Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Successful VV-ECMO Support in a Patient with a Challenging Diagnosis of Miliary Tuberculosis
*Corresponding author: Margarida Costa e Silva and Raul Neto, Pulmonology Department, Vila Nova de Gaia/Espinho Hospital Center, Raul Neto, Intensive Care Department, Vila Nova de Gaia/Espinho Hospital Center, Portugal.
Received: December 02, 2021; Published: December 16, 2021
DOI: 10.34297/AJBSR.2021.15.002077
Abstract
Experience with extracorporeal membrane oxygenation in tuberculosis is scarce. We report the case of a 46-year-old woman with miliary tuberculosis successfully rescued with a short course of veno-venous extracorporeal membrane oxygenation (VV-ECMO). Seven days after admission due to fever and hypoxemic respiratory failure, multifactorial progressive deterioration of gas exchange led to ICU admission and decision to intubate. Mycobacterium tuberculosis was identified after repeated bronchoalveolar lavage sampling. Respiratory failure progressed and she evolved with shock. VV-ECMO support and anti-bacillary drugs were started. Patient was weaned from ECMO after 3 days, extubated at day 9 and discharged from hospital at day 38. ECMO may be a support option for severe respiratory failure due to tuberculosis, when invasive positive-pressure ventilation fails.
Keywords: Extracorporeal Membrane Oxygenation; ECMO; Tuberculosis; Tuberculosis, Miliary; Respiration, Artificial; Intensive Care Units
Introduction
Miliary tuberculosis, a potentially fatal form of disseminated tuberculosis resulting from massive lymphohematogenous dissemination of Mycobacterium tuberculosis [1,2] is a challenging and often missed diagnosis. A high index of clinical suspicion and aggressive diagnostic procedures are often necessary. Invasive procedures encompass appreciable risks, especially in the critically ill patient. Acute respiratory distress syndrome (ARDS) is rare in tuberculosis, [3-5] occurring in about 3-10% of patients [3] and mortality ranges between 60 and 90% [6]. VV-ECMO support may be an option, despite scarce evidence [3,5]. We report a case of successful VV-ECMO rescue in a patient with severe multifactorial respiratory failure with a challenging diagnosis of miliary tuberculosis.
Case Description
A 46-year-old Indonesian woman living in Portugal since 2018, presented to the emergency department with a 15-day history of fever, non-productive cough, headache, and fatigue. She had no relevant past medical history or exposures and was not on any medication or recreational drugs. She had no known history of contact with COVID-19 patients. Besides fever, physical examination was unremarkable. Initial workup revealed hypoxemic respiratory failure (paO2 55.1mmHg breathing room air) and slightly elevated C-reactive protein (6,91mg/dL). Real time polymerase chain reaction (PCR) for SARS-CoV-2 and HIV-ELISA test were negative. A CT scan showed widespread micronodules with random distribution, suggestive of a miliary pattern (Figure1A).
She was admitted for investigation. Sputum smear, mycobacterial culture and nucleic acid amplification testing were negative. Bronchoscopy was performed with no relevant findings and PCR for M. tuberculosis on bronchoalveolar lavage (BAL) were negative. Extensive BAL and blood workups regarding other infectious, malignant and immunomodulated diseases were uneventful.
At day seven, she was transferred to the ICU due to sudden worsening respiratory failure (RF) (tachypnea, PaO2/ FiO2<100mmHg). Empirical piperacillin/tazobactam and azithromycin were added, presuming superimposed hospitalacquired bacterial infection. After an unsuccessful 16-hour trial of high-flow oxygen therapy, she was intubated and mechanically ventilated (Vt 6ml/kg, PEEP 10cmH20). CT angiogram ruled out pulmonary emboli and air-leak syndromes, showing worsened micronodules and new-onset symmetrical ground glass opacities with anteroposterior density gradient and small bilateral pleural effusion (Figure1B).
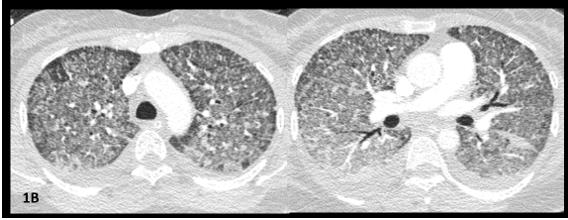
Figure 1B: CTPA images. Worsening of micronodules and appearance of symmetrical ground glass opacities with anteroposterior density gradient; small bilateral pleural effusion.
We performed another bronchoscopy for BAL analysis after intubation, keeping in mind the high suspicion index for miliary tuberculosis, that was poorly tolerated. It confirmed the primary hypothesis (positive PCR for M. tuberculosis; no evidence of viral, fungal, or bacterial infections). She immediately started empirical rifampicin (600mg IV), isoniazid (300mg IV), ethambutol (1200mg, oral), and pyrazinamide (1500mg, oral).
Despite protective-lung ventilation strategy (including neuromuscular blockage, esophageal pressure catheter guidedventilation, prone position) she rapidly evolved with severe refractory global RF and shock (1 mcg/kg/min norepinephrine plus steroids). Point-of-care ultrasonography showed adequate biventricular function and vascular filling, no pericardial effusion or pneumothorax. VV-ECMO support was initiated 30h after intubation (initially: flow:3L/min, sweep-gas flow: 3L, 100% O2 on oxygenator, adjusted in the first hours to 3.5-4L/min of flow and of 4L/min of sweep gas). Mechanical Ventilation (MV) was adjusted to ultra-low tidal volume strategy (4ml/kg). Arterial blood gas analysis showed immediate improvement of hypercapnia but persistence of severe hypoxemia. Bronchodilators and diuretics were optimized. Amikacin and levofloxacin were added, due to uncertainty regarding effective serum concentrations of HRZE under ECMO.
Patient improved steadily: vasopressors and neuromuscular blockade were stopped after 2 days; ECMO cessation on day 3, maintaining lung protective MV, successfully extubated at day 9, and transferred to general ward 48h after. She was discharged home after 38 days, under tuberculosis treatment with no evidence of adverse effects.
Discussion
We report a rare case of severe acute respiratory failure due to miliary tuberculosis in a young immunocompetent woman who was successfully supported with a short course of VV-ECMO due to worsening RF. This reinforces the severity of tuberculosis-related ARDS, as well as the deleterious effect of the necessary diagnostic and support measures. Acute respiratory failure represents one of the most severe complications of late tuberculosis [7].
The diagnosis of miliary tuberculosis is globally challenging due to low rates of positive microscopy for acid-fast bacilli in sputum (32%-36%) and BAL (31%) [3]. Although transbronchial biopsies may enhance the probability of a proper diagnosis (up to 79%) [3], this was not our first option due to risk of complications and clinical deterioration. Molecular methods (like PCR) on BAL have showed 60-80% sensitivity for tuberculosis in smear-negative patients, representing a promising alternative to more invasive techniques [3]. Nonetheless, BAL sampling is not risk-free, particularly in the critically ill [8]. Furthermore, many conditions, including malignancies and hypersensitivity pneumonitis, can present with a miliary pattern on chest imaging and must be differentiated from miliary tuberculosis [9]. Our case presented some atypical findings, such as diffuse ground-glass opacities, adding additional difficulties by widening the number of differential diagnoses.
To the best of our knowledge there are only nine reported cases of patients with pulmonary tuberculosis on ECMO support [3-7,10- 13]. Those are extremely diverse in terms of medical background, clinical phenotype, degree of immunosuppression, adjuvant offlabel therapies or superimposed events precipitating the need for ECMO support. However, it is well-defined that mortality is higher in tuberculosis patients requiring MV vs. respiratory failure from other causes (25,9%-81,0%) [4,14]. Most of the referred cases describe prolonged therapy with VV-ECMO, and results from the EOLIA trial show a mean ECMO duration of 15 ± 13 days [15]. Our patient was only on ECMO support for 3 days, which was only reported in two other cases in literature [5,6].
Since the scarce evidence, we can only hypothesize reasons for successful short-term improvement with ECMO support. The singular pathophysiology of tuberculosis-associated ARDS might be one [5], and early treatment may have avoided progression to an organized/fibrotic stage. Timely institution of MV with ultra-low tidal volumes could also have helped preventing ventilator inducedlung injury and minimized lung stress and strain. Repeated BAL sampling and fluid overloading right before cannulation probably played a role in the acute deterioration and rapid improvement once those aggravating factors were corrected, as superimposed bacterial infection, supported by inflammatory markers kinetics (Table1).
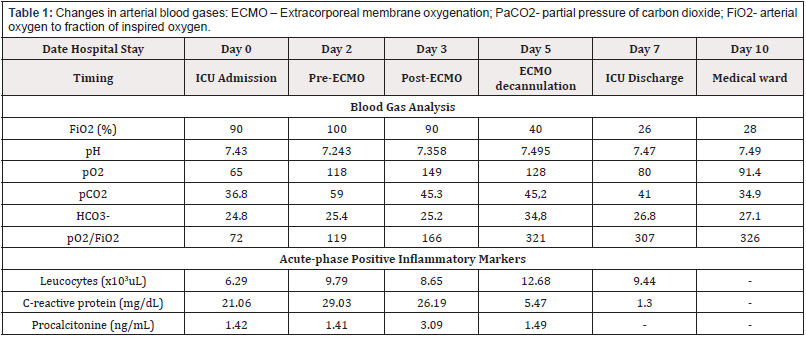
Table 1: Changes in arterial blood gases: ECMO – Extracorporeal membrane oxygenation; PaCO2- partial pressure of carbon dioxide; FiO2- arterial oxygen to fraction of inspired oxygen.
As the successful eradication of tuberculosis is dependent on adequate blood levels of anti-tuberculosis drugs [3], another management challenge in this ECMO supported patient was the unknown drug pharmacokinetics, as the ECMO circuit increases the volume distribution for most parenteral drugs [16] and we were not able to monitor drug levels. Little is known about the use of antibacillary drugs in this context [3,16]. Pharmacokinetics was only described in a single case report [17], were the authors described the use of a similar regimen but were able to use therapeutic drug monitoring to achieve better treatment concentrations. Their patient required nearly 23 mg/kg/day of rifampin as compared to the standard of 10 mg/kg/day and 50% more pyrazinamide and ethambutol than recommended for her weight [17]. On the other hand, levofloxacin concentrations used were standard [17]. As we were not able to perform serum concentrations monitoring, we decided to put her under a 6-drug regimen (addition of levofloxacin (group 3) and amikacin (group 2) to HRZE classic regimen.
Conclusion
Accurate diagnosis of tuberculosis is crucial for a successful treatment and VV-ECMO support was useful in the management of severe RF in a patient with miliary tuberculosis, even as a shortterm strategy. Careful adjustment of anti-tuberculosis drugs is mandatory during ECMO support to ensure effective eradication.
Statement Confirming Patient Consent
Patient provided and signed the informed consent form.
Competing Interest’s Statement
The authors have no competing interests or funding incentives to declare.
References
- Surendra K Sharma, Alladi Mohan (2017) Miliary Tuberculosis. Microbiol spectr 5(2).
- Boushab Mohamed Boushab, Leonardo Kishi Basco (2019) Miliary tuberculosis and acute respiratory distress syndrome. J clin tuberc other mycobact dis 16: 100113.
- Edda Vesteinsdottir, Gunnar Myrdal, Kristinn O Sverrisson, Sigurbjorg J Skarphedinsdottir, Olafur Gudlaugsson, et al. (2019) ARDS from miliary tuberculosis successfully treated with ECMO. Respir Med Case Rep 26: 165-167.
- Norihito Omote, Yasuhiro Kondoh, Hiroyuki Taniguchi, Tomoki Kimura, Kensuke Kataoka, et al. (2016) Acute respiratory distress syndrome due to severe pulmonary tuberculosis treated with extracorporeal membrane oxygenation: A case report and review of the literature. Respir Med Case Rep 19: 31-33.
- Nguyen Gia Binh, Toshie Manabe, Dao Xuan Co, Pham The Thach, Dang Quoc Tuan, et al. (2019) Tuberculosis-induced acute respiratory distress syndrome treated with veno-venous extracorporeal membrane oxygenation. Respir Med Case Rep 28: 100900.
- Sonia Erika Frick, Christoph Flothmann, Benjamin Preiswerk, Renate Behr, Michele Genoni (2015) Extracorporeal Membrane Oxygenation in Miliary Tuberculosis and AIDS: A Case Report. Thorac Cardiovasc Surg Rep 4(1): 18-20.
- V Cogliandro, G Lapadula, A Bandera, A Muscatello, R Marcolin, et al. (2014) ECMO: an alternative support for acute respiratory failure caused by tuberculosis? Int J Tuberc Lung Dis 18(7): 879-881.
- Toufik Kamel, Julie Helms, Ralf Janssen Langenstein, Achille Kouatchet, Antoine Guillon, et al. (2020) Benefit-to-risk balance of bronchoalveolar lavage in the critically ill. A prospective, multicenter cohort study. 46(3): 463-474.
- Surendra K Sharma, Alladi Mohan, Abhishek Sharma (2012) Challenges in the diagnosis & treatment of miliary tuberculosis. The Indian journal of medical research 135(5): 703-730.
- Max Andresen, Pablo Tapia, Marcelo Mercado, Guillermo Bugedo, Sebastian Bravo (2013) Catastrophic respiratory failure from tuberculosis pneumonia: Survival after prolonged extracorporeal membrane oxygenation support. Respir Med Case Rep 10: 19-22.
- Seok In Lee, Hyun Joong Hwang, So Young Lee, Chang Hyu Choi, Chul Hyun Park, et al. (2017) Veno-veno-arterial extracorporeal membrane oxygenation for acute respiratory distress syndrome with septic-induced cardiomyopathy due to severe pulmonary tuberculosis. J Artif Organs 20(4): 359-364.
- Sung Jin Nam, Young Jae Cho (2016) The successful treatment of refractory respiratory failure due to miliary tuberculosis: survival after prolonged extracorporeal membrane oxygenation support. Clin Respir J 10(3): 393-399.
- T Mauri, G Foti, A Zanella, M Bombino, A Confalonieri, et al. (2012) Long-term extracorporeal membrane oxygenation with minimal ventilatory support: a new paradigm for severe ARDS? Minerva anestesiol 78(3): 385-389.
- Denise R Silva, Diego M Menegotto, Luis F Schulz, Marcelo B Gazzana, Paulo Tr Dalcin (2010) Mortality among patients with tuberculosis requiring intensive care: a retrospective cohort study. BMC infect dis 10: 54.
- Alain Combes, David Hajage, Gilles Capellier, Alexandre Demoule, Sylvain Lavoué, et al. (2018) Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med 378(21): 1965-1975.
- Sherwin J, Heath T, Watt K (2016) Pharmacokinetics and Dosing of Anti-infective Drugs in Patients on Extracorporeal Membrane Oxygenation: A Review of the Current Literature. Clin Ther 38(9): 1976-1994.
- Hyung Sook Kim, Eun Sook Lee, Young Jae Cho (2014) Insufficient serum levels of antituberculosis agents during venovenous extracorporeal membrane oxygenation therapy for acute respiratory distress syndrome in a patient with miliary tuberculosis. ASAIO J 60(4): 484-486.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.