Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Effect of Juniperus virginiana Essential Oil on Candida albicans Biofilm Formation
*Corresponding author: Zahra Jahanshiri, Department of Mycology, Pasteur Institute of Iran, Iran
Received: December 29, 2021; Published: January 13, 2022
DOI: 10.34297/AJBSR.2022.15.002098
Abstract
Candida albicans is one of the most important natural microbiotas in human. It is responsible for creating various types of candidiasis as opportunistic yeast. Biofilm formation is one of the pathogenic factors of Candida albicans that triggers infection from any surface on which it is formed. In this study, the effect of Juniperus virginiana essential oil (JVEO) on growth, germ tube production and biofilm formation against C. albicans ATCC 10231 was investigated. JVEO effectively inhibited C. albicans biofilm formation with a minimum inhibitory concentration (MBIC50) of <1250μg/ml. Moreover, it suppressed hyphal growth and germ tube and biofilm formation in that strain. Transcriptomic analysis carried out by qRT-PCR revealed that the expression of HWP1, ALS3 adhesion related genes and CPH1, CYR1, UME6, HGC1 and EED1 hypha formation and maintenance related genes were all suppressed by JVEO. Furthermore, in the cAMP-dependent protein kinase pathway, RAS1 was down regulated but EFG1 was not affected. These results suggest that JVEO may be a potential therapeutic treatment and control of Candida biofilm formation.
Keywords: Candida Albicans; Juniperus Virginiana; Biofilm; Gene
Research Article
Biofilms are special microbial structures that play significant roles in triggering infectious diseases and in different deviceassociated infections [1,2]. Candida species are considered to be the most important agents of fungal biofilm which cause infection. The biofilm formation on abiotic and biotic surfaces is shown to be the most important property of these species. Candida species are commensal organisms that are found in healthy individuals (25-75%), with any tissue damage and inflammation [3]. These species include 50-70% of the commensal organisms which may cause a wide range of superficial to life-threatening infections [4,5]. The presence of biofilm can aggravate the infection due to the establishment of a structure with high tolerance to antifungal agents comparing to planktonic cells and act as a resource for the cells to disseminate to different parts of the body, including the bloodstream. This leads to systemic infections. Moreover, C. albicans biofilm represents great resistance to main antifungals such as fluconazole and amphotericin B [6-8]. So, there exists an imperative need to investigate new antifungal compounds against C. albicans biofilms. Plants are rich sources of agents with biological effects as have been used for many years in traditional medicine. The natural extract or essential oil obtained from various plants contains many organic compounds with therapeutic usage [9,10]. Terpenoids, terpenes and aromatic substances are three main components of essential oils. From very ancient times these oily liquids have been shown to have strong activity against fungi and bacteria as reported in a number of studies [11,12]. It has been demonstrated that essential oils have significant role in the pharmaceutical industries; moreover, these can be measured as a principal alternative in controlling microorganisms including fungi [13]. Juniperus virginiana is a juniper which is distributed and native in the eastern of USA. J. virginiana is identified with aromatic smell and aptitude to prevent flying insects [14].
Ethanolic and superficial fluid extraction of J. virginiana has been shown to possess inhibitory effect against wood decay fungi [15]. Many studies have been done to demonstrate potential strategies for eradicating and controlling biofilm production in C. albicans [16]. Furthermore, various studies have been carried out to discover the compounds or products with natural base and antibiofilm or antifungal activity against C. albicans. For instance, Raut et al. [17] have identified eight terpenoids with inhibitory effect on mature biofilm of C. albicans. It has been reported that filamentation, intercellular adherence, and biofilm development were inhibited by phenazines produced by P. aeruginosa [18]. Wong et al. [19] have discovered a small molecule with activity against Candida spp both in vitro and in vivo. Adhesion is the initial step of biofilm formation that is followed by cell proliferation, hypha formation, extracellular matrix production, eventually, cell dispreading [20]. Numerous studies have been conducted on the effect of natural materials on cell adhesion, hypha formation and biofilm formation in C. albicans [21,22]. In the present study, we aimed to investigate the activity of Juniperus virginiana essential oil (JVEO) against growth and biofilm formation of C. albicans and we evaluated causal mechanisms by estimating the expression of the key genes involved in adhesion and biofilm formation.
Materials and Methods
C. albicans ATCC10231 was obtained from pathogenic fungi culture collection of the Pasteur Institute of Iran, cultured on Sabouraud dextrose agar (1% w/v peptone, 4% w/v dextrose, and 1.8% w/v agar) medium and incubated at 28°C for 48h. To prepare cell suspension, the strain was cultured in YPD (1% yeast extract, 2% peptone, and 2% dextrose) liquid medium in orbital shaker at 300C for 24h. Fungal cells were harvested and washed in sterile phosphate buffer saline (PBS). The cell suspensions were prepared in RPMI 1640 (Biosera, France) medium and used for liquid fungal cultures in quantitative antifungal and biofilm assays. Different concentrations (2500 to 78.12μg /ml) of the essential oil were diluted in methanol (MeOH) and used in all experiment in this study. Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of JVEO were carried out by the broth microdilution method according to CLSI standard M27-A3 [23]. Serial two-fold concentrations of JVEO (2500 to 78.12μg /ml) were prepared, and RPMI 1640 with MOPS (pH 7.0) was used to perform the test with final inoculums concentrations of 0.5-2.5×103 CFU/ml in 96-well tissue culture plate, incubated at 35°C for 48h. After incubation time, the optical density was measured at 600 nm for the analysis of minimum inhibitory concentrations (MICs) with a plate reader (BioTek, USA). MFC was measured by taking 50μl of content of each clear well including JVEO at a concentration above the MIC and culturing on YPD plates. The plates were incubated at 37°C for 48h [22]. Biofilm formation test was carried out using method describe previously by Ramage et al. [24] with slight modification. C. albicans ATCC 10231 was cultured on Sabouraud dextrose agar plates for 24 h at 37°C.
The fungal suspension at 1× 106 CFU/ml concentrations in RPMI-1640 was transferred (200 μl) into selected wells of flat bottom 96-well microtiter plates and incubated at 37°C for 90 min for initial adhesion. After incubation, non-adherent cells were removed and fresh RPMI 1640 medium containing different concentration of JVEO (2500 to 78.12 μg /ml) was added in a final volume of 200 μl per each well. The biofilm formation was determined after 24 h at 37°C using XTT assay. The metabolic activity was measured spectrophotometrically at 490 nm using a microplate reader (BioTek, USA). A time-kill study of C. albicans ATCC 10231 was carried out according to Quan et al. [25]. C. albicans cells were prepared at inoculums size of 1-5×105 CFU/ml in RPMI 1640 medium. The test solutions were arranged in 2 × MIC, 1× MIC and 1/2× MIC concentration of JVEO and incubated for 1, 2, 4, 8, and 24 h at 35°C with agitation in 200 rpm. A 50 μl aliquot was taken from all samples and serially diluted 10-fold in sterile saline and cultured on YPD agar medium for 48h at 35°C. Colony numbers were established after incubation. All experiments were done in triplicate. The effect of JVEO on germ tube formation of C. albicans was carried out according to the method described by D’Auria et al. [26]. C. albicans was cultured in YPD medium for 24h. The fungal cells were harvested and adjusted in concentration 1×105 Cells/ ml in RPMI 1640 medium and treated by different concentration of essential oil from 2500 to 78.12 μg/ml. The cultures were incubated in 37°C for 3h with mild shaking. The germ tube formation ability was determined by optical microscopy observation. About 250 cells were observed from treated fungal cells of each concentration. C. albicans 10231 cells (1.0×106 cells/ml) in RPMI-1640 were introduced into tissue culture flask and incubated in 37°C for 90 min. After incubation time, non-adherent cells were removed and adherent cells were treated with different concentrations (1250, 625 and 312.5 μg /ml) of JVEO in RPMI 1640 and incubated at 37°C for 24h [21]. After removing the medium, biofilm was lightly washed by sterile PBS buffer and the cells were scrapped from the bottom of the flask. The yeast cells were homogenized by glass beads. Total RNA was extracted using GITC (Guanidium isothiocyanate) reagent and treated with RNase-free DNase [27]. The cDNA was prepared using 1000 ng RNA (normalized with equal amount of RNA weight in all reactions) using Revert Aid M-MuLV reverse transcriptase according to the manufacturer’s protocol (Fermentase cDNA kit). The expression of selected genes was determined by Real-Time quantitative RT-PCR. The primers were shown in Table 1. The amplification was performed using the SYBR Green master mix in 25 μl reactions containing 20ng cDNA and 0.2μM of each primer by a Rotor gene 6000 (Corbett) sequence detection system. Realtime RT-PCR program included an initial incubation at 95°C for 10 s, followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 15 sec [21]. The reactions were repeated in triplicate. The gene expression was normalized by 18S rRNA gene, and the folding changes were determined using the relative threshold method (2−ΔΔCT). All data were analyzed by One way ANOVA using GRAPHPAD PRISM 6 (GraphPad Prism Software Inc, San Diego, CA, USA). The differences with P < 0.05 were considered to be significant.
Results and Discussion
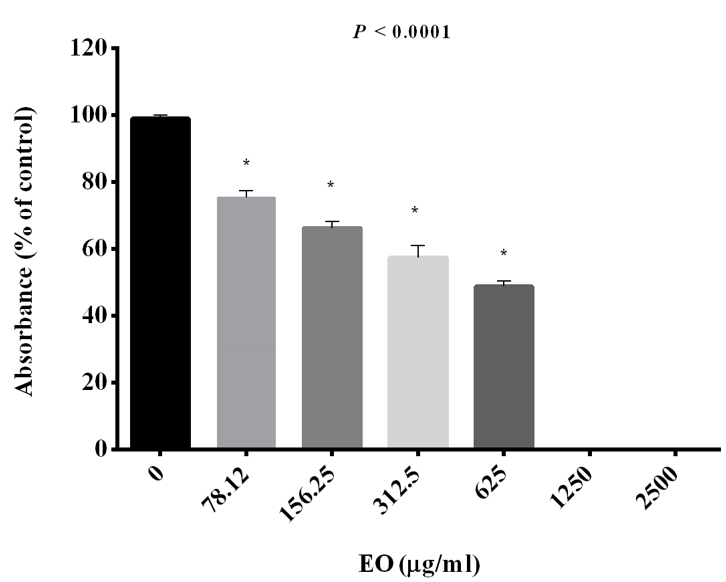
Figure 1: The effect of different concentration of JVEO on growth of C. albicans ATCC10231. Data are expressed as the mean ± standard deviation of the independent assays in triplicate. All samples compared to the EO-free sample as control.
This study shows that essential oil obtained from aerial parts of eastern red cedar, Juniperus virginiana L. (Cupressaceae), suppresses the growth and biofilm formation in C. albicans with emphasizing the expression of the key genes involved in adhesion and biofilm formation. C. albicans is considered the major fungal pathogen responsible for Candida infections in human [28]. Biofilm formation is one of the important pathogenic factors of C. albicans. It has been demonstrated that 65%–80% of Candida infections are related to the biofilm formation [29]. A special structure of biofilm is suitable as source of recurrence and represented tolerance to antifungal agents; so, the development of antifungal strategies to avoid biofilm formation or biofilm elimination has become a challenge [30]. Our results showed that essential oil at different concentration inhibited the growth of C. albicans ATCC10231. The results revealed that the fungal growth was inhibited dose dependently with increasing concentrations of the EO. JV essential oil was a potent growth inhibitor with an MIC and MIC50 of 1250 and 625 μg /ml respectively at 48h (Figure 1).
1250 μg /ml was MIC and MFC concentration of JVEO. The results of the time-kill assay revealed the strong fungicidal activity of JVEO against C. albicans ATCC 10231 in 1250μg/ml concentration and the fungicidal endpoint was observed in 2 h while amphotericin B showed slower value in 1μg/ml concentration which killed ˃99% after 24h (Figure 2). To evaluate the biofilm inhibitory activity of EO, we used XTT for measuring the metabolic activity of the yeast cells. (Figure 3) showed that JVEO inhibited biofilm formation after the adding of EO to yeast cells after 90 min of adhesion in compare with control (non treated). EO exhibited strong inhibitory effect on C. albicans biofilm formation in dose dependently manner, with the MIC of 625 < MBIC50 < 1250 μg/ml (Figure 3). The effect of EO on biofilm formation was improved as the concentration of EO was increased; therefore, no biofilm formation was observed in maximum concentration of JVEO (2500 μg/ml). Many studies revealed that natural compounds such as phytochemicals are able to inhibit biofilm formation in C. albicans [31-35]. They reported that the mechanism of action of these compounds were inhibition of the hypha formation and disruption of adhesion; as two essential steps of biofilm formation mechanism. Some studies have been carried out on the antimicrobial and antifungal effects of JVEO previously [12,15]. Here we investigated that JVEO inhibits growth, germ tube and finally biofilm formation in C. albicans. Moreover, Germ tube formation was evaluated as an important factor in biofilm formation in different concentration of EO. The results revealed that JVEO inhibits germ tube formation in different concentration of dose-dependently manner. However, at an EO concentration of 156.25 μg/ml, it revealed >50% inhibitory effect in germ tube formation (Figure 4) we showed that the anti-biofilm mechanisms of JVEO were reduction of the germ tube and hypha formation. To discover the molecular mechanism that affects morphological transition, we evaluated the expression of the genes involved in cell adhesion and yeast to hypha transition after treatment with different concentration (312.5, 625, 1250 μg/ml) of EO (Figure 5). The genes involved in hypha formation, cell adhesion, cAMP dependent protein kinase and MAP-kinase, include HWP1, EFG1, RAS1, ALS3, CPH1, ECE1, HST7, EED1, CYR1, UME6, HGC1. As shown in (Figure 5) JVEO strongly suppressed the transcription levels of hypha-specific genes such as HWP1, ECE1 and ALS3 in biofilm growth in dose-dependent manner.
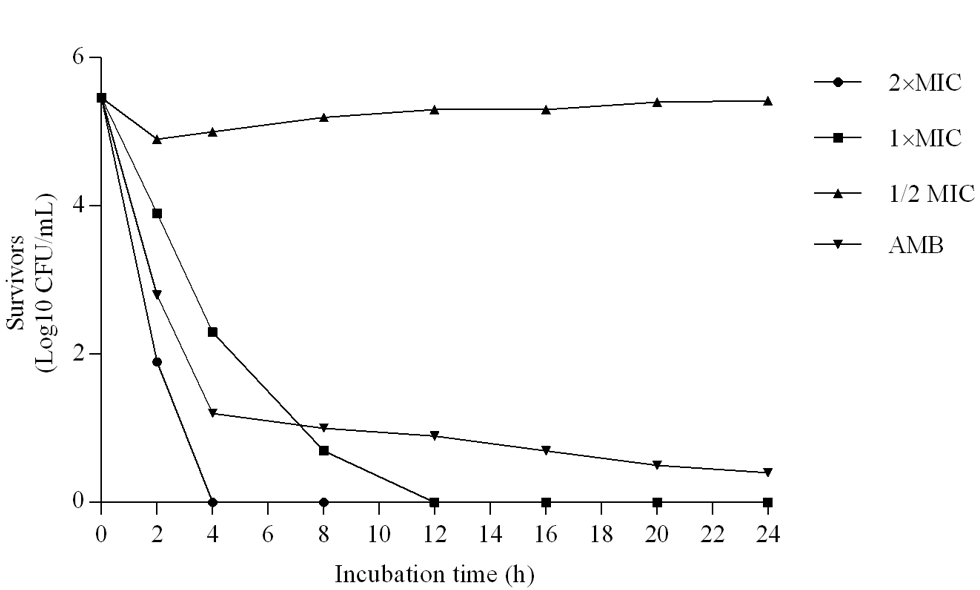
Figure 2: Time-kill curves for Candida albicans by JVEO and amphotericin B (AmB). 2×MIC, 1× MIC, 1/2MICof JVEO and 1μg/ml AmBtrol.
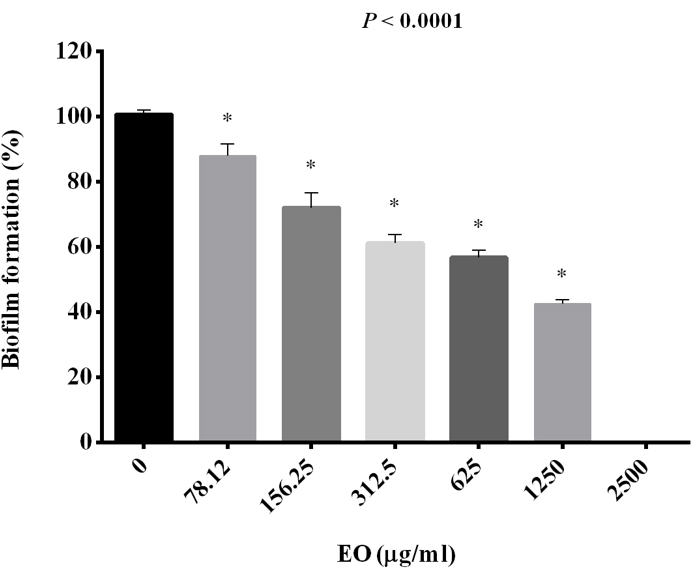
Figure 3: The effect of different concentration of JVEO on biofilm formation. Data represented the mean ± standard deviation of the independent assays in triplicate. All samples compared to the EO-free sample as control.
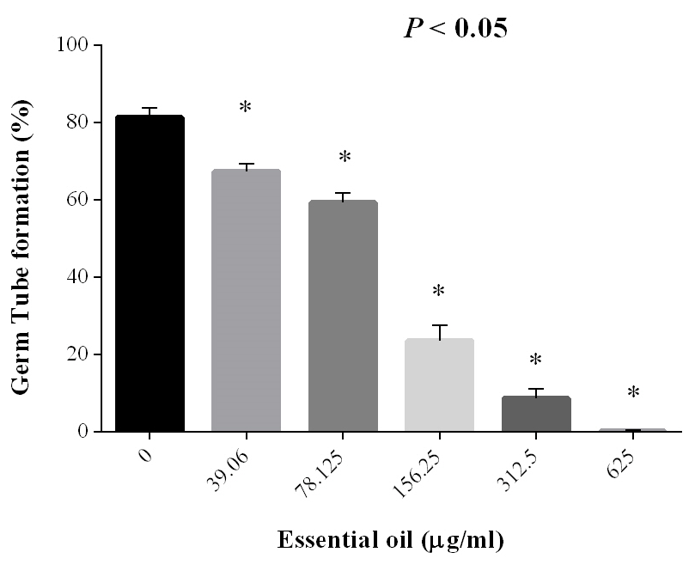
Figure 4: The inhibitory effect of JVEO on the germ tube formation of Candida albicans. The bars represent the mean ± SD values. Asterisks show statistically significant differences with the control.
It has been demonstrated that hypha formation contributes as one of the major virulence of C. albicans [36] and any disruption in this process leads to produce abnormal biofilm formation that is simply removed from the surface [37]. The first step of biofilm formation in C. albicans, is adhesion. Agglutinin-like sequence (ALS) is one of the families of adhesion molecules which include eight members (ALS1-ALS9). This family is expressed by ALS genes that encode cell wall glycosyl-phosphatidylinositol proteins which are required for the attachment of the yeasts to the surfaces [16,31]. Within this family, ALS3, due to its important role in biofilm production, has been shown to be of great importance among other genes in the family. Furthermore, ALS3, is represented to be highly expressed in oral epithelial cell infection [38,39]. HWP1, another adhesion protein, is a manoprotein which exists in the cell wall of hyphal cells and plays critical role in Candida biofilm formation [16,31] moreover, EAP1, SSA1 and SAPS are other principal proteins that play essential role in adhesion and invasion mechanisms [40].
The results revealed that among genes involved in the signal transduction pathways which lead to the expression of hyphaspecific genes, RAS1, CPH1 and CYR1 showed decreased expression although EFG1, HST1 were not affected. The genes involved in longterm maintenance of hyphal growth such as UME6, HGC1 and EED1 were down regulated after treatment with EO. Our results showed that the expression of important genes implicated in adhesion and hypha formation was affected by JVEO. Real-time PCR revealed that HWP1, RAS1, ALS3, ECE1, UME6 and HGC1 were down regulated strongly in dose dependently manner. RAS1, ALS3 and ECE1 represented high expression in the highest concentration. Different transcriptional genes regulate biofilm formation in C. albicans including: BCR1, TEC1 and EFG1 [41]. The genes consist of SAPs, LIP and PLBs also have essential role in colonization and disseminated infection by effect on the host cell membrane [42]. The data showed that the expression of CPH1, EED1 and CYR1 genes was suppressed but not as much as previous genes. The expression of EFG1 didn’t change much. HST7 showed increased relative expression in two concentrations (312.5 and 1250 μg/ml) (Figure 5). In general, qRTPCR indicated that treatment with JVEO can strongly affect significant genes in relation to hypha and biofilm formation in C. albicans. Terpinen-4-ol is an isomer of terpineol that is found in the essential oil of aromatic plants such as tea tree oil. It revealed biological properties such as antimicrobial, antioxidant and anti-inflammatory activity [43,46]. Here we studied on JVEO the highest percentage of which is reported to be Terpinen-4-ol previously [47]. Based on this, it can be concluded that the antibiofilm effect of the extract is due to the presence of this compound. Given the widespread prevalence of fungal infections, finding effective ways to more adequately treat patients is essential today. This is achieved by studying the various pathogenic properties of these species, including biofilm formation.
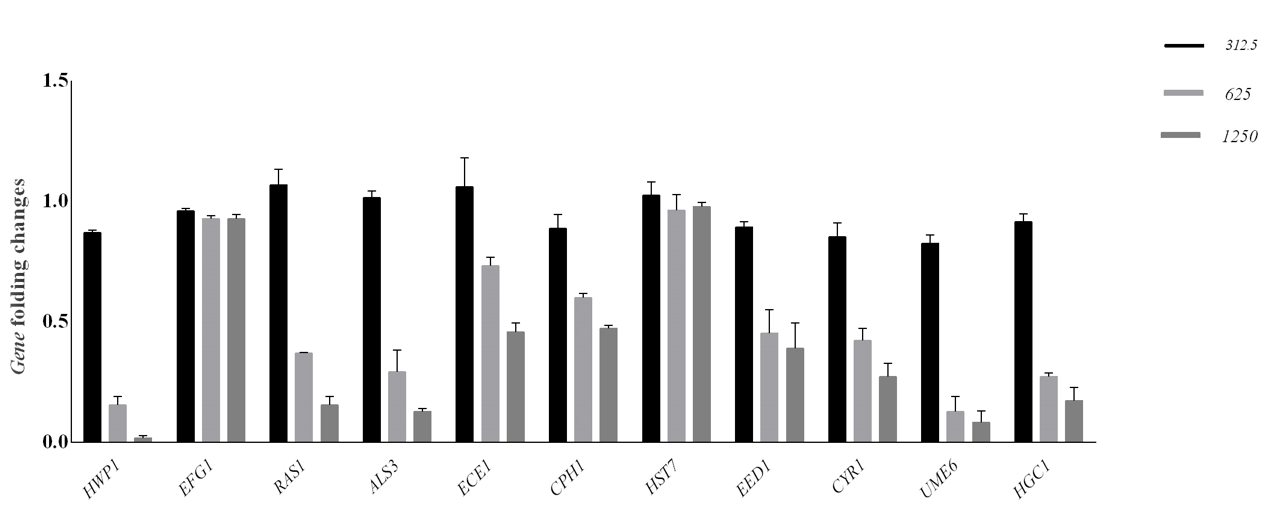
Figure 5: Transcription levels of biofilm involved genes in C. albicans after treatment with three concentrations of JVEO. The mRNA levels were normalized on the basis of their 18S rRNA levels. Significant differences were observed for mRNA levels for each of gene between the treated and the non-treated samples. Data are expressed as the mean ± standard deviation of the independent assays in triplicate.
Conclusion
Depending on the results, Juniperus virginiana essential oil represents potent activity against growth and germ tube formation of C. albicans; and inhibits biofilm formation in these fungi, it may be possible to use this essential oil for control and treatment of infections caused by Candida biofilm.
Acknowledgment
This work was supported by the Research Deputy of Pasteur Institute of Iran. This work was financially supported by Pasteur Institute of Iran. The authors wish to thank Dr. Fatemeh Arastehnazar from Iranian Blood Transfusion Organization for language editing.
Competing Interest’s Statement
The authors declare that they have no conflict of interest.
References
- Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis (8)9: 881-890.
- Hall Stoodley L, Costerton WJ, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2(2): 95-108.
- Barros PP, Ribeiro FC, Rossoni RD, Junqueira JC, Jorge AOC (2016) Influence of Candida krusei and Candida glabrata on Candida albicans gene expression in in vitro Arch Oral Biol 64: 92-101.
- Azie N, Neofytos D, Pfaller M, Meier Kriesche HU, Quan PS, et al. (2012) The PATH (Prospective Antifungal Therapy) alliance® registry and invasive fungal infections: update. Diagn Microbiol Infect Dis 73(4): 293-300.
- Pfaller AM, Diekema JD (2007) Epidemiology of invasive Candidiasis: a persistent public health problem. Clin Microbiol Rev 20(1): 133-163.
- Chandra J, Mukherjee KP, Leidich DS, Faddoul FF, Hoyer LL, et al. (2001) Antifungal resistance of candida biofilms formed on denture acrylic in vitro. J Dent Res 80(3): 903-908.
- Tobudic S, Lassnigg A, Kratzer C, Graninger W, Presterl E (2010) Antifungal activity of amphotericin B, caspofungin and posaconazole on Candida albicans biofilms in intermediate and mature development phases. Mycoses 53(3): 208-214.
- Nett E J, Sanchez H, Cain TM, Ross MK, Andes RD (2011) Interface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulation. Eukaryot Cell 10(12): 1660-1669.
- Nascimento G G F, Locatelli J, Freitas P C, Silva G L (2000) Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol 31: 247-256.
- Raina R, Verma P K, Peshin R, Kour H (2019) Potential of Juniperus communis L as a nutraceutical in human and veterinary medicine. Heliyon 5(8): 02376.
- Mondello F, De Bernardis F, Girolamo A, Cassone A, Salvatore G (2006) In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and –resistant human pathogenic Candida species. BMC Infect Dis 3(6):158.
- Cavaleiro C, Pinto E, Goncalves M J, Salgueiro L (2006) Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus and Candida strains. J Applied Microbil 100(6): 1333-1338.
- Bizzo RH, Hovell AMC, Rezende CM (2009) Essential oils in Brazil: general aspects, development and perspectives. Quim Nova 32: 588-594.
- Eller JF, Clausen CA, Green F, Taylor S L (2010) Critical fluid extraction of Juniperus virginiana L. and bioactivity of extracts against subterranean termites and wood-rot fungi. Ind Crops Prod 32: 481-485.
- Mun S P, Prewitt L (2011) Antifungal activity of organic extracts from Juniperus virginiana Heartwood against Wood Decay Fungi. For Prod J 61: 443-449.
- Mafalda C, Miguel CT (2018) Candida biofilms: Threats, Challenges, and Promising Strategies. Front Med 5:28.
- Raut J S, Shinde R B, Chauhan N M, Karuppayil S M (2013) Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling 29(1): 87-96.
- Morales D K, Grahl N, Okegbe C, Dietrich L E P, Jacobs N J, et al. (2013) Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. mBio 4(1): 00526-12.
- Wong S S, Kao R Y, Yuen K Y, Wang Y, Yang D, et al. (2014) In vitro and in vivo activity of a novel antifungal small molecule against Candida infections. PLoS One 9(1): e85836.
- Nobile JC, Mitchell PA (2005) Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol 15(2): 1150-1155.
- Yan Y, Tan F, Miao H, Wang H, Cao Y Y (2019) Effect of Shikonin Against Candida albicans Front Microbiol 10: e1085.
- Hsu C C, Lai W L, Chuang K C, Lee M H, Tsai Y C (2013) The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Med Mycol 51(5): 473-482.
- (2011) CLSI reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. Approved Standard, Clinical Laboratory Standards Institute (CLSI). CLSI document M27-A2.
- Ramage G, Saville S P, Wickes B L, Lopez Ribot J L (2002) Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol 68(11): 5459- 5463.
- Quan H, Cao Y Y, Xu Z, Zhao J X, Gao P H, et al. (2006) Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob Agents Chemother 50(3): 1096-1099.
- D Auria F D, Tecca M, Strippoli V, G Salvatore, L Battinelli, et al. (2005) Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Med Mycol 43(5): 391-396.
- Ho H C, Shiau, P F, Liu F C, Chung J G, et al. (1998) Purification, characterization and complete amino acid sequence of nuclease C1 from Cunninghamella echinulata echinulata. Eur J Biochem 256(1): 112-118.
- Tscherner M, Schwarzmüller T, Kuchler K (2011) Pathogenesis and antifungal drug resistance of the human fungal pathogen Candida glabrata. Pharmaceuticals 4: 169-86.
- Davies D (2003) Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2(2): 114-122.
- Kojic EM, Darouiche RO (2004) Candida infections of medical devices. Clin Microbiol Rev 17(2): 255-267.
- Braga P C, Culici M, Alfieri M, Dal Sasso M (2008) Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int J Antimicrob Agents 31(5): 472-477.
- Janeczko M, Maslyk M, Kubinski K, Golczyk H (2017) Emodin, a natural inhibitor of protein kinase CK2, suppresses growth, hyphal development and biofilm formation of Candida albicans. Yeast 34(6): 253-265.
- Alalwan H, Rajendran R, Lappin D F, Combet E, Shahzad M, et al. (2017) The Anti-Adhesive effect of curcumin on Candida albicans biofilms on denture materials. Front Microbiol 8: 659.
- Sapaar B, Nur A, Hirota K, Yumoto H, Murakami K, et al. (2014) Effects of extracellular DNA from Candida albicans and pneumonia-related pathogens on Candida biofilm formation and hyphal transformation. J Appl Microbiol 116(6): 1531-1542.
- Wu SC, Wang Y, Liu N, Dong G Q, Sheng C Q (2017) Tackling Fungal Resistance by Biofilm Inhibitors. J Med Chem 60(6): 2193-2211.
- Thompson D S, Carlisle P L, Kadosh D (2011) Coevolution of morphology and virulence in Candida species. Eukaryot Cell 10(9): 1173-1182.
- Nobile JC, Mitchell A P (2006) Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol 8(9): 1382-1391.
- Murciano C, Moyes LD, Runglall M, Tobouti P, Islam A, et al. (2012) Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS One 7(3): e33362.
- Zordan R, Cormack B (2012) Adhesins on opportunistic fungal pathogens. In RA Calderone, CJ Clancy (Eds.), Candida and candidiasis. ASM Press 243-259.
- Wachtler B, Wilson D, Haedicke K, Dalle F, Hube B (2001) From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. Plos One 6(2): 17046.
- Finkel J, Mitchell A (2011) Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 9(2): 109-118.
- Barros ML, Boriollo FM, Alves CA, Klein I M, Gonçalves B R, et al. (2008) Genetic diversity and exoenzyme activities of Candida albicans and Candida dubliniensis isolated from the oral cavity of Brazilian periodontal patients. Arch Oral Biol 53(12):1172-1178.
- Astani A, Reichling J, Schnitzler P (2010) Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother Res 24(5): 673-679.
- Wedge D E, Tabanca N, Sampson B J, Christopher Werle, Betul Demirci, et al (2009) Antifungal and insecticidal activity of two juniperus essential oils. Nat Prod Commun 4(1): 123-127.
- Cha JD, Jeong M R, Jeong S I, Sang Eun Moon, Bong Seop Kil, et al. (2007) Chemical composition and antimicrobial activity of the essential oil of Cryptomeria japonica. Phytother Res 21(3): 295-299.
- Barra A, Coroneo V, Dessi S, Cabras P, Angioni A (2007) Characterization of the volatile constituents in the essential oil of Pistacia lentiscus l. from different origins and its antifungal and antioxidant activity. J Agric Food Chem 55(17): 7093-7098.
- Tahghighi A, Maleki Ravasan N, Dinparast Djadid N, Alipour H, Ahmadvand R, et al. (2019) GC-MS analysis and anti-mosquito activities of Juniperus virginiana essential oil against Anopheles stephensi (Diptera: Culicidae). Asian Pac J Trop Biomed 9: 168-175.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.