Patients Following Aneurysmal Subarachnoid Hemorrhage: Recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care 15(2): 211.
- Macdonald RL, Schweizer TA (2017) Spontaneous subarachnoid haemorrhage. Lancet 389(10069): 655-66.
- Mas J L, Derumeaux G, Guillon B, Massardier E, Hosseini H, et al. (2017) Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med 377(11): 1011-1021.
- Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, et al. (2017) Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med 377(11): 1033-1042.
- Mojadidi Mohammad Khalid, Elgendy Akram Y, Elgendy Islam Y, Mahmoud Ahmed N, Elbadawi Ayman, et al. (2017) Transcatheter Patent Foramen Ovale Closure After Cryptogenic Stroke. JACC Cardiovasc Interv 10(21): 2228-2230.
- Maaijwee NAMM, Rutten Jacobs LCA, Schaapsmeerders P, van Dijk EJ, de Leeuw F E, et al. (2014) Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol 10(6): 315-325.
- Ekker MS, Verhoeven JI, Vaartjes I, Jolink WMT, Klijn CJM de, et al. (2019) Association of Stroke Among Adults Aged 18 to 49 Years with Long-term Mortality. JAMA 321(21): 2113 -2123.
- Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, et al. (2016) Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol 15(9): 913-924.
- Foraker RE, Olivo Marston SE, Allen NB (2012) Lifestyle and Primordial Prevention of Cardiovascular Disease: Challenges and Opportunities. Curr Cardiovasc Risk Rep 6(6): 520-527.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.