Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Chronic Obstructive Pulmonary Disease (COPD) as a Systemic Disease: A Multiparametric Approach
*Corresponding author:Ornella Salimbene, Department of Environment, Land, and Infrastructure Engineering (DIATI), Politecnico of Torino, 10129, Italy.
Received: April 25, 2022; Published: May 09, 2022
DOI: 10.34297/AJBSR.2021.15.002210
Abstract
The World Health Organization predicts that Chronic Obstructive Bronchopathy Disease (COPD) will become the third leading cause of death in 2030 worldwide. COPD, is a ‘preventable and treatable’ pathology, characterized by an air flow limitation that is not entirely reversible. Flow restriction is generally progressive and associated with a pulmonary inflammatory response correlated with major risk factors such as tobacco smoke, followed by occupation and air pollution. The purpose of this research is to investigate and evaluate the correlations between the entity of bronchopulmonary disease and the inflammation index (CRP), antioxidant potential (CoQ10) and the cardiac overload index (NT-proBNP). To develop this research protocol, 26 patients with different severity of COPD (BODE Index) were enrolled. The experimental results show a direct correlation between antioxidant potential and oxygen content in the blood, an inverse correlation between cardiac overload and distance traveled in meters (6-minute walking test). Finally, all patients showed a systemic inflammatory state directly proportional to the state of blood oxygenation. Although the study included a limited number of patients and lacked a control group, it was possible to identify an ideal model of patients with COPD from which to draw valid conclusions.
Keywords: COPD; C-reactive protein (CRP); Coenzyme CoQ10; NT-proBNP
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide [1,2]. In the United States, the Third National Health and Nutrition Survey (NHANES II) reveals a prevalence of 6.9% for mild COPD and 6.6% for the Moderate COPD in people between 25 and 75 years old. The highest occurrence is in males and increases with age [3]. Many authors showed a prevalence of about 18% of obstructive pulmonary airflow disease in an Italian population of 4353 patients. It is estimated that the prevalence of COPD will increase globally in the coming decades due to the increase in smoking, especially in developing countries [2,4,5]. Furthermore, the percentage of hospitalizations increases proportionally to a lower socio-economic status [6,7]. According to the Legambiente report ‘Mal’Aria 2018’ (Europe calls, Italy responds) one of the main causes of the increase in the disease is also air pollution due to natural factors and especially human activities.
The main sources of pollutant emissions are the production of energy and the transformation of fuels, road transport, nonindustrial combustion, the use of solvents, production processes and the treatment and disposal of waste. Exposure to particulate matter at a young age causes tracheitis, bronchitis, asthma, in percentages ranging around 24%, acute exposure causes exacerbations. In adults, chronic exposure causes asthma, chronic obstructive pulmonary disease (COPD) and neoplasia not only of the lung but also of other parts of the body. At the state of the art, there is sufficient scientific evidence that for every increase of 10 micrograms per cubic meter of Pm2.5 there is in the long term in Italy a 7% increase in total mortality, 10% in cardiovascular and respiratory mortality, 9% of that for lung cancer (Department of Pneumology, Mauritian Hospital, Turin). COPD has a very important socio-economic impact in society, estimates by the National Heart Lung and Blood Institute (INHLB) show an annual cost of $ 23.9 billion for COPD, more than half for health services provided, 4.7 billion for morbidity, 4.5 billion related to premature mortality. Hospitalization remains the main economic burden [8].
COPD- Systemic Inflammatory Disease
The chronic inflammatory process that affecting the peripheral airways and it is responsible for the structural alteration that occurs during COPD is well-known [9]. The American Thoracic Society and the European Respiratory Society underline the systemic inflammatory nature of COPD, referring to the definition of the Global Initiative on Obstructive Lung Diseases (GOLD), which underlined for the first time the concept of ‘inflammatory pathology’ [4].
Several studies analyzing the plasma levels of various markers have highlighted the presence of systemic inflammation in COPD. Gan WQ, et al. [10] noted the correlation between COPD, FEV1 or FVC and the levels of systemic inflammatory makers, such as C-reactive Protein (CRP), fibrinogen, tumor necrosis factor (TNF-α), interleukin-6 (IL-6) and white blood cell count [10]. Broekhuizen et al., [11] emphasized a more compromised energy metabolism, an increased disability, demonstrated by a reduced effort tolerance and a more marked respiratory stress in those COPD patients with a CRP plasma level> 4.21 mg/L, regardless of the entity of the airflow obstruction, expressed in terms of FEV1. Moreover, patients with increased CRP levels, show a reduced post-bronchodilator FEV1, compared to patients with normal CRP levels due to reduced reversibility in FEV1 after inhalation of a Beta-agonist [11].
COPD- Multi-Dimensional Disease
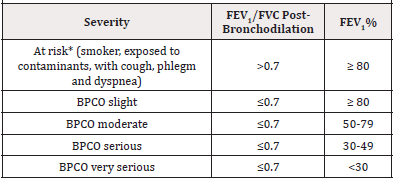
American Thoracic Society (ATS) and the European Respiratory Society (ERS) guidelines are recommended to evaluate the severity of COPD through a single physiological measure (Table 1) the forced expired volume in 1 sec (FEV1) [1]. This functional parameter is an excellent predictor of lung morbidity and mortality.
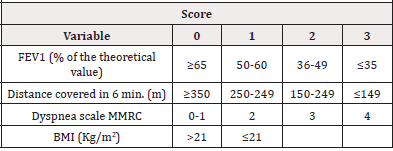
However, COPD is a heterogeneous and complex disease, in fact it is associated with other alterations and clinical manifestations not strictly related to the severity of the air flow limitation. Several studies highlight a weak correlation between the severity of the air flow limitation and the reduction of effort capacity [12], pulmonary arterial hypertension [13] and the weakness of the peripheral musculature [14]. To highlight parameters capable of assessing the severity of COPD and predicting the outcome of the disease, Celli et al., 2006, demonstrated that a new multi-dimensional staging system, such as the BODE index (Body-mass index, degree of airflow Obstruction and Dyspnea, and Exercise capacity index) is able to evaluate the respiratory and systemic expressions of the disease, better than observed only with FEV1. The BODE index is determined through the following variables (Table 2)
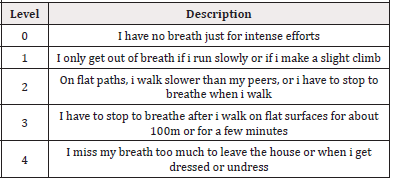
Table 2.Dyspnea Scale. Mahler DA et al. Am Rev. Respir Dis 1987,135:1229 (adapted by Salimbene I, et al., 2020).
a) Body Mass Index (BMI), expressed Kg/m2 b) FEV1, expressed in % of the theoretical c) Distance covered in 6-minute Walking Test (6mWT), expressed in meters d) Medical Research Council dyspnea modified scale (MMRC)
Using these variables and the score respectively attributed to each one, it is possible to define the BODE Index as shown in the Table 3.
Based on all the parameters expressed in Table 3 are defined
four groups of patients as following
1. Group 1 (score from 0 to 2)
2. Group 2 (score from 3 to 4)
3. Group 3 (score from 5 to 6)
4. Group 4 (score from 7 to 10)
Celli BR et al. [1], highlighted how patients with BODE index 4 have a mortality rate of 80% estimated at 52 months. In addition, the authors pointed out that through the BODE index it is possible to predict the risk of death at 0.74%, compared to the value of 0.65% obtained using the FEV1 as an analysis parameter for the risk of death [15]
Oxidative Stress in COPD
COPD increases the risk of cardiovascular disease by 2-3 points [16]. Various studies have shown that the function of the endothelium in these patients is abnormal in both lungs [17] and in the renal systemic circulation [18,19], cigarette smoking is a clear risk factor for both COPD and cardiovascular disease. Some authors suggest that the persistent low level of systemic inflammation that occurs in COPD, may significantly contribute to the pathology of these cardiovascular abnormalities. If so, this may have important therapeutic implications in the management of these patients, since anti-inflammatory therapy would bring benefits not only for the chronic pulmonary inflammatory process, but also for the prevention of cardiovascular diseases. The Lung Heath Study showed that 25% of deaths in the USA are due to cardiovascular disease (patients aged around 50, with FEV1 equal to 79% of the predicted). Systemic inflammation associated with COPD can play a fundamental role in the comorbidities associated with this condition. Possible explanations are the following: the systemic dissemination of local lung inflammation, the hypoxiainduced production of inflammatory mediators and the effects of pulmonary hyperinflation [20]. Rabinovich et al. [22] have shown that moderate exercise in COPD results in an increase in the inflammatory response in the muscle [21,22].
So skeletal muscle could be a resource for systemic inflammation in COPD; tissue hypoxia and sedentary lifestyle would seem implicated. Inflammatory markers implicated in COPD, which systematically increase are IL-6, IL-8, TNFα and fibrinogen [23-25]. Particular attention was paid to reactive protein C, a reagent that in the acute phase is produced by the liver in response to circulating IL-6, used in serum as a biomarker of systemic inflammation. CRP levels are associated with an increase in mortality in patients with COPD and in the general population. In stable COPD patients there is also an inverse correlation between CRP and airway obstruction and BMI [26,27]. The increase in CRP is an independent risk factor for coronary artery disease, myocardial infarction and stroke [28], in addition CRP is associated with a worse prognosis in acute coronary syndrome [29]. CRP is produced by the hepatocytes stimulated by IL-6. It is generally ascertained that IL-6 is elevated in response to vascular damage and associated inflammatory response, CRP is also found in atheromatous lesions and can also have a causal role in atherogenesis [30]. It is important to underline that no study has significantly proven the hypothesis that the increase in oxidative stress in COPD increases cardiovascular risk.
Oxidative stress is understood as an imbalance between the production of reactive O2 species (ROS: including free radicals, reactive oxygen and nitrogenic species) and protective antioxidants (superoxide dismutase and glutathione peroxidase). Oxidation causes apoptosis, cell destruction and necrosis, but also increases inflammation through the activation of inflammation of gene expression for inflammatory mediators and adhesion molecules. Oxidative stress increases with the severity of air obstruction, as measured by the products of lipid peroxidation in the sputum induced in patients with COPD [31]. Like inflammation, also pulmonary oxidative stress, associated with COPD, extends systematically.
Coenzyme Q10 as the Main Indicator of Oxidative Stress
Coenzyme Q (vitamin Q or ubiquinone), is an organic molecule and more precisely a quinone with isoprenic side chains, similar in structure to vitamin K and E. It is present in cereals, soy, nuts, vegetables, meat, fish, vegetable oils, wheat germ and its introduction with food is essential. Blood levels of coenzyme Q10 are higher among vegetarians, it is an essential molecule to produce ATP, but also an important antioxidant in the intra-extra cellular compartments. According to various hypotheses, the coenzyme Q supplement may be able to block the oxidative stress related to the aging process. Various studies have shown that CoQH2-10 is one of the antioxidants that decreases first in the condition of increased oxidative stress.
Since the latter is defined as an alteration of the oxidant/ antioxidant balance in favor of the former, the% of CoQ-10 on the total coenzyme Q10 (% CoQ-10) may well indicate the degree of oxidative stress. Thanks to the simultaneous discovery in plasma of CoQ10 and CoQH2-10, with simple and reliable measurement methods it has been shown how oxidative stress increases in patients with COPD, but also hepatitis, coronary artery disease, Parkinson’s and amyotrophic lateral sclerosis. Coenzyme Q10 has also been used in cancer with brilliant results [32], in muscular dystrophy, in angina pectoris [33].
Materials and Methods
As described in the abstract section, the aim of the research is to deepen the understanding of the relationships between the entity of the lung disease and the inflammation index, antioxidant potential (CoQ10) and cardiac overload index (NT-proBNP) by investigating it in a group of COPD patients with different BODE index. In this regard, 26 patients (23 males and 3 females) with stable COPD of different severity, were treated at home with inhalation therapy with bronchodilators and/or steroids. The patients (PTS) being treated at the ‘Agostino Gemelli’ hospital in Rome (Italy) and included in the research protocol, were organized into three groups according to the BODE index.
The first group included 11 patients with BODE index between 0 and 2, the second group included 7 patients with BODE index between 3 and 4, and the third group included 8 patients with BODE index between 5 and 10 (Figure 1). The choice of combining patients belonging to the BODE 3 and 4 group (according to the classification of Celli et al. [1] in a single group, was dictated by the small size of the sample and therefore by the need to quantitatively homogenize the three study groups.
The demographic characteristics and standard deviation (SD)
of the patients are summarized as follows:
a) average age = 66 years (SD 10)
b) average weight = 76.2 kg (SD 11.1)
c) average height = 1.68 m (SD 0.07)
d) average body mass index (BMI) = 27 Kg/m2 (SD 3.7)
Subjects with one or more of the following criteria were excluded from the study: evidence of left ventricular failure and valvulopathy, history of coronopathy, diabetes, evidence of neurological diseases, lung and upper airway infection in the previous 4 weeks, concomitant restrictive pneumopathy, serum creatine> 2.5 mg/dl or dialysis, systolic blood pressure <85 mmHg, pregnancy, addiction to alcohol or drugs, taking specific drugs (hypnotics, anxiolytics, psychosis, antidepressants, nausea, dizziness, antipileptics, drugs for parkinsonism, for dementia) (Figure 1).
Study Design Method
The assessment of the performance of the study for each patient was organized into 3 days (from day -1 to day 1) and is summarized as follows:
Day -1
a. Evaluation of lung function (global spirometry with helium
dilution method, pharmacological bronchodilation test, carbon
monoxide diffusion test)
b. Determination of nutritional status through BMI
c. Evaluation of effort tolerance, through 6-mininute walking test
d. Assessment of dyspnea through the use of the modified UK
Medical Research Council scale
e. Evaluation of gas exchange with blood gas analysis;
Electrocardiographic evaluation
f. Self-completion of the health questionnaire (S. George)
g. Determination of the BODE Index
Day 0
1) Collection of venous blood for NT-proBNP and reactive protein
C
2) Positioning of the pulse oximeter for dynamic monitoring
Day 1
Blood Gas Analysis: The dosage of the hormone NT-proBNP and reactive protein C, takes place using a nephelometric method. Plasma levels of CoQ10 were measured by high resolution liquid chromatography (HPLC) method, proposed by the International CoQ10 Association [34]. The reference values are 0.7 -1 μg/mL. The statistical analysis was carried out by analyzing the variance between the groups. The T student was used for paired data for intra-subject changes, instead linear regression for correlation analyzes.
Results and Data
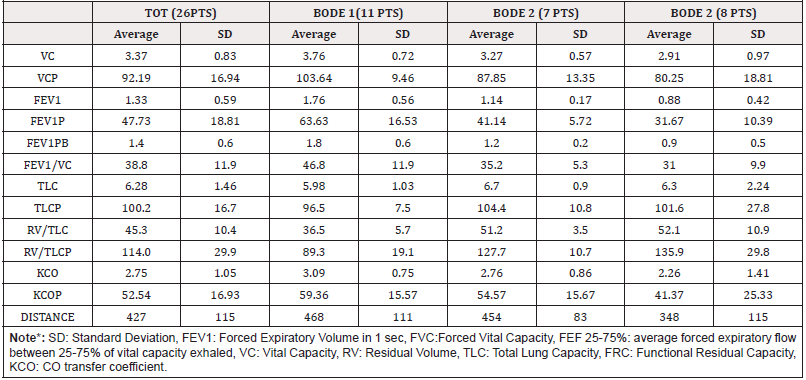
Severe airflow obstruction was observed in all groups with the following specific characteristics: an average forced expiratory volume in 1 sec (FEV1) equal to about 48% compared to the theoretical value, a reduction in transfer of CO (expression of capillary bed compromise), an average distance in the norm during the execution of 6-minute walking test. There is no air trapping (RV/TLC as % of the predicted equal to 114.0, Table A).
The gas exchanges are moderately compromised with a mild hypoxemia and normocapnia, as evidenced by the results reported in Table B relating to the blood-analytical and saturimetric indices. Overall, Table C highlights the inflammatory state, the extent of cardiac overload, the antioxidant potential and the thyroid function of the patients, with respect to the severity index of lung disease. The average NT-proBNP value of the total sample (26 PTS) is 88.1 pg/mL (SD= 82.7), moreover the average CRP value of the total sample is 6.2 mg/L (SD= 5.4), it is an expression of a systemic inflammatory state.
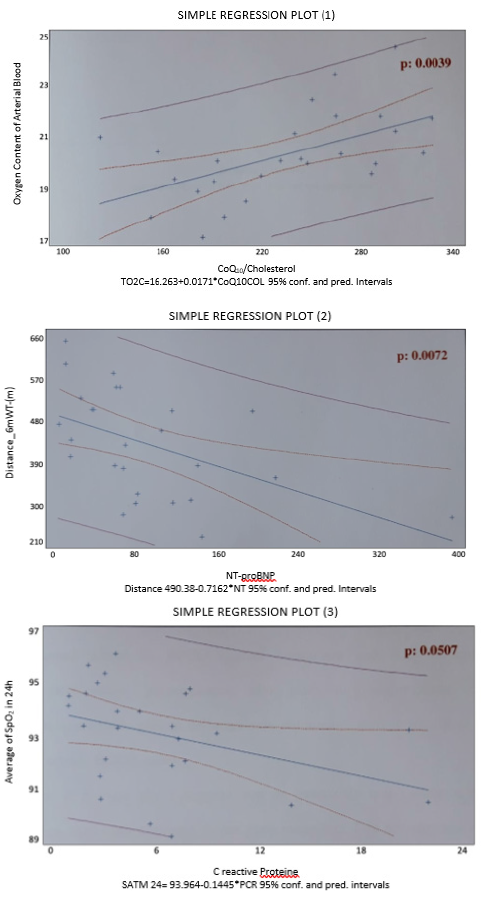
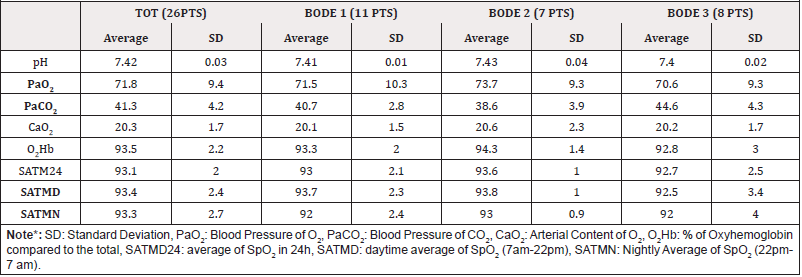
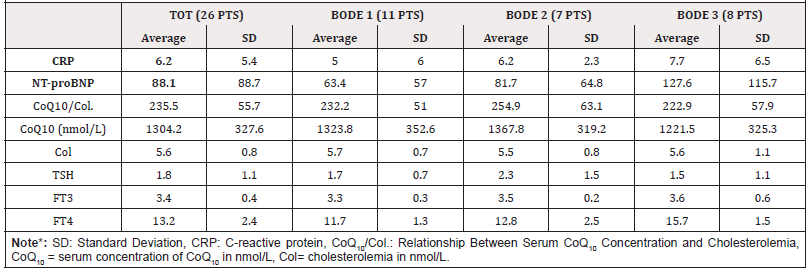
From the analysis of the parameters and the comparison of the three considered groups (Table B) considered, the following results emerged: the three groups do not differ for PaO2 (the respective mean values are 71.5 mmHg, 73.7 mmHg and 70.9 mmHg), the patients in the BODE 3 group have a significantly higher PaCO2 (44 mmHg) than the BODE 1 group (40.7 mmHg, p= 0.011) and 2 (38.6 mmHg, p = 0.011); in general the average nocturnal saturation is significantly reduced, compared to the daytime (92.3 mmHg vs 93.4 mmHg, p= 0.046) especially in the subjects of the BODE 2 group (92.0 vs 93.7 mmHg, p= 0.024). The three groups have an overlapping inflammatory state, they do not differ in terms of cardiac overload and antioxidant potential. Figure 2 shows the graphs of simple regression plot with the correlation of the following parameters a) The arterial content of O2 and coenzyme Q10 (corrected for cholesterolemia, simple regression plot 1) b) The distance covered in meters during 6 mWT and the cardiac overload index (simple regression plot 2) c) The oxyhemoglobin saturation in 24h and the systemic inflammatory state (simple regression plot 3).
Conclusions and Implications
It has been concluded that COPD patients studied in this pilot study have a systemic inflammatory state indirectly proportional to the blood oxygenation state, regardless of the severity of lung disease, estimated using the multi-parametric BODE index system. In addition, subjects with better antioxidant potential have been found to have higher arterial oxygen content. This direct correlation is present regardless of the severity of COPD. It is not possible, however, to estimate the extent of the oxidative stress of the population under study, as the serum concentration of CoQ10 reflects the sum of the reduced (the true antioxidant) and oxidized portions. Finally, the population considered shows cardiac overload inversely related to the distance traveled in meters, (during the execution of the 6-min. Walking test).
Several studies have shown an increase in type B natriuretic peptide in COPD patients [35] in particular have highlighted the possibility of using the plasma concentration of type B natriuretic peptide (BNP) as a prognostic marker and screening parameter for pulmonary arterial hypertension (PH) in patients with chronic lung disease. More precisely, Leuchte et al. [35] studying the ability of BNP levels to predict the presence of significant PH (defined by the presence of mean pulmonary arterial pressure> 35 mmHg), observed that a plasma BNP threshold value of 33 pg/mL, presents a sensitivity rate of 0.87 and a specificity rate of 0.81 [35]. The research conducted is limited to the small number of patients recruited to the lack of a control group and to the impossibility of using the serum dosage of coenzyme Q10 as a measure of oxidative stress. Nonetheless, the use of the highly sensitive nephelometric method for the serum CRP dosage and the multiple exclusion criteria adopted, aimed at eliminating those pathologies that influence the outcomes of interest, have allowed to identify an ideal model of COPD patients from which to draw valid observations.
Acknowledgments
The authors would like to acknowledge the support of the Dept. of Pneumology of the Policlinico ‘Agostino Gemelli’ of Rome (Italy), Dr. Giuseppe Maria Corbo and Dr. Valente Salvatore for the supervision, collaboration and organization of the study protocol.
Conflict of Interest
None.
References
- Celli B R, Mac Nee W (2006) Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal 27(1): 242.
- Murray CJL, Lopez Ad (1997) Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 349(9064): 1498-1504.
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC, et al. (2002) Chronic obstructive pulmonary disease surveillance-United States 1997-2000. MMWR Surveill Summ 51(6): 1-16.
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, et al. (2001) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. American J Respir Crit Care Med 163(5): 1256-1276.
- Feenstra TL, Van Genugten MLL, Hoogenveen RT, Wouters EF, Rutten Van Molken, et al. (2001) The impact of aing and smoking on the future burden of chronic obstructive pulmonary diseses. Am J Respir Crit Care Med 164(4): 590-596.
- Vestbo J, Prescott E, Lange P (1996) Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 153(5): 1530-1535.
- Prescott E, Vestbo J (1999) Socioeconomic status and chronic obstructive pulmonary disease. Thorax 54: 737-741
- (1996) Division of Epidemiology, National Heart, Lung and Blood Institute.
- Hoggs JC (1993) Bronchiolitis in asthma and chronic obstructive pulmonary disease. Clin Chest Med 14(4): 733-740.
- Gan WQ, Man SF, Senthilselvan A, Sin DD (2004) Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 59(7): 574-580.
- Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM (2006) Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 61(1): 17-22.
- Hay JG, Stone P, Carter J, Church S, Eyre Brook A, et al. (1992) Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary disease. European Respiratory J 5(6): 659-664.
- France A J, Prescott RJ, Biernacki W, Muir AL Mac Nee W, et al. (1988) Does right ventricular function predict survival in patients with chronic obstructive lung disease?. Thorax 43: 621-626.
- Decramer M, Gosselink R, Troosters T, Verschueren M, Evers G, et al. (1997) Muscle weakness is related to utilization of health care resources in COPD patients. European Respiratory Journal 10(2): 417-423.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Orca M, et al. (2004) The Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index in Chronic Obstructive Pulmonary Disease. New England Journal of Medicine 350(10): 1005-1012.
- Sin DD, Paul Man SF (2003) Why Are Patients with Chronic Obstructive Pulmonary Disease at Increased Risk of Cardiovascular Diseases? The Potential Role of Systemic Inflammation in Chronic Obstructive Pulmonary Disease. Circulation 107(11): 1514-1519.
- Dinh Xuan AT, Higenbottam TW, Clelland CA, Pepke Zaba J, Cremona G, et al. (1991) Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N Engl Journal Med 324(22): 1539-1547.
- Howes TQ, Deane CR, Levin GE, Baudouin SV, Moxham J, et al. (1995) The effects of oxygen and dopamine on renal and aortic blood flow in chronic obstructive pulmonary disease with hypoxemia and hypercapnia. Am J Respir Crit Care Med 151(2 Pt 1): 378-383.
- Baudouin SV, Bott J, Ward A, Deane CR, Moxham J, et al. (1992) Short term effect of oxygen on renal haemodynamics in patients with hypoxaemic chronic obstructive airways disease. Thoracic 47(7): 550-554.
- Wouters EF (2005) Local and systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2(1): 26-33.
- Agusti AG (2003) Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 21(2): 347-360.
- Rabinovich RA, Ardite E, Troosters T, Carbo N, Alonso J, et al. (2001) Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164(7): 1114-1118.
- Palange P, Testa U, Huertas A, Calabro L, Antonucci R, et al. (2006) Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J 27(3): 529-541.
- Schols AM, Buurman WA, Staal Van Den Brekel AJ, Dentener MA, Wouters EF, et al. (1996) Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 51(8): 819-824.
- Gan WQ, Man SFP, Senthilselvan A, Sin D (2004) Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 59(7): 574-80
- Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM (2006) Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 61(1): 17-22.
- Sin DD, Man SF (2003) Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 107(11): 1514-1519.
- Luc G, Bard JM, Juhan Vague I, Ferriers J, Evans A, et al. (2003) C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol 23(7): 1255-1261.
- Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB, et al. (1997) Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet 349(9050): 462-466.
- Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, et al. (2000) C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol 20(9): 2094-2099.
- Paredi P, Kharitonov SA, Leak D, Ward S, Cramer D, et al. (2000) Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 162(2 Pt 1): 369-373.
- Gaby AR (1996) The Role of Coenzyme Q 10 in Clinical Medicine: Part I. Altern Med Rev 1: 11-17.
- Kamikawa T, Kobaiashi A, Yamashita T, H Hayashi, N Yamazaki, et al. (1985) Effects of coenzyme Q10 on exercise tolerance in chronic stable angina pectoris. Am J Cardiol 56(4): 247-251.
- Mosca F, Fattorini D, Bompadre S, Littarru GP (2002) Assay of coenzyme Q10 in plasma by a single dilution step. Anal Biochem 305(1): 49-54.
- Leuchte HH, Baumgartner RA, Nounou MEI, Michael Vogeser, Claus Neurohr, et al. (2006) Brain Natriuretic Peptide Is a Prognostic Parameter in Chronic Lung Disease. Am J Resp Crit Care Med 173(7): 744-750.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.