Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Cardioprotective Property of Extracts of Dialium guineense Stem Bark in Rats Exposed to CCl4
*Corresponding author: Abu OD, Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria.
Received:August 16, 2022; Published: August 22, 2022
DOI: 10.34297/AJBSR.2022.16.002296
Abstract
The aim of this study was to investigate the cardioprotective property of extracts of Dialium guineense stem bark in rats exposed to carbon tetrachloride (CCl4). A total of twenty-five (25) adult male Wistar rats, which weighed between 170 and 190 g (mean weight = 180±10 g) were randomly assigned to five groups (5 rats per group): normal control, CCl4 control, silymarin, aqueous extract and ethanol extract groups. Cardiac injury was induced with CCl4 (single oral dose of 1.0 mL/kg body weight, bwt). Rats in the silymarin group were administered 100 mg silymarin/kg bwt (standard cardioprotective drug), while those in the two treatment groups received 1000 mg/kg bwt of aqueous or ethanol extract for a period of 28 days. Activity of aspartate aminotransferase (AST), level of nitric oxide (NO) as well as atherogenic index of plasma (AIP) were measured. Rat heart was also subjected to histopathological examination. The results showed that the activity of AST as well as levels of low-density lipoprotein cholesterol (LDL-C), AIP, atherogenic coefficient (AC) and cardiac risk ratio (CRR) were significantly higher in CCl4 control group than in normal control group, but they were markedly reduced after treatment with extracts of D. guineense stem bark (p < 0.05). However, the extracts significantly promoted the release of NO from the coronary endothelium of rats exposed to CCl4 (p < 0.05). Carbon tetrachloride (CCl4) markedly disrupted the normal architecture of rat’s hearts inducing fibrosis, necrosis, and haemorrhage. However, treatment with aqueous and ethanol extracts of the medicinal plant led to marked regeneration of cardiomyocytes. The toxic cardiac injury induced by CCl4 was significantly blocked by the plant extracts.
Keywords: Aspartate aminotransferase, Cardiac function, Dialium guineense, Nitric oxide, Tissue histology
Introduction
Cardiac injury induced by drugs is becoming an increasingly important concern, since it constitutes serious risk to human health [1]. Cardiac dysfunction caused by cardiotoxic agents such as CCl4 may lead to heart failure, myocardial ischemia, arrhythmias, hypertension, myocarditis, pericarditis, and thromboembolism [2]. Oxidative and nitrative stress, as well as protein adduct formation are established mechanisms of chemical-induced cardiotoxicity. The latter leads to cardiomyocyte inflammation, altered calcium homeostasis, programmed cell death (apoptosis), swelling of cardiomyocytes, nuclear splitting, vacuolization, and alteration in signaling pathways. These events result in diseases of the heart muscle including heart failure, which could lead to death [3].
Medicinal plants are sources of natural antioxidants which are used for the treatment of different ailments. These plants produce important therapeutic effects [4]. One of such plants is Dialium guineense. It is a tall, tropical, fruit-bearing tree, belonging to the Leguminosae family, and has small, typically grape-sized edible fruits with brown hard inedible shells. In Africa, it grows in dense forests along the southern edge of the Sahel [5]. The plant grows naturally in West African countries, Central African Republic, and Sudan [6,7]. Different parts of the medicinal plant are used against several diseases [5]. Extracts of the plant have been shown to be reservoirs of important phytochemicals [8-10]. At present little or nothing is known about the cardioprotective capacity of extracts of Dialium guineense stem bark. The aim of this study was to investigate the cardioprotective property of extracts of D. guineense stem bark in rats exposed to CCl4.
Materials and Methods
Chemicals
Analytical grade chemicals and reagents were used in this study, and they were purchased from Sigma-Aldrich Ltd. (USA).
Moreover, the patient had a history of prostate hypertrophy, ureterocele and bladder diverticula, whilst a previous urological examination revealed urinary retention. Due to suspicion of bladder papilloma’s, an appropriate urological examination was scheduled. Two months ago, the patient was admitted in a tertiary hospital with a diagnosis of prostatitis. However, the isolation and identification of a specific pathogen was not achieved. Ciprofloxacin and amikacin were prescribed as empirical antibiotic therapy.
Collection of Plant Material
The stem barks of D. guineense were procured from Auchi, Edo State, Nigeria. Identification and authentication of the plant took place at the herbarium of the Department of Plant Biology and Biotechnology, University of Benin, Benin City, Nigeria (No. UBHD330).
Plant Preparation and Extraction
The stem barks of D. guineense were washed and shade-dried at room temperature. The plant material was then pulverized using mechanical blender. Extraction of the stem bark was carried out as described in literature [11,12].
Experimental Rats
A total of twenty-five (25) adult male Wistar rats, which weighed between 170 and 190 g (mean weight = 180±10 g) were purchased from Anatomy Department, University of Benin, Benin City, Nigeria. The rats were housed in metal cages under standard laboratory conditions, and they were allowed free access to rat feed and clean drinking water. Prior to commencement of the study, the rats were acclimatized to the laboratory environment for a period of seven days. The study protocol was approved by the University of Benin Faculty of Life Sciences Ethical Committee on Animal Use.
Experimental Design
The rats were assigned to five groups (5 rats per group): normal control, CCl4 control, silymarin, aqueous extract and ethanol extract groups. Cardiac injury was induced with CCl4 (single oral dose of 1.0 mL/kg bwt). Rats in the silymarin group were administered 100 mg silymarin/kg bwt (standard cardioprotective drug), while those in the two treatment groups received 1000 mg/kg bwt of aqueous or ethanol extract for a period of 28 days.
Blood and Tissue Sample Collection and Preparation
At the end of the treatment period, the rats were euthanized. Blood samples were collected from the anesthetized rats via cardiac puncture in heparinized sample bottles. Rat hearts were also excised and used to prepare 20 % tissue homogenate. The blood and homogenate were centrifuged at 2000 rpm for 10 min to obtain supernatants which were used for biochemical analysis.
Biochemical Analyses
Activity of AST, and levels of NO and LDL-C were measured [13-15]. Atherogenic index of plasma (AIP), AC and CRR were also determined [16-17].
Histological Examination of the Tissues
Portions of the heart were serially sectioned and fixed in 10 % formalin for 48 h. The specimen was then dehydrated using varied concentrations of ethanol and cleared in three changes of xylene before embedment in paraffin. Serial sections (4 μm thick) were made and stained with haematoxylin and eosin (H&E) according to standard method. Histological assessment was performed under light microscopy using an image analyzer (Image Proplus, version 3.0).
Statistical Analysis
Count data are expressed as mean±SEM (n=5). The statistical analysis was performed using SPSS (version 20). Groups were compared using Duncan multiple range test. Statistical significance was assumed at p<0.05.
Results
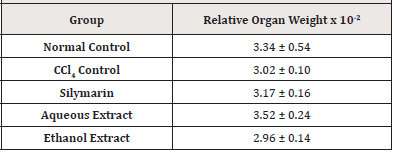
Effect of Extracts of D. guineense Stem Bark on Relative Organ Weight
There were no significant differences in relative organ weight among the groups (p > 0.05) (Table 1).
Note*: Data are relative organ weights and are expressed as mean ± SEM (n=5).
Effect of Extracts of D. guineense Stem Bark on Cardiac Function
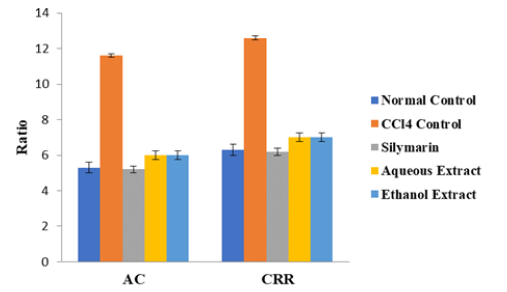
As shown in Figures 1-3, the activity of AST as well as levels of LDL-C, AIP, AC and CRR were significantly higher in CCl4 control group than in normal control group, but they were markedly reduced after treatment with extracts of D. guineense stem bark (p < 0.05). However, the extracts significantly promoted the release of NO from the coronary endothelium of rat’s hearts exposed to CCl4 (p < 0.05) (Figures 1 -4).
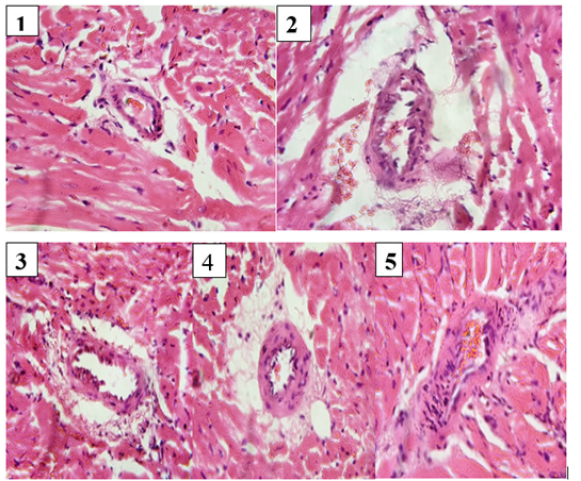
Histology of normal control rat heart revealed bundles of myocardial fibres, interstitial space, and coronary artery (normal cardiac architecture), while that of CCl4 control showed congested coronary artery, mild myocyte hypertrophy and fibrosis. Interstitial inflammatory cells infiltrate, necrosis and haemorrhage were visible. Histopathological examinations of silymarin and the two treatment groups revealed markedly reduced interstitial oedema and significant reduction in cellular debris. There was also mild collagen deposition.
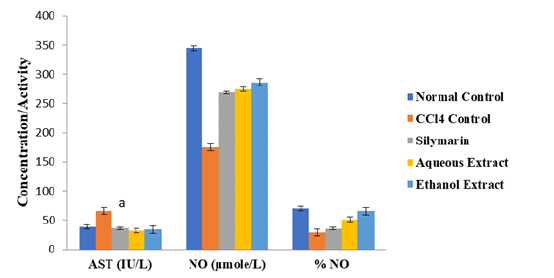
Figure 1:Effect of Extracts of D. guineense Stem Bark on Markers of Cardiac Function. Note*: Data are markers of cardiac function and are expressed as mean ± SEM. ap < 0.05, when compared with CCl4 control group.
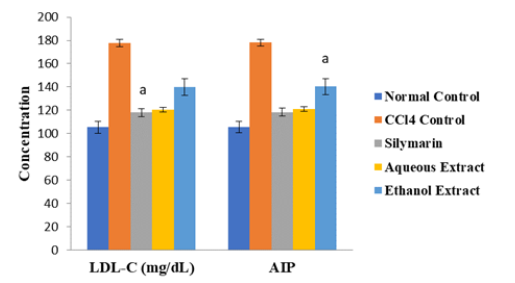
Figure 2:Comparison of the Levels of LDL-C and AIP Among the Groups. Note*: ap < 0.05, when compared with CCl4 control group.
Discussion
Carbon tetrachloride (CCl4) is a well-known substance for induction of tissue injury via generation of free radicals. The compound is highly toxic to organs/tissues such as liver, kidneys, heart, lung, testis, brain and blood [18]. It is biotransformed by hepatic microsomal cytochrome P450 to trichloromethyl radical, which initiates lipid peroxidation [19,20]. Free radicals’ formation, a rate limiting process in tissue peroxidative damage, is the generally accepted mechanism of CCl4-induced cardiotoxicity [21]. This leads to loss of myocardial structural integrity and depressed cardiac function [21]. The aim of this study was to investigate the cardioprotective property of extracts of D. guineense stem bark in rats exposed to CCl4. The results showed that CCl4 administration significantly increased lipid profile (LDL-C and AIP) and these results agree with those of previous reports [22]. Oxidative stress impairs the cell’s ability to remove free cholesterol as high-density lipoprotein cholesterol, thus increasing the amounts of cellular free cholesterol. Antioxidants have been shown to inhibit all these mechanisms [23]. A high LDL-C level is associated with increased risk for heart disease and stroke. Low-density lipoprotein (LDL) transports cholesterol to the arteries, and when its level is elevated, this cholesterol accumulates in the blood vessel walls and contribute to the formation of plaque [24]. Treatment with aqueous and ethanol extracts of D. guineense stem bark significantly decreased all the parameters of cardiovascular disease risk factors, measured in this study. The effects were comparable to those of the standard cardioprotective drug, silymarin. The protective property of the extracts may be due to the abundant phytochemicals which act as antioxidants [8,10&25]. Previous studies have elucidated the mechanistic association of antioxidant effect with hyperlipidemic cardiomyopathy [26,27].
Histological examination of the myocardium, valves and cardiac blood vessels is often as important as the gross examination. The diagnostic features and categories of heart disease are many and varied, possibly more than any other organ. In this study, histopathological findings of heart tissues further established the results regarding the effects of extracts of the medicinal plant on anatomical (heart mass index) and biochemical parameters. Heart sections of normal control rats revealed normal structures of heart, while rats exposed to CCl4 showed congestion, degeneration of myocardial tissue, and necrosis as a confirmation of cardiotoxicity. Reversals of such damage were observed in silymarin- and extracts-treated rats. It is likely the extracts possess antiplatelet activity. Platelets, platelet products and thombosis play important roles in the occurrence of acute occlusive vascular events, including myocardial infarction (MI) and ischemic stroke, since the disruption of platelet- and fibrin-rich atherosclerotic plaque may be followed by aggressive platelet deposition and, ultimately the development of a thrombus that can precipitate an acute occlusive event. Decreased platelet aggregation could be a plausible mechanism for the cardioprotective effects of the extracts [28].
Conclusion
The results of this study indicate that aqueous and ethanol extracts of D. guineense stem bark possess cardioprotective property against CCl4-induced cardiotoxicity. Further study aimed at the isolation, identification, and characterization of the active component(s) of the extracts and elucidation of their possible molecular mechanism of action is recommended.
References
- Dessalvi CC, Deidda M, Mele D, Bassareo P, Esposito R, et al. (2018) Chemotherapy-induced cardiotoxicity: new insights into mechanisms, monitoring, and prevention. J Cardiovascu Med 19(7): 315-323.
- Colombo A, Meroni, CA, Cipolla CM, Cardinale D (2013) Managing cardiotoxicity of chemotherapy. Curr Treat Options Cardiovasc Med 15(4): 410-424.
- Iqubal A, Iqubal MK, Sharma S, Mohd Asif Ansari, Abul Kalam Najmi (2019) Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: old drug with a new vision. Life Sci 218: 112-131.
- Shori AB (2015) Screening of antidiabetic and antioxidant activities of medicinal plants. J Integr Med 13(5): 297-305.
- Dalziel JM, Hutchison J (1973) Flora of West Tropical Africa. 1(2nd Edn.). The White friars Press Ltd pp. 561.
- Bero J, Ganfon H, Jonville MC, Frederich M, Gbaguidi F, et al. (2009) In vitro antiplasmodial activity of plants used in Benin in traditional medicine to treat malaria. J Ethnopharmacol 122(3): 439-444.
- Arogba SS, Ajiboro A, Odukwe I (2006) A physicochemical study of Nigerianvelvet tamarind (Dialium guineense L.) fruit. Journal of the Science of Food and Agriculture 66(4): 533-534.
- Abu OD, Onoagbe IO, Obahiagbon O (2020) Qualitative phytochemical screening and proximate analysis of Dialium guineense stem bark. IAR Journal of Agriculture Research and Life Sciences 1(4): 108-112.
- Abu O.D, Onoagbe IO, Obahiagbon O (2020) In Vitro Antioxidant Activities of Extracts of Dialium Guineense Stem Bark. American Journal of Sciences and Engineering Research 3(4): 68-75.
- Abu OD, Onoagbe IO, Obahiagbon O (2020) Phenolic contents of extracts of Dialium guineense stem bark. American Journal of Sciences and Engineering Research 3(4): 92-96.
- Abu OD, Imafidon KE, Iribhogbe ME (2015) Biochemical effect of aqueous leaf extract of Icacina trichanta Oliv. on urea, creatinine and kidney oxidative status in CCl4-induced Wistar rats. Nigerian Journal of Life Sciences 5(1): 85-89.
- Abu OD, Imafidon KE, Iribhogbe ME (2017) Aqueous leaf extract of Icacina trichanta Oliv ameliorates CCl4-induced liver toxicity in Wistar rats. Journal of the Nigerian Society of Experimental Biology 17(3): 107-111.
- Reitman S, Frankel SA (1957) Colorimetric method for the determination of serum glulamic oxaloacetic and glulamic pyranc transaminases. Am J Chin Pathol 28(1): 56-59.
- Marcocci L, Packer L, Droy Lefaix MT, Sekaki A, Gardes Albert M (1994) Antioxidant action of Ginkgo biloba extract EGb 761. Methods in Enzymol 234: 462-475.
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6): 499-502.
- Ikewuchi CJ, Ikewuchi CC (2009) Alteration of Plasma Lipid Profiles and Atherogenic Indices by Stachytarpheta jamaicensis L. (Vahl). Biokemistri 21(2): 71-77.
- Frohlich J, Dobiášová M (2003) Fractional Esterification Rate of Cholesterol and Ratio of Triglycerides to HDL-Cholesterol Are Powerful Predictors of Positive Findings on Coronary Angiography. Clinical Chem 49(11): 1873-1880.
- Adaramoye OA (2009) Comparative effects of vitamin E and kolaviron (a biflavonoid from Garcinia kola) on carbon tetrachlorideinduced renal oxidative damage in mice. Pak J Bio Sci 12(16): 1146-1151.
- Shenoy KA, Somayaji SN, Bairy KL (2001) Hepatoprotective effects of Ginkgo biloba against carbon tetrachloride induced hepatic injury in rats. Indian J Pharmacol 33: 260-266.
- Adewole S, Salako A, Doherty O, Naicker T (2010) Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. Afr J Biomed Res 10: 34-41.
- Plaa GL, Witschi H (1976) Chemicals, drugs, and lipid peroxidation. Annu Rev Parmacol Toxicol 16: 125-141.
- Swamy AHV, Patel UM, Koti BC, Gadad PC, Patel NL, et al. (2013) Cardioprotective effect of Saraca indica against cyclophosphamide induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Indian J Pharmacol 45(1): 44-48.
- Gesqui`ere L, Loreau N, Minnich A, Davignon J, Blache D (1999) Oxidative stress leads to cholesterol accumulation in vascular smooth muscle cells. Free Radic Biol Med 27(1-2): 134-145.
- Reiser R, Probstfield JL, Silvers A, Scott LW, Shorney, ML, et al. (1985) Plasma lipid and lipoprotein response of humans to beef fat, coconut oil and safflower oil. Am J Clin Nutr 42(2): 190-197.
- Abu OD, Onoagbe IO, Obahiagbon O (2020) Analyses of metal and amino acid compositions of aqueous and ethanol stem bark extracts of Dialium guineense. Journal of Biogeneric Science and Research 6(4): 1-3.
- Bhatt L, Sebastian B, Joshi V (2017) Mangiferin protects rat myocardial tissue against cyclophosphamide induced cardiotoxicity. J Ayurveda Integr Med 8(2): 62-67.
- Mythili Y, Sudharsan PT, Sudhahar V, Varalakshmi P (2006) Protective effect of d,l-alpha-lipoic acid on cyclophosphamide induced hyperlipidemic cardiomyopathy. Eur J Pharmacol 543(1-3): 92-96.
- Kamanyi A, Mbiantcha M, Nguelefack TB (2009) Antinociceptive and anti-inflammatory activities of extracts from the stem bark of Croton macrostachyus (Euphorbiaceae) in mice and rats. Journal of Complementary and Integrative Medicine 6(1): 1-17.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.