Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Chemical Profile and Anti-Metabolic Syndrome Potentials of Methanol Extract of Sida Acuta Leaves in Wistar Rats
*Corresponding author:Idakwoji PA, Department of Biochemistry, Faculty of Natural Sciences, Kogi State University, Anyigba, Nigeria
Received:July 27, 2022; Published: August 10, 2022
DOI: 10.34297/AJBSR.2022.16.002293
Abstract
This study investigated the possible ameliorative potentials of methanol extract of Sida acuta leaves (MESA) against high- fat diet (HFD) induced metabolic syndrome in rats. Forty (40) male albino rats were randomized into 4 groups of 10 animals each. Group 1 served as normal control and the rats were fed standard rat diet for 90 days. Rats in Group 2 were fed HFD for 90 days and served as negative control. Groups 3 and 4 rats were fed HFD for 90 days and administered 200 and 400mg/kg MESA concurrently for 90 days. The effects of treatment on some components of metabolic syndrome (hyperglycaemia, insulin resistance obesity and dyslipidaemia) were investigated through the measurement of body weight, fasting blood sugar (FBS), serum insulin level, total cholesterol (Tchol.), triacylglyceride (TAG), High density lipoprotein (HDL) and Low-density lipoprotein (LDL) concentrations. Results showed that administration of the extract at doses of 200 and 400mg/kg for 90 days significantly (p< 0.05) reduced body weight, FBS, insulin level, TAG and LDL as well as significantly (p< 0.05) increased serum levels of HDL. It was concluded that since, the methanol extract of Sida acuta leaves exerted beneficial effects on blood glucose, plasma insulin concentration, body weight, BMI and serum lipids of HFD-fed rats. It possesses significant anti-metabolic syndrome potentials.
Keywords: Metabolic Syndrome; High Fat Diet; Hyperglycaemia; Sida Acuta; Wistar Rats
Abbreviations: MESA: Methanol Extract of Sida Acuta Leaves; FBS: Fating Blood Sugar; BW: Body Weight; BMI: Body Mass Index; Tchol: Total Cholesterol; TAG: Triacylglyceride; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein
Introduction
Obesity, dyslipidemia, glucose intolerance, and coronary heart disease are all symptoms of metabolic syndrome, which lowers quality of life and raises mortality and morbidity risks [1]. The prevalence of metabolic syndrome has dramatically increased globally as a result of modern lifestyles [2] and an increase in the intake of high-sugar diets, particularly fructose [3]. According to[4] the prevalence of the metabolic syndrome and its components is influenced by a person’s genetic background, nutrition, degree of physical activity, use of tobacco, family history of diabetes, and educational attainment. The syndrome is made up of a number of variables, including insulin resistance, visceral obesity, atherogenic dyslipidemia, endothelial dysfunction, hereditary vulnerability, high blood pressure, hypercoagulable condition, and chronic stress. There are numerous well-established experimentally induced obesity models that can be used to examine a drug’s efficacy in treating obesity. However, the most relevant experimental model that represents human obesity is diet-induced obesity [5].
In this study we used a suitable animal model that mimics the symptoms of human metabolic syndrome to test the potential pharmacological properties of Sida acuta in the management of obesity, diabetes, hypertension and related metabolic disorders. Despite being readily available, modern pharmaceutical treatments for the treatment of dyslipidemia and cardiovascular diseases are expensive and have been found to have substantial side effects that discourage patient compliance. Therefore, there is need to find alternative therapies principally from herbal sources because compared to synthetic medicine they are cheap and having least side effects. Sida acuta belongs to the family Malvaceae. It is a weed that commonly dominates enhanced pastures, waste, and anxious places roadsides [6]. The plant is native to Mexico and Central America but has spread throughout the tropics and subtropics [7]. In traditional medicine, the plant is often alleged to treat diseases such as fever, headache, skin diseases, diarrhoea, and dysentery. The described pharmacological properties of the plants engage the anti-plasmodial, anti-bacterial, anti-fungal, antioxidant, cytotoxic activities, and many other properties. Some studies resulted in the isolation of single compounds while the others just demonstrated the activity of the crude extracts [8]. There is lack of available data justifying its role in protecting vascular endothelium damage in rats induced by high fat diet (HFD). Therefore, this experiment was designed to investigate the ameliorative effect of Sida acuta extract on HFD-induced metabolic syndrome in Wistar albino rats.
Materials and Methods
Plant Material
The leaves of Sida acuta were sourced from its natural habitat in Dekina area of Kogi State and identified by Mr. Gbenga Akanni of Biological Science Department, Lokoja, Kogi State.
Chemicals and Drugs
Methanol (BDH, England) and all other chemicals used were purchased from Sigma Chemical, St. Louis USA. A digital glucometer and its corresponding test strips (Fine Test®, Infopia Co., Ltd. USA) were purchased from HealthSeal® pharmacy store in Lokoja Local Government Area, Kogi State.
Preparation of Methanol Leaves Extract of Sida acuta
The leaves of Sida acuta were rinsed in order to remove debris, dried under shade for 5 days and subsequently pulverized using a manual blender. The resulting powdered material was extracted with methanol using cold maceration method. After 72h, the mixture was filtered using Whatmann filter paper (No 1) to obtain the filtrate. The filtrate was concentrated and evaporated to dryness on a hot water bath at 45℃ to obtain the methanol extract of Sida acuta Leaves (MESA).
Phytochemical Screening
The phytochemical composition of the extract was determined using the methods of Sofowara [9].
Acute Toxicity Study
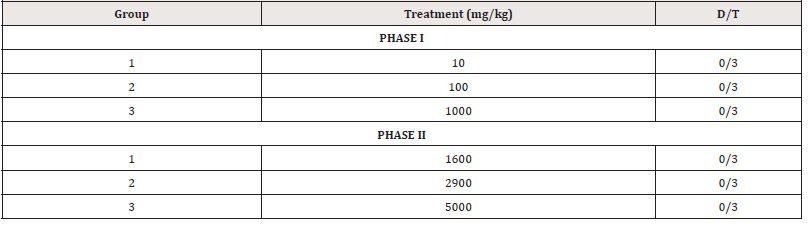
The oral median lethal dose (LD50) of the extract was determined in rats according to the method described by Lorke [10].
Experimental Design
Forty (40) albino rats were randomized into 4 groups of 10 animals each and fed/ treated as follows:
Group 1:Animals were fed with a standard rat die (30% protein, 35% carbohydrates, 25.5% fat and 9.5% crude fibre) for 90 days.
Group 2:Animals were fed with HFD (15.5% protein, 18.5% carbohydrates, 62.5% fat and 3.5% crude fibre. Fat component was derived from margarine) for 90 days.
Group 3:Animals were fed with HFD for 90 days but treated with MESA (200 mg/kg/day) concurrently for 90 days.
Group 4:Animals were fed with HFD and treated with MESA (400 mg/kg/day) concurrently for 90 days.
Measuement of Body Weight and BMI
Body weight and body length Body (measured as nose-anus
length) were measured at the beginning and end of the study. They
were used to calculate the body mass index (BMI) of the rats as
follows:
Body mass index (BMI) = body weight (g)/ length2 (cm2).
Determination of Fasting Blood Sugar
Fasting blood sugar was assessed by the using a glucometer (Fine Test®) with its corresponding strips.
Prior to assessing fasting blood glucose, the animals were fasted overnight, but were allowed free access to water.
Estimation of Serum Lipid Profile
On the 28th day of the experiment, all the rats were euthanized by chloroform inhalation and blood samples were collected by cardiac puncture. The blood was collected into plain serum tubes, allowed to clot and centrifuged for 10 minutes at 3500rpm. Total cholesterol (TC), triacylglycerol (TAG) and high-density lipoprotein (HDL) concentrations will be determined according to the method of16 while low density lipoprotein (LDL) levels will be calculated using Friedwald equation [11].
Plasma insulin determination
This was done according to the method described by Engvall and Perlmann [12].
Statistical analysis
All data were expressed as Mean ± SD and statistical differences between means were determined by one- way ANOVA followed by Duncan post –hoc test for multiple comparison tests using SPSS. Values were considered significant at p≤0.05.
Results
Phytochemical Screening
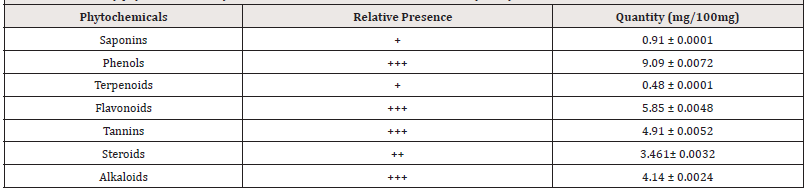
The Preliminary phytochemical analysis of the extract revealed the presence of saponins, phenols, terpenoids, flavonoids, tannins, steroids, and alkaloids in varying quantities (Table1) and (Table 2).
Key: + Slightly Detected, ++ Moderately Detected, +++ Highly Detected, ND- Not Detected.
D/T: Number of deaths/ number of rats treated.
Effect of the methanol extract of Sida acuta leaves (MESA) on FBS of High- fat Diet- fed Rats
The effect of MESA on the FBS of high- fat diet- fed rats is shown in Table 3. After 90 days of feeding the rats in the negative control with the high- fat diet, the group showed significant (p≤0.05) increase in FBS compared to normal control rats. Treatment with MESA at 200 and 400mg kg for 90 days significantly (p≤0.05) reduced FBS compared to negative control. There was no significant (p>0.05) difference in FBS of rats treated with MESA (400mg/ kg) compared to normal control (Table 3).
Effect of the methanol extract of Sida acuta leaves (MESA) on Body Weight (BW) of High- fat Diet- fed Rats
After 90 days of feeding the rats in the negative control with the high- fat diet, the group showed significant (p≤0.05) increase in BW compared to normal control rats. Treatment with MESA at 200 and 400mg kg for 90 days significantly (p≤0.05) reduced BW compared to negative control (Table 4).
Effect of the methanol extract of Sida acuta leaves (MESA) on Body Mass Index (BMI) of High- fat Diet- fed Rats
The effect of MESA on the BMI of high- fat diet- fed rats is shown in Table 5. Feeding of rats in the negative control with the high- fat diet for 90 days produced a significant (p≤0.05) increase in BMI compared to normal control rats. MESA at 200 and 400mg kg dosedependently and significantly (p≤0.05) reduced BMI compared to negative control (Table 5).
Effect of the methanol extract of Sida acuta leaves (MESA) on the serum lipid profile of High- fat Diet- fed Rats
The effects of MESA on the serum lipid profile of high-fat dietfed rats are presented in Table 6. The mean concentrations of the total cholesterol (Tchol), triacylglyceride (TAG), high density lipoprotein (HDL) and low-density lipoprotein (LDL) of the negative control were significantly higher (P<0.05) than those of the normal control. MESA at both doses used significantly decreased (P<0.05) serum concentrations of Tchol., TAG and LDL with a corresponding increase in HDL compared to negative control. There was no significant (p>0.05) difference in effects of MESA (400mg/ kg) compared to normal control (Table 6).
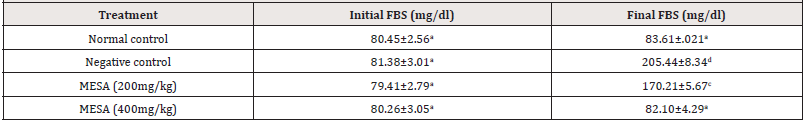
Table 3:Effect of the methanol extract of Sida acuta leaves (MESA) on FBS of High- fat Diet- fed Rats.
Data is represented as mean ± S.D. Mean values having different lowercase letters down the columns as superscripts are considered significant (p< 0.05).
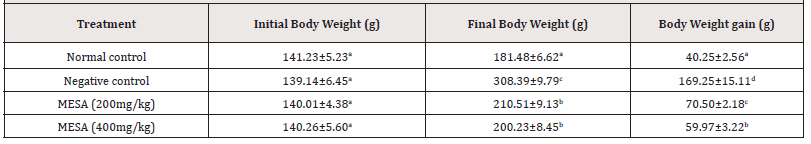
Table 4:Effect of the methanol extract of Sida acuta leaves (MESA) on Body Weight (BW) of High- fat Diet- fed Rats.
Data is represented as mean ± S.D. Mean values having different lowercase letters down the columns as superscripts are considered significant (p< 0.05).
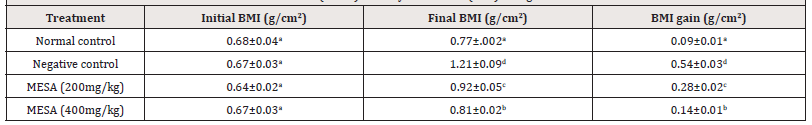
Table 5:Effect of the methanol extract of Sida acuta leaves (MESA) on Body Mass Index (BMI) of High- fat Diet- fed Rats.
Data is represented as mean ± S.D. Mean values having different lowercase letters down the columns as superscripts are considered significant (p< 0.05).
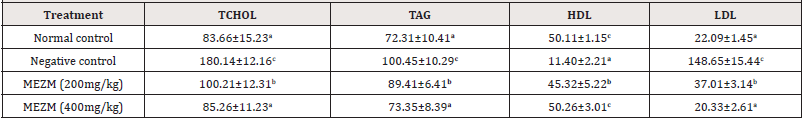
Table 6:Effect of the methanol extract of Sida acuta leaves (MESA) on the serum lipid profile of High- fat Diet- fed Rats.
Data is represented as mean ± S.D. Mean values having different lowercase letters down the columns as superscripts are considered significant (p< 0.05).
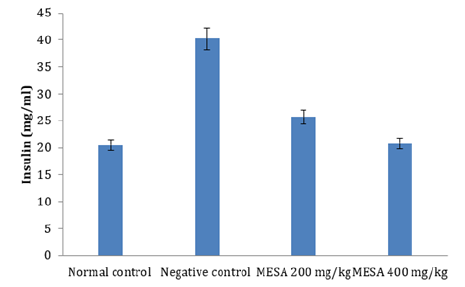
Effect of the methanol extract of Sida acuta leaves (MESA) on Plasma Insulin Level of High- fat Diet- fed Rats
The effect of MESA on the insulin level of high- fat diet- fed rats is shown in Figure 1. 90 days of feeding of the negative control rats with the high fat diet, significantly (p≤0.05) increased the insulin level compared to normal control. Treatment with 200 and 400mg/ kg of MESA for 90 days produced a significant (p<0.05) decrease in insulin level compared to negative control. There was no significant (p>0.05) difference in plasma insulin of rats treated with MESA (400mg/ kg) when compared to normal control (Figure 1).
Discussion
The metabolic syndrome is a serious health issue globally. Rapid urbanization, surplus energy intake, increasing obesity, and sedentary life habits are the major factors responsible for metabolic syndrome. Metabolic syndrome increases the risk of developing type 2 diabetes mellitus, cardiovascular diseases, stroke and death. Effective preventive approaches include lifestyle changes, primarily weight loss, diet, and exercise, and the treatment comprises the appropriate use of pharmacological agents to reduce the specific risk factors [13-16]. Traditionally, many medicinal plants have been used to treat the individual components of the metabolic syndrome. In this study we used a suitable animal model that mimics some symptoms of human metabolic syndrome to test the potential beneficial properties of the methanol extract of Sida acuta leaves.
Preliminary phytochemical analysis of the extract revealed the presence of tannins, alkaloids, saponins, terpenoids, steroids, and flavonoids in varying proportions. Over the last decades, the role of these phytochemicals in features of metabolic syndrome has been extensively investigated. Due to their multiple properties, these plant-derived natural compounds have demonstrated to provide positive effects in obesity, diabetes, renal and in cardiovascular diseases. The effectiveness of the extract investigated in this study could be attributed to the presence of these phytochemicals.
The acute toxicity study of the extract (10-5000mg/kg) produced no significant physical signs of toxicity such as writhing, weakness, anorexia, gasping, and palpitation, reduction in body weight, decreased respiratory rate or death in both phases of the study. Hence, the oral median lethal dose (LD50) of the extract was therefore estimated to be greater than 5000 mg/kg. The implication of this is that the extract is safe when consumed acutely even at a high dose. However, long term toxicity studies are required to establish the safety of this extract.
This study confirmed that HFD might be a good way to initiate insulin resistance [11,17] which is the consequence of a number of defects including impaired insulin secretion by the pancreatic cell, resistance of peripheral tissues to the glucose utilizing effect of insulin and augmented hepatic glucose production [18,19]. This was evident in this study as an increase in FBS and plasma insulin concentration was observed in rats fed with HFD. In this study, intake of HFD for 90 days also significantly increased the body weight and BMI. Dyslipidaemia was also observed, and this could be as a result of an increase in the influx of greater amounts of non-esterified fatty acids to liver causes an increase in triacylglycerol level which may cause dyslipidemic change [20]. It has been demonstrated that changes in lipid concentrations and lipoprotein fractions are associated with the increased risk for obesity-related metabolic conditions. It has also been found that obesity is a significant risk factor for the development of cardiovascular diseases [21,22].
Treatment of HFD- fed rats with 200 and 400 mg/kg of methanol extract of Sida acuta leaves for 90 days, significantly reduced the FBS and insulin concentration. This observation could possibly be attributed to the relatively high antioxidant activity of the leaves. The extract might have also produced its antihyperglycaemic activity through the release of insulin by inhibiting the ATPsensitive potassium channels in the membrane of the residual beta cells just like sulfonylureas and meglitinides. It is also possible that the extract might have potentiated the action of insulin to stimulate glucose uptake and utilization by tissues, especially by the liver, skeletal muscle, and adipose tissue [23].
In this study, the HFD also increased the body weight and BMI of rats. This observed increase could be as a result of fat deposited due to variations between the energy intake and energy expenditure in the rats. Treatment with 200 and 400mg/kg of the extract effectively reduced the body weight and body mass index of the obese rats. In view of this, we can safely say that the methanol extract of Sida acuta leaves is capable of reversing obesity, a major factor in metabolic syndrome. Elevated levels of TC, TAG and LDL with concomitant reduction in HDL characterize the dyslipidemic changes reported for HFD. Indeed, TC, TAG, LDL and HDL indicate disordered lipid metabolism and predisposition to cardiovascular disease. These alterations could predispose the risk of developing atherosclerosis and cardiovascular diseases while reduction in HDL cholesterol could intensify the development of atherosclerosis and cardiovascular diseases. Indeed, results of this study revealed that administration of 200 and 400 mg/kg of the extract had a favorable effect on the lipid profile of HFD- fed rats as it decreased serum concentrations of TC, TAG and LDL and also increased HDL serum concentration as compared to negative control. The observed reduction in serum cholesterol may be attributed to the levels of polyphenolic (flavonoids, tannin and saponins) compounds present in the extract. The reversal of HFD-fed rats mediated alterations in lipid profile by the extract suggests anti-dyslipidemic activity.
Conclusion
The methanol extract of Sida acuta leaves exerted beneficial effects on blood glucose, plasma insulin concentration, body weight, BMI and serum lipids of HFD-fed rats. Therefore, the extract possesses significant anti-metabolic syndrome potentials.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Reddy SS, Karuna R, Baskar R, Saralakumari D (2008) Prevention of insulin resistance by ingesting aqueous extract of Ocimum sanctum to fructose-fed rats. Horm Metab Res 40(1): 44-49.
- Hydrie MZI, Basit A, Shera AS, Hakeem R, Hussain A (2010) Dietary patterns associated with risk for metabolic syndrome in urban community of Karachi defined by cluster analysis. Pak J Nutr 9(1): 93-99.
- Anoop Misra, Lokesh Khurana (2008) Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 93(11): 9-30.
- Adrian J Cameron, Jonathan E Shaw, Paul Z Zimmet (2004) The metabolic syndrome: prevalence in worldwide populations Endocrinol Metab Clin North Am 33(2): 351-375.
- M Tschöp, M L Heiman Rodent (2001) obesity models: an overview. Exp Clin Endocrinol Diab 109(6): 307-319.
- Mann A, Gbate M, Umar AN (2003) Sida acuta subspecie acuta. Medicinal and economic plant of Nupeland, Jube Evans Books and Publication p. 241.
- Holm LG, Plucknett DL, Pancho JV, JP Herberger (1977). The World’s Worst Weeds: distribution and biology. University Press of Hawaii, Honolulu, USA.
- Ajeet, Singh, Navneet (2018) Pharmacological Application of Sida acuta (Burm). Pharmacological Benefits of Natural Products. 1st Edn, Chapter-9: 144-155
- Sofowara A (2006) Medical plants and traditional medicine in Africa. Reprint edition, Spectrum Books Ltd., Ibadan.
- Lorke D (1983) A new Approach to Practical Acute Toxicity Testing. Archives of Toxicology 54(4): 275-287.
- Satsuki Tanaka, Tatsuya Hayashi, Taro Toyoda, Taku Hamada, Yohei Shimizu, et al. (2007) High-fat diet impairs the effects of a single bout of endurance exercise on glucose transport and insulin sensitivity in rat skeletal muscle. Metabolism 56(12): 1719-1728.
- Nawel Hamza, Bénédicte Berke, Catherine Cheze, Abdel Nacer Agli, Philip Robinson, et al. (2010) Prevention of type 2 diabetes induced by high fat diet in the C57BL/6J mouse by two medicinal plants used in traditional treatment of diabetes in the east of Algeria. J Ethnopharmacol 128(2): 513-518.
- Alberti KGMM, Robert H Eckel, Scott M Grundy, Paul Z Zimmet, James I Cleeman, et al. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; And international association for the study of obesity. Circulation 120(16): 1640-1645.
- K George MM Alberti, Paul Zimmet, Jonathan Shaw, IDF Epidemiology Task Force Consensus Group (2005) Themetabolic syndrome - a new worldwide definition. Lancet 366(9491): 1059-1062.
- Jobien K Olijhoek, Yolanda van der Graaf, Jan Dirk Banga, Ale Algra, Ton J Rabelink, et al. (2004) The Metabolic Syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur Heart J 25(4): 342-348.
- Darwin Deen (2004) Metabolic syndrome: time for action. Am Fam Physician 69(12): 2875-2882.
- Anne M Flanagan, Jackie L Brown, Consuelo A Santiago, Pauline Y Aad, Leon J Spicer, et al (2008) High-fat diets promote insulin resistance through cytokine gene expression in growing female rats. J Nutr Biochem 19(8): 505-513.
- G I Shulman (2007) Cellular mechanisms of insulin resistance. J Clin. Invest 106(2): 171-176.
- Baquer NZ (1998). Glucose over utilization and underutilization in diabete sand effects of antidiabetic compounds. Anales de la Real Academia de Farmacia 64: 147-180.
- Scott M Grundy (2004) Obesity, metabolic syndrome and cardiovascular disease. J Clin Endo Metab 89(6): 2595-2600.
- Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z (2004) Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes 28(4): 58-65.
- Martin Fried, Vojtĕch Hainer, Arnaud Basdevant, Henry Buchwald, Mervyn Deitel, et al. (2008). Interdisciplinary European guidelines on surgery for severe obesity. Obes Facts 1(1): 52-59.
- Gerich JE (2000) Physiology of glucose homeostasis. Diabetes Obes Metab 2(11): 345-350.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.