Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Autoimmune Syndrome Through the Prism of Targeted AT-Mediated Proteolysis: Innovative Ideas, Philosophy, and Tools for Practitioners of The Next Step Generation
*Corresponding author: Sergey Suchkov, MINO MGUPPI, Evdokimov Moscow State Medical & Dental University, EPMA (European Association for Prediction, Prevention and Personalized Medicine), Russia.
Received: November 29, 2021; Published: February 04, 2022
DOI: 10.34297/AJBSR.2022.15.002114
Abstract
A feature of the modern laboratory-based personalized and precision diagnostics is a rapid intro-duction of the full-scale highly informative predictive and prognostic diagnostic technologies in-to the daily clinical practice. This trend is particularly evident in clinical immunology and biotechnology. The key link in the pathogenesis of autoimmune diseases is autoantibodies (auto-Abs), which have different levels of specificity and functionality, constituting the most important portion of a unified diagnostic strategy in the practice of an endocrinologist.
Based on recent scientific research in the field of diseases of autoimmune nature, we would as-sume that catalytic Abs (abzymes) can serve as an informative translational tool for the pre-early diagnosis of autoimmune syndromes. Thus, by studying, screening and discovering new bi-omarkers being specific for autoimmune diseases, there is not only an opportunity for a timely start of treatment and development of biodesign, but also the implementation of the promising philosophy of Personalized & Precision Medicine (PPM) based on biomarkers-related platforms (Ab-based ones, in particular), into clinical practice [1]. This article looks as a summary of current knowledge and prognostic prospectives towards cata-lytic Abs in autoimmunity and thus some autoimmune clinical cases, their role in pathogenesis, and the exploitation of both whole molecules and their constituent parts in developing highly ef-fective targeted drugs of the future to come, and thus the therapeutic protocols being individual-ized.
Keywords: Catalytic Antibodies (abzymes), Antibody-proteases, Protabzymes, Biomarkers, per-sonalized and precision medicine, Autoimmune syndrome, Autoimmunity
Introduction
Through the accumulation of knowledge about the functioning of the immune system, as well as the deep understanding of biochemical processes in the physiologically normal state and in pa-thology, particularly specific autoAbs, called catalytic Abs (ABZYMES), were identified. Abzymes belong to an absolutely new group of physiologically active substances with dual characteristics: they represent a pool of canonical autoAbs and possess catalytic activity. Among them, proteolytic and DNA-hydrolyzing autoAbs are of special value. Abzymes are an important pathogenic factor in the progression of clinical autoimmunity syndrome. The presence of autoAbs against various autoantigens (autoAgs) is accompanied by their high catalytic potential. The in-crease in this activity correlates with serum levels of the autoAbs, clinical manifestations of auto-immune disorders, disease severity and the rate of progressing disability. Abzymes are crucial for immune homeostasis regulation as well. And they can be of practical value in the development of modern immunodiagnostic tools and schedules of targeted immunotherapy.
Catalytic Abs are generated spontaneously by the immune system in the absence of immunization by an exogenous peptide or a protein. Catalytic IgG and IgM have been documented in sera of healthy individuals. Under physiological conditions catalytic Abs participate directly in the elim-ination of the biochemical and metabolic wastes released by the human metabolism [2]. However, on the other hand, the pathological role of autoAbs in autoimmune diseases is widely recognized. In this review, we focus only on three types of enzymes documented in such autoimmune diseas-es as autoimmune thyroiditis (AT), autoimmune myocarditis (AM) and multiple sclerosis (MS).
Editorial Viewpoint
Medical abzymology has made a great contribution to the development of general autoimmunity theory: it has put the Ab as the key brick of the theory to the level of physiological functionality by providing such Ab with the ability to catalyze and mediate direct and independent cytotoxic effect on cellular and molecular targets. Natural abzymes while being a pool of canonical Abs and possessing catalytic activity, belong to an absolutely new group of physiologically active substances whose features and properties are evolutionary consolidated in one functionally active biomolecule. Meanwhile, due to the rapid development of high-precision diagnostic methods for monitoring autoimmune diseases at the early and pre-early stages, a wide palette of autoAbs as valuable bio-indicators can be detected even in subclinical and latent clinical symptoms. However, whilst ana-lyzing the value of the information obtained, scientists found that not the entire mass of autoAbs is a reliable indicator of the development of an autoimmune syndrome. AutoAbs with proteolytic activity are singular, and it is those autoAbs that specify the form of the pathology.
It is recognized that Abs initiate a wide range of reactions, including reactions to different non-self- or modified selfcompounds. In addition, molecules with small, detailed differences can be distinguished by their reactivity with different Abs [3]. Because of the highly evolved mecha-nism of the immune system, which is capable of producing structurally and functionally complex compounds, including Abs, there are great prospects for biologists, chemists, bioengineers and practitioners. Experts in science and medicine, operating with high-tech tools and pre-processed and mined information, provide the basis for understanding biomolecular structure within the functions of living systems.
Features of Ig-Hydrolyzing Abzymes and Their Screening
AutoAbs detectable within the family of autoAbs are able to cleave autoAgs including autolo-gous thyroglobulin (TG) specifically displaying catalytic activity similar to canonical proteases (Protabzymes or Ab-proteases). Ab-proteases with TGhydrolyzing (Figure 1).
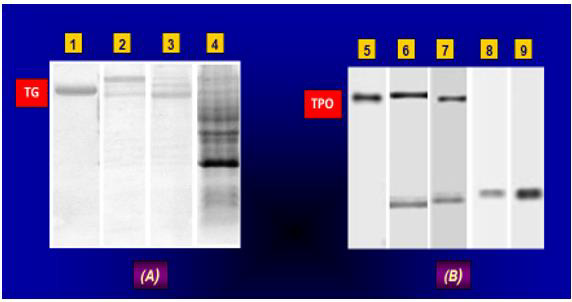
Figure 1: Ab-mediated hydrolysis of allogeneic TG and TPO.
Electrophoresis was done in SDS-PAGE; (А) и (B) – electrophoresis of proteolytic products of TG and TPO, respectively; Lanes (1) amd (2) –
human reference ТГ and ТРО, respectively; Lanes (2)/(3) and (4) – anti-ТГ autoAbs isolated from sera of AIT and DTG patients, respectively;
(6)/(7)andи (8)/(9) – anti-ТРО autoAbs isolated from sera of DTG and AIT patients, respectively; TG – thyroglobulin; ТРО – thyroid peroxidase;
Аbs – antibodies; PAGE – polyacrilamide gel; AIT and DTG – autoimmune thyroiditis and diffuse toxic goiter, respectively; HC – healthy controls;
SDS – sodium dodecyl sulphate.
Activity reveals an inverse correlation with the severity of autoAbs.
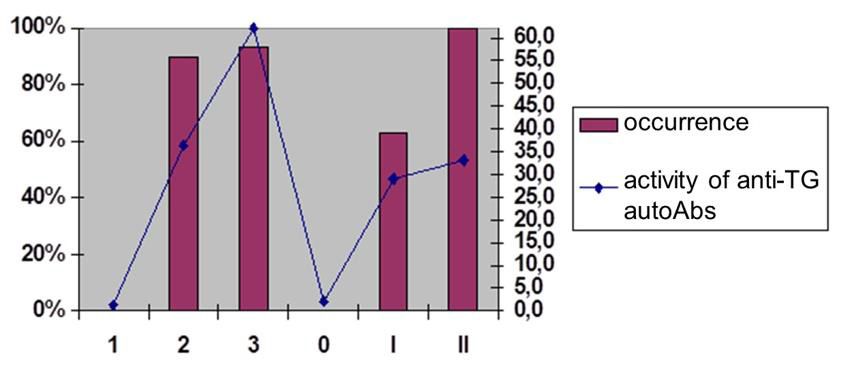
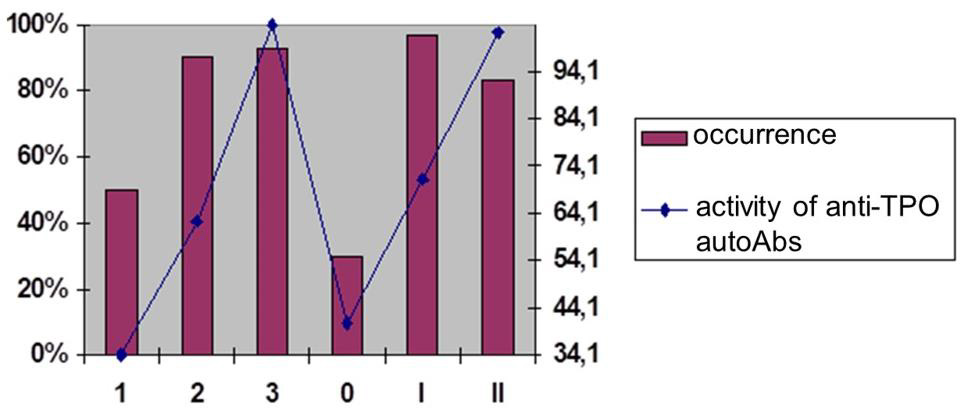
The family of antithyroid autoAbs also includes Abs with a functional resource, such as abzymes or Abs with proteolytic properties, which are extremely informative objects for monitoring pa-tients with autoimmune thyroiditis (AIT) and Basedow’s disease. Among the extensive family of antithyroid autoAbs we should distinguish the Abs to TG (anti-TG autoAbs) (Figure 2A- 2D) and Abs to thyroid peroxidase (anti-TPO autoAbs), as well as combinatorial Abs, detected in most patients with autoimmune thyroiditis.
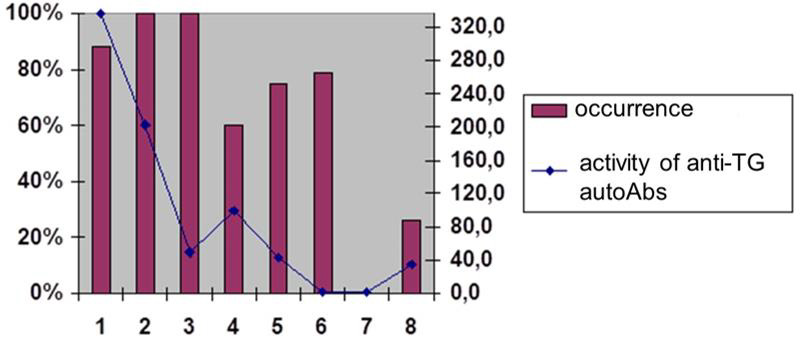
Figure 2A: The prevalence and activity of anti-TG AT-proteases among AIT patients Preva-lence and activity of anti-TG AT-proteases among AIT patients.
TG is a well-known thyroid hormone precursor being the target to be attacked by the specific au-toAbs in autoimmune thyroid conditions. The pathogenic significance of antithyroid autoAbs differs, but in cooperation with cytotoxic tools such autoAbs would much contribute into the development of auto aggression conditions, thereby reducing the total volume of the functionally significant part of the thyroid. At the same time, some of the antithyroid Abs are able to ignore the traditional tools of cytotoxicity, filling this “gap” by having their own “armed bullets” prepacked within their infrastructure.
Abs with proteolytic properties (abzymes) belong to this unique category of natural autoAbs with a resource of “functionality” (for example, catalytic/proteolytic or cytotoxic potential). Such Ab are detected at the pre-early (subclinical) stages and without any cooperation with other tools of the patient’s immune effective arsenal can attack the glandular tissue, actively participating in the pathogenesis of autoimmune thyroid diseases. Among currently known antithyroid autoAbs (an-ti-TG autoAbs, anti-TPO autoAbs, combinatorial autoAbs) were detected in 90%, 76% and 20% of AIT patients and in 40%, 30% and 90% of patients with thyrotoxicosis, indicating the exceptional importance of using such Abs as bioindicators and biopredictors [4].
Clearance of TG in the blood by anti-TG Ab-proteases may facilitate the reduction of autoimmune reactions against this protein and be of potential benefit for patients. However, anti- TG Abs have been shown to access the thyroid; administration of anti-TG Abs and implantation of TG-Ab-secreting hybridomas in experimental animals results in thyroid dysfunction. In clinical thyroidology the standard Serologic Tandem consisting of two types of such Abs (an-ti-TPO and anti-TG autoAbs), sensitive but insufficiently specific biomarkers of AIT and TDG, is of particular value for monitoring. For this reason, the indicators illustrating the serological dynamics of such Abs are considered criterion-relevant only when repeatedly elevated numbers in the course of long-term monitoring of a patient [5-8].
Studying the role of Ab-proteases in the pathogenesis of autoimmune thyroid pathology and evaluation of associative relationships between Ab-proteases and peculiarities of the clinical and immunological manifestations of the pathology is a promising area of research and translational applications. Meanwhile, a significant part of the population of antithyroid autoAbs endowed with proteolytic activity belongs to the objects of special clinical significance in AIT and TDG patients [9-11]. It should be noted that in tandem, Ab-proteases have the property of mutually potentiating their activity, and this property is most pronounced in active destructive process in the thyroid (longterm thyrotoxicosis, manifest hypothyroidism with hypotrophy, as well as atrophic forms of AIT): in these patients the Ab-protease activity in tandems is significantly higher than that in patients who are monoseuropositive for Ab-proteases. Accordingly, subclinical diagnosis based on the detection of certain types of antithyroid autoAbs (primarily with a functional resource) can help to identify the risks of autoimmune thyroid pathology, and early intervention can prevent the transition of a mild (preclinical) form of autoimmune disease into an irreversible process with the development of severe autoimmune syndrome.
Antithyroid autoAbs with proteolytic activity are no less significant in patients with TDG, although the total activity of such Abs (Ab-proteases) in TDG is significantly lower than in AIT. Abproteases are predominantly concentrated in patients with manifest and complicated TDG, as well as in patients with terminal goiter. Thus, in subclinical TDG and early stages of goiter, Ab-proteases are markedly less active, and their spectrum is limited to the presence of only one type of Ab-proteases (TPO-specific). At the same time, as the disease progresses, the goiter increases and thyrotoxicosis develops, there is an increase in the activity of Ab-proteases not only in noncovariant, but also in serological tandems.
Thus, the dynamics of Ab-protease content in TDG shows a pronounced association with the development of the clinical picture of the disease, primarily with an increase in goiter size and the scale of destructive processes in the thyroid against the background of thyrotoxicosis. Thus, the Ab-protease screening procedure can be considered as a highly sensitive and specific criterial test for predictive diagnosis and monitoring of patients with AIT and TDG, as well as those in risk groups, which allows the diagnosis not only within the boundaries of major thyroid nosologies, but also much wider, covering a number of syndromal forms, including different variants of functional thyroid autonomies. It is on the basis of the obtained knowledge about the specific role of autoAbs that it will be possible to create the newest test-systems based on Ab-proteases with a high level of functionality and to introduce targeted testkits into clinical (including endo-chronological) practice for the purposes of serodiagnosis, seromonitoring and seroprediction.
Antibodies As a Molecular Diagnostic Tool in Autoimmune Myocarditis
Autoimmune myocarditis (AM) is one of the key unresolved issues in cardiology and now re-quires new approaches using immunogen diagnostic methods. In contrast to the systemic forms of autoimmune pathology, AM occupies the diametrically opposite pole of the classification scheme of autoimmune diseases and refers to organ-specific forms of autoimmune pathology. The current situation in cardiology practice is dictated by insufficient information about the pathogenetic significance of different categories of antimyocardial autoAbs on the one hand and weak interaction between clinical immunology and cardiology on the other.
Antimyocardial autoAbs constitute a rather heterogeneous group of ATs, including entire families of ATs against beta 1- adrenergic and muscarinic receptors, mitochondrial ATP/ADPtranslocase, sarcolemma proteins, ANT-translocator, etc. but most often, against alpha- and beta-chains of myosin [12]. Meanwhile, detection of high-affinity antimyocardial IgG-class autoAbs in the sera indicates an important and direct role of myocardial autoAg in the induction of autoAb synthesis in AM. Such autoAbs are known to be a consequence of switching the synthesis of natural IgM-class au-toAbs, which have low avidity and are detected at the preclinical stages of AM, to the synthesis of high-affinity IgG-class autoAbs containing a wide range of idiotypic determinants (including pathogenic properties) in their structure and characteristic for the developed clinical picture of the disease [13].
As mentioned above, at least two classes of anti-myocardial autoAbs are detected in these forms of autoimmune pathology, namely, autoAbs against alpha- and beta-chains of cardiomyosin. Exactly the presence of autoAbs to the alpha-chain satisfies the criteria of organ-specific autoim-mune syndrome, illustrating the possibility not only of adequate diagnostics and monitoring but also of seroprediction of the disease course. We also identified and described autoATs with proteolytic activity against cardiomyosin (cMyo), which were detected in the blood of patients with autoimmune myocarditis (AM) (Figure 3).
Therapeutic Trends in The Future to Come
As the pathophysiological significance of anti-cardiac autoAbs directed against various Ags has become increasingly clear, attention has shifted to methods to suppress their effects. Therapeutic strategies have included induction of tolerance to various cardiac Ags, immunosorption, and administration of intravenous iIg.
MBP-Hydrolyzing Abzymes and Their Role in The Destruction of Myelin
Multiple sclerosis (MS) is just one of the chronic autoimmune diseases resulting in a destruction of myelin by different substances, including autoAbs. At present, a spectrum of myelin-associated autoAbs occurring in MS patients has been confirmed to be very large. The crucial step in the pathogenesis of MS is a primary myelin damage which is mediated by autoAbs to trigger a release of separate and pathogenically valuable myelin-associated epitopes into the bloodstream. Along with canonical Abs, some of the families proven to occur are Abs possessing with catalytic activity (catAbs or abzymes) and thus to belong to Abs with a feature of functionality. As regard CatAbs, it is multivalent Igs, presumably, of IgG isotype, endowed with a capacity to hydrolyze the Ag substrate. The property mentioned is buried in the Fab-fragment of the Ig molecule and is appearing to sound as a functional property of the Ab molecule. In this sense, proteolytic Abs (or Ab-proteases) as a significant portion of the big family of abzymes represent Abs endowed with a capacity to provide targeted proteolytic effect [14].
It was noted that the greatest value is a sequence specificity of Ab-proteases which was ana-lyzed by screening peptide cleavage products due to SELDI protocol. Ab-mediated proteolysis of myelin basic protein (MBP) results in generating a set of peptides with molecular weight ranged in various but fixed boundaries to suit common principles of the molecular architectonics of MBP (Figure 4). The final statistical data revealed five sites of preferential proteolysis. Among them the most immunogenic and encephalitogenic sites of immunodominant category responsible for generating aggressive bursts of demyelination are concentrated in three areas of MBP molecule: 43-170 – immunodominant area of the chain to include the sites defined: 81-103 -is a sequence-specific immunogen, 82-98 - is a subsequence-specific highly immunogenic and highly encephalitogenic site [15] (Figure 5A-5C).
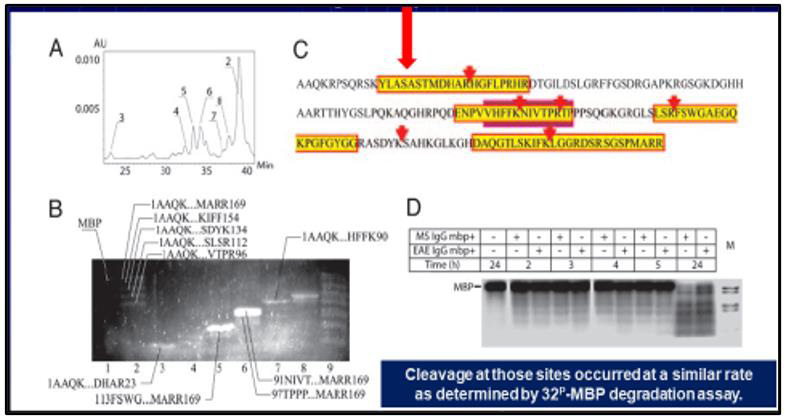
Figure 4: MBP-targeted Ab-proteases in MS sera.
Ab-mediated proteolysis of MBP results in generating a set of peptides with MW ranged in various but fixed boundaries to suit com-mon
principles of the molecular architectonics of MBP. The final statistical data revealed FIVE sites of preferential proteolysis
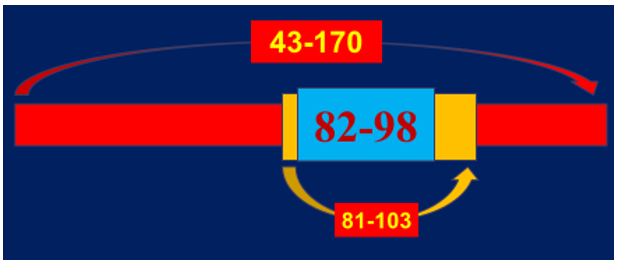
Figure 5A: MBP-related sites to be attached by MBP-targeted MBP Ab-proteases in MStargeted Ab-proteases in MS sera. Most of those sites (as a set defined) are located within the 43-170 region of MBP which are just immunodominant!
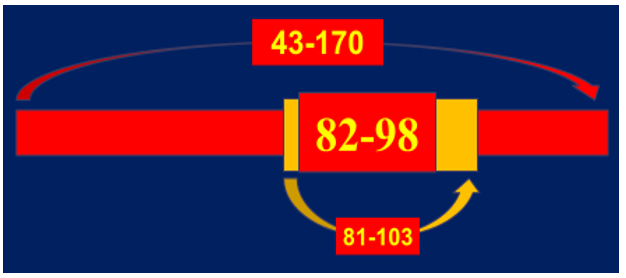
Figure 5B: MBP-related sites to be attached by MBP-targeted MBP Ab-proteases in MStargeted Ab-proteases in MS sera. Two sites from the set located within 43-170 sequence, would comprise 81-103 and 82-98 subsequences that whilst being sequence-specific, highly immunogene-ic and encephalitogeneic both as well, proved to be specific and major inducers of very aggressive EAE in SJL mice.
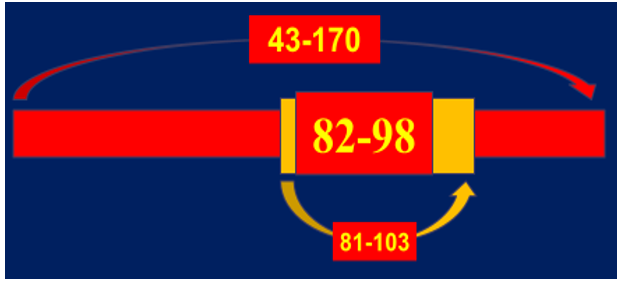
Figure 5C: MBP-related sites to be attached by MBP-targeted MBP Ab-proteases in MStargeted Ab-proteases in MS sera Both 81-103 and 82-98 subsequences have been proven to be major MBP targets to be attacked by Ab proteases obtained from patients with secondary-progradient courses of MS, progression phase (SPPP), and with remittent course of MS, exacerbation phase (REP), both are known as most aggressive ones!
The re-orientation of the sequence specificity in MBP-targeted Ab-mediated proteolysis during the evolution and progression of MS to focus on the more important targeted sites for proteolysis might be an early prognostic and/or predictive sign to monitor demyelination progressing and thus the clinical illness to come. So, the ACTIVITY of Ab-proteases in combination with the SEQUENCE SPECIFICITY to attack well-defined but separate sets of sequences and sub-sequences defined would confirm a high impact of Abproteases to monitor both clinical and subclinical courses of chronic autoimmune inflammation (MS) to predict stepwise transformations and the clinical illness finally!
As it is broadly known, canonical autoAbs play neither predictive nor discriminative role to affect the subclinical stage of MS. In contrast, sequence-specific Ab-proteases have proved to be greatly informative and thus valuable biomarkers to monitor MS at both subclinical and clinical stages! Therefore, the proposed predictive value of MBP-targeted Ab-proteases for the development of MS is being challenged! The translational potential of this knowledge is in the rational design of new diagnostic tools and new therapeutics based on principles of artificial biocatalysts and biodesign (Figure 6).
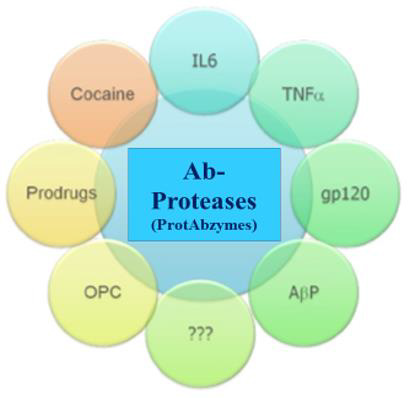
Figure 3: Prospectives of Ab-Proteases as Artificial Biocatalysts in vivo.
IL, interleukin; TNF, tumor necrosis factor; gp120, a glycoprotein exposed on the surface of the HIV envelope. OPC, oligodendro-cyte precursor
(progenitor) cell; AβP, anti-amyloid beta protein Abs.
In this sense, Ab-proteases can be programmed and reprogrammed. The latter would suit the needs of the body metabolism or could be designed for the development of principally new catalysts with no natural counterparts to suit the requests and standards of regeneration and remyelination. It goes without saying, that tremendous value are Ab-proteases directly affecting the physiologic remodeling of tissues with multilevel architectonics (for instance, myelin) whilst securing the re-quests and standards of regeneration. Moreover, by changing sequence specificity of the Ab-mediated proteolysis one may reach reduction of a density of negative cleavages made by Ab-proteases in MBP and thus could minimize the overall negative effect within the myelin sheath and, finally, minimizing scales of demyelination.
The prospects for progress in developing novel approaches for the diagnosis and treatment of MS have been greatly encouraged by several observations, including
a) The identification of a major immunogenic region in MBP, peptide 82–98.
b) The demonstration that both B and T cell re-sponses to MBP are focused on this region, particular for patients who express HLA-DR2.
c) clinical studies suggesting that patients treated with MBP peptide 82–98 can be made tolerant to the protein in association with delayed disease progression or reduced disease activity over time [13-15].
Conclusion
So, the activity of Ab-proteases and its dynamics tested would confirm a high subclinical and predictive value of the tools as applicable for monitoring protocols. The new targeted therapies for autoimmune and inflammatory diseases would require greater understanding of a patient or a person-at-risk to get the therapy personalized for either of those subsets. Accordingly, now it is time to identify biomarkers of newer generations and to create simultaneously a fundamentally new strategy based upon subclinical recognition of those biomarkers long before the disease clinically manifests itself. To achieve the implementation of PPM concept into the daily practice, it is necessary to create a new strategy based upon recognition, testing and monitoring of biomarkers of the newest generation [16].
In conclusion, we are experiencing a Renaissance primarily driven by next-generation biotechnologies. In this sense, autoAbs (abzyme) is a type of Abs with catalytic activity being found not only in healthy humans and but also in patients with autoimmune diseases. So, further studies on Ab-mediated autoAg degradation and other targeted Ab-mediated proteolysis may provide biomarkers of newer generations and thus a supplementary tool for assessing the disease progression and predicting disability of the patients and persons-at-risks [17].
References
- TA Bodrova, DS Kostyushev, EN Antonova, Sh Slavin, DA Gnatenko, et al. (2012) Introduction into PPPM as a new paradigm of public health service: an integra-tive view. EPMA 3(1): 16.
- Alexander G Gabibov, Natalya A Ponomarenko, Eugenia B Tretyak, Mikhail A Paltsev, Sergey V Suchkov, et al. (2006) Catalytic autoantibodies in clinical autoimmunity and modern medicine. Autoimmun Rev 5(5): 324-330.
- Friboulet A, Avalle B, Debat H, Thomas D (1999) A possible role of catalytic antibodies in metabolism. Immu-nol Today 20(10): 474-475.
- Natalya A Ponomarenko, Oxana M Durova, Ivan I Vorobiev, Elena S Aleksandrova, Georgy B Telegin, et al. (2002) Catalytic antibodies in clinical and experimental pathology: human and mouse models. J Immunol Methods 269(1-2): 197-211.
- Takami HE, Miyabe R, Kameyama K (2008) Hashimoto's Thyroiditis. World J Surg 32(5): 688-692.
- Wen WB, Liu FY (2007) Autoantibodies highly increased in patients with thyroid dys-function. Cell Mol Immunol 4(3): 233-236.
- Demirbilek H, Kandemir N, Gonc EN, Ozon A, Alikasifoglu A, et al. (2007) Hashimoto's thyroiditis in children and adolescents: a retrospective study on clinical, epidemiological and la-boratory properties of the disease. J Pediatr Endocrinol Metab. 20(11):1199-1205.
- W Wen, F Liu (2007) Autoantibodies Highly Increased in Patients with Thyroid Dysfunction. Cell Mol Immunol 4 (3): 233-236.
- Caforio AL, Mahon NJ, Tona F, Mc Kenna WJ (2002) Circulating cardiac autoantibodies in dilated cardiomy-opathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 20024(4): 411-417.
- Rose NR, Burek CL (2000) Autoantibodies to thyroglobulin in health and disease. Appl Biochem Biotechnol. 83(1-3): 245-251.
- Bulow Pedersen I, Laurberg P, Knudsen N, Jorgensen T, Perrild H, et al. (2005) A population study of the association between thyroid autoantibodies in serum and abnor-malities in thyroid function and structure. Clin Endocrinol (Oxf) 62(6): 713-720.
- Jasani B, Ternynck T, Lazarus JH, Phillips DI, Avrameas S, et al. (1999) Parkes AB Natural anti-body status in patients with Hashimoto's thyroiditis. J Clin Lab Immunol 51(1): 9-20.
- Caforio AL, Mahon NJ, Mckenna WJ (2001) Cardiac autoantibodies to myosin and other heart-specific auto-antigens in myocarditis and dilated cardiomyopathy”. Autoimmunity 34(3): 199-204.
- Ponomarenko NA, Durova OM, Vorobiev II, Belogurov AA Jr, Kurkova IN, et al. (2006) Autoantibodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc Natl Acad Sci U S A 103(2): 281-286.
- Weber MS, Hemmer B, Cepok S (2011) The role of antibodies in multiple sclerosis. Biochim Biophys Acta 1812(2): 239-245.
- SV Suchkov, ZS Alekberova, FN Paleev, TE Naumova, VK Misikov, et al. (2005) Achievements and prospects of clinical abzymology. Vestn Ross Akad Med Nauk (9): 38-43.
- Séverine Padiolleau-Lefèvre, Raouia Ben Naya, Melody A Shahsavarian, Alain Friboulet, Béran-gère Avalle, et al. (2014) Catalytic antibodies and their applications in biotechnology: state of the art”. Biotechnol Lett 36(7): 1369-1379.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.