Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
In Vitro Characterization of a Novel Medical Device in Simulated Vaginal Environment
*Corresponding author:Giulia Schiavo, Farmaceutici Damor Spa, Via emilio scaglione 27, Napoli, NA, 80123, Italy.
Received: July 17, 2022; Published: August 01, 2022
DOI: 10.34297/AJBSR.2022.16.002288
Abstract
Bacterial vaginosis, aerobic vaginitis and candidiasis are the most common infections in women in fertile age, characterized by an imbalanced vaginal microbiota population and vaginal discharge. The therapeutic approach could vary from antibiotics to antiseptics with the risk of developing resistance. For this reason, a novel hydrogel formulation, Fitostimoline® Septagel, has been developed for the local treatment of irritative-inflammatory-dystrophic states of the vaginal area. The aim of this study was to evaluate, by an in vitro assay, the efficacy of Fitostimoline® Septagel on the most common vaginitis and vaginosis pathogen.
Agar spot overlay test and the survival of Streptococcus agalactiae, Escherichia coli and Candida albicans pathogens were detected in a simulated vaginal model. Furthermore, to determine the physical properties of Fitostimoline® Septagel, pH, viscosity and the swelling index of the gel were analyzed.
This study demonstrates the in vitro effectiveness of Fitostimoline® Septagel as an adjuvant treatment of vaginitis and vaginosis. The results show that Fitostimoline® Septagel has a positive effect on the pH of the environment in which it is introduced, it maintains its hydrogel properties for the entire duration of the application, guaranteeing adhesion to the place of application and counteracting the proliferation of pathogens of bacterial and fungal origin.
Introduction
This study demonstrates the in vitro effectiveness of Fitostimoline® Septagel as an adjuvant treatment of vaginitis and vaginosis. The results show that Fitostimoline® Septagel has a positive effect on the pH of the environment in which it is introduced, it maintains its hydrogel properties for the entire duration of the application, guaranteeing adhesion to the place of application and counteracting the proliferation of pathogens of bacterial and fungal origin.
AV presents an highly symptomatic inflammatory state with a yellow vaginal discharge and a peculiar smell. BV and AV are generally determined by a vaginal pH beyond the healthy vaginal pH range of 3.8 – 4.5, above 4.5 [5-8].
BV affects women in their reproductive age and can either be symptomatic or asymptomatic. Approximately 50% of women are symptomatic and experience vaginal malodor, discharge, itching and increased vaginal pH. BV can increase the risk of contracting many sexually transmitted infections [9].
An estimated 75% of women experience at least one in the life episode of vulvovaginal candidiasis, and 40 to 45% two or more either [10]. The initial symptoms of the different types of vaginitis are the same, such as local itching and burning, leukorrhea, dysuria, and dyspareunia. The first line therapeutic approach, that is frequently based on self-medication [11], should aim to treat these symptoms with formulations able to block the pathologic progression without inducing bacteria resistance [12]. In order to achieve this purpose, a novel hydrogel formulation, Fitostimoline® Septagel, a medical device based on 0.1% Polyhexanide biguanide (PHMB) and Rigenase®, has been developed by the research and development team of Farmaceutici Damor indicated for the local treatment of irritative-inflammatory-dystrophic states of the vaginal area (vaginitis/vaginosis).
Hydrogels are hydrophilic polymers able to swell in physiological environment as they are made of three-dimensional viscoelastic networks that retain water many times their dry weight [13,14]. The effectiveness of a gel formulation applied in the human vagina depends on its adaptation to the environment based on specific conditions of temperature, pH and vaginal fluid. The characteristics and composition of this fluid determine, to a great extent, the interactions between resident microbiota and urogenital pathogens [15,16]. The hydrogel formulation of Fitostimoline® Septagel has been developed in order to allow a long persistence in the application site.
Rigenase®, a Farmaceutici Damor’s trademark, is a Triticum vulgare extract that exercises a refreshing and lenitive action, and in addition, promotes tissue repairing and cellular microenvironment recovery, concurring to the reconstitution of the normal vaginal flora and of the physiologic acid pH [17]. In addition, Rigenase® exhibits antioxidant capacity by tissue-repairing activity and moisturizing action [18].
In line with the above mentioned issues, the aim of this study is to evaluate if Fitostimoline® Septagel, thanks to its specific physical-chemical properties, can reasonably be recommended as an adjuvant approach in the treatment of vaginitis and vaginosis. To address the aim of the study, we investigated the activity of Fitostimoline® Septagel by mimicking a vaginal model, reproducing, in vitro, the normal vaginal conditions and in case of infection.
Materials and Methods
Fitostimoline® Septagel
Fitostimoline® Septagel is a medical device for vaginal use contained in an aluminum tube provided with disposable applicators. Each tube contains: Rigenase®, PHMB, Hydroxyethylcellulose, Glycerin, Phenoxyethanol, Macrogol 400, Purified water. pH: 4 - 6. Treatment frequency: one application (5 g) every 72 hours.
Mimic vaginal model (MVM)
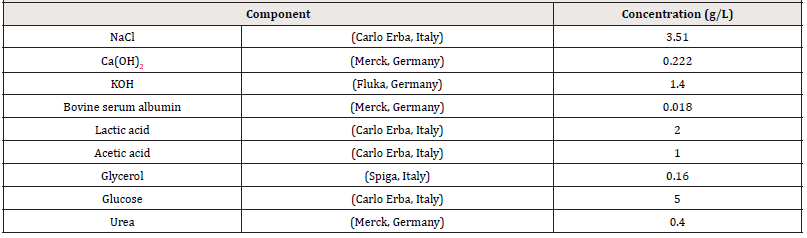
Three simulated vaginal fluids (SVF) were prepared (Table 1), [19], adjusting to a pH of 4.2 (normal conditions), 5.5 and 6.5 (in case of infection) [20,21], using HCl (Carlo Erba, Italy) or NaOH (Carlo Erba, Italy), keeping at 37°C.
Physicochemical evaluation of Fitostimoline® Septagel in mimic vaginal model
For three different mimic vaginal environments (pH 4.2; 5.5 and 6.5), pH and viscosity were investigated at contact times of 8 – 24 – 48 – 72 hours at 37°C; 100 ml of each SVF were prepared and 100 g of Fitostimoline® Septagel were weighed and mixed with 15 mL of each SVF.
pH Determination
The pH was measured using a pH meter (Mettler-Toledo) which was calibrated prior to each use with buffer solution pH 2.0, 7.0 and 12.0.
Viscosity
Viscosity (expressed in mPa/s) of all samples was determined by using Rotational Viscometer Visco Star plus, sample spindle L4 and speed of 6 rpm; at 37°C.
Swelling index in SVF
To determine the swelling index of the prepared topical hydrogel in SVF, one gram of Fitostimoline® Septagel was soaked into 5 ml of SVF incubated at 37°C for 72 hours. After contact times (t) of 8 – 24 – 48 – 72 hours, after removing the excess SVF, the samples were weighed again. The following formula was used to calculate the swelling ratio [22].
Swelling ratio = (Ws − W0/W0) × 100 (1) where Ws is the weight of the swollen hydrogel at contact time t and W0 is the initial weight.
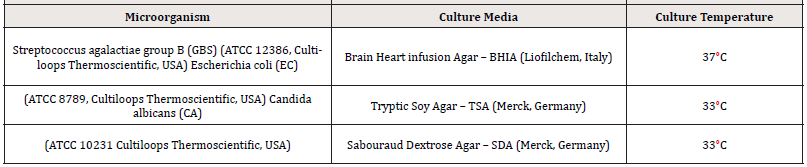
Test Organisms
Table 2 summarizes the respective growing conditions of the two bacteria and the yeast used in the current study. Each loop containing the lyophilized microorganism was initiated in tubes of 10 mL of Tryptic Soy Broth (TSB, Merck, Germany), after 24-hours incubation at their respective growth temperatures in aerobic conditions, 1 mL of the suspension was transferred to 9 mL of fresh TSB incubated for 48 - 54 hours (six replicates), then centrifuged at 3000 rpm for 15 minutes. The cell pellet from two tubes was resuspended in 18 mL SVF (for each pH).
Survival of Pathogens in Mimic Vaginal Model
Survival of pathogens in three different mimic vaginal environments (pH 4.2; 5.5 and 6.5) was investigated according to Borges et al. [23]. Each SVF was inoculated 2% (v/v) for every pathogen’s suspension (prepared as described in “Test Organisms” session) and incubated at 37°C. After contact times of 8-24-48- 72 hours aliquots were withdrawn for further enumeration by ten-fold dilutions in TSB (performed as positive controls too), colony-forming units (CFU)/mL were determined after 24 hours incubation plated on media and temperatures described in (Table 2). Results were expressed in a logarithmic scale as Log10.
Microbiological evaluation of Fitostimoline® Septagel Effect in Mimic Vaginal Model
Survival of pathogens in three different mimicked vaginal environments (pH 4.2; 5.5 and 6.5) was investigated in the presence of Fitostimoline® Septagel. Keeping the proportion of 5 g of Septagel to 0.75 mL of SVF [24], for each pH, 100 g of Fitostimoline® Septagel were weighed and mixed with 15 mL of each pathogen’s suspension (prepared as described in “Test Organisms’’ session) and incubated at 37°C. After contact times of 8 – 24 – 48 – 72 hours aliquots were withdrawn for further enumeration by a first dilution 1:100 in a sterile neutralizer ((3.6 g/L potassium dihydrogen phosphate; 7.2 g/L disodium hydrogen phosphate dihydrate; 4.3 g/L sodium chloride; 1 g/L peptone; 30 g/L Tween 80; 30 g/L saponin; 0.5 g/L histidine; 3 g/L lecithin; 5 g/L sodium thiosulfate pentahydrate) and then ten-fold dilutions in TSB (performed as positive controls too), CFU/g were determined after 24 h incubation plated on media and temperatures described in Table 2. Results were expressed in a logarithmic scale as Log10.
Agar-Spot-Overlay Test Method
The microbiostatic activity (barrier effect) of Fitostimoline® Septagel was evaluated by determining the zone of growth inhibition using a modified agar overlay assay. The method described was set up to develop a bilayer system, which could provide a reproducible assay to evaluate antimicrobial Septagel activity [25]. For the basal layer, BHIA agar plates were prepared by pouring approximately 10 mL of medium agar into 90 mm Petri dishes and allowed to solidify. Approximately 10 microliters of each SVF and 10 micrograms of Fitostimoline® Septagel diluted with each SVF as described above, immediately after incubation at 37°C were placed onto the surface of the basal layer as spots. The plates were left for 30 minutes at room temperature to ensure adequate diffusion.
Each microorganism was streaked onto BHIA 90 mm plates for 18–24 h at 33°C (37°C for S. agalactiae). Subsequently, one isolated colony was picked from the plate and suspended in Ringer’s Solution (Liofilchem, Italy) and adjusted to equal turbidity of 0.5 McFarland standard (3.0 McFarland standard for C. albicans). Each suspension was used to seed BHIA medium stabilized at 45°C (0.1 mL microorganism test suspension/100 mL medium). 10 mL of each obtained cultured medium was poured to overlay the basal layer plates prepared as above described.
The final inoculum size for the top agar layer was 1 × 106 CFU/ mL of bacteria and 2.5×105 CFU/mL of yeast, respectively. After solidification of the top agar, incubation of the plates occurred at strain-specific temperatures (Table 2) for 24 hours. The agar overlay tests were independently repeated three times as a qualitative assay of antimicrobial activity of Fitostimoline® Septagel.
Statistical Analysis
The data analyzed are reported as the mean ± SD for approximately normally distributed continuous variables, as the median (interquartile range [IQR]: 25th percentile, 75th percentile) for severely skewed continuous variables and as numerical values (percentages) for categorical variables. Normal distribution of the data was assessed via normal probability plots and confirmed with the skewness/kurtosis test for normality. Statistical significance was determined by a p value <0.05. In the statistical analysis, differences for continuous variables were evaluated using two-sample t-test for approximately normally distributed variables and Mann-Whitney U test for severely skewed variables. Chi-square or Fisher tests were used to measure associations between dichotomous and categorical variables. All analyses were performed using SPSS 26.0.
Results
Physicochemical Evaluation of Fitostimoline® Septagel in Mimic Vaginal Model
Previous studies showed that 5 g of Fitostimoline® Septagel was able to properly cover the human vaginal epithelium, immediately after insertion, within the first 30 minutes and without sexual intercourse [26]. The fluid ambient volume present in the vagina is approximately 0.50 – 0.75 mL [19]. Therefore, a simulated Fitostimoline® Septagel application for 72 hours (recommended treatment dose) was obtained to mimic the maximum dilution which is likely to occur in the vagina after the gel administration. In our trial we applied 5 g of gel in the following vaginal conditions: temperature 37°C and 0.75 mL of female genital tract secretion, - simulated vaginal fluid (SVF) – at a pH 4.2 (normal conditions), and at a pH 5.5 and pH 6.5 mimicking the infection state [24].
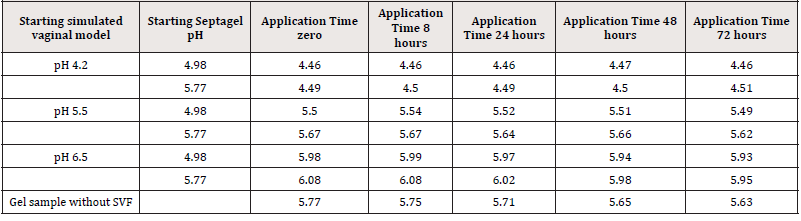
Due to the pH range of the formulation, two independent tests were performed with two samples of Fitostimoline® Septagel. The pH of the first hydrogel was 4.98 and 5.77 for the second one. For each formulation and for each SVF, 100 g of Fitostimoline® Septagel were weighed and mixed with 15 mL of SVF. A sample without SVF dilution was incubated at 37°C for 72 hours as control. No statistical significance was recorded for this analysis. Results are summarized in Table 3.
Figure 1 shows pH values during the application time at 37°C in the three different simulated vaginal environments for both hydrogel formulations; no significant differences were observed for trends of pH values for the two formulations.
The blue line indicates an application in a mimic vaginal model at pH 4.2, the red line at pH 5.5 and the green line at pH 6.5. (a) First gel formulation: pH 4.98. (b) Second gel formulation: pH 5.77.
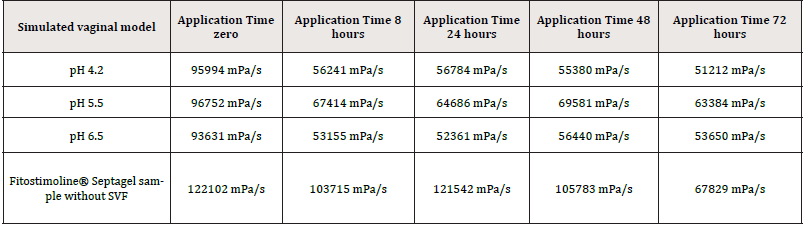
Viscosity is a structural gel property and it determines its adhesion capacity in vivo. This parametron is temperature dependent. All simulated gel applications were found to have viscosities in a range between 69581 mPa/s and 51212 mPa/s and as a general observation, the incorporation of simulated vaginal fluid resulted in decreasing the gel viscosity as listed in Table 4. No significant difference was detected in this analysis.
Figure 2 shows the formulation viscosity trend. On this parametron the pH is almost irrelevant, while the temperature has an impact on the viscosity values. In the figure the temperature variation, highlighted by the black arrow, shows the moment in which the gel has been put into contact with the fluid and the relative measurement after 8h at 37°C. Despite the temperature variation, the viscosity values remained high and constant for the entire duration of the application, with no statistically significant comparison.
The blue line indicates an application in a mimic vaginal model at pH 4.2, the red line at pH 5.5 and the green line at pH 6.5.
Swelling is one of the fundamental hydrogel properties [27]. This feature is correlated to the polymer matrix used to create the gel, and allows to absorb many times more water than its dry mass [28]. In order to test this capacity a swelling index was performed in SVF at application contact times. Table 5 summarizes the results. The values show 3 replicates ± standard deviation.
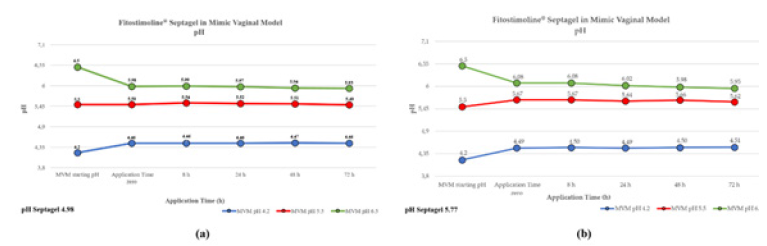
Figure 1: Evaluation of pH variation after application of Fitostimoline® Septagel at different contact times.
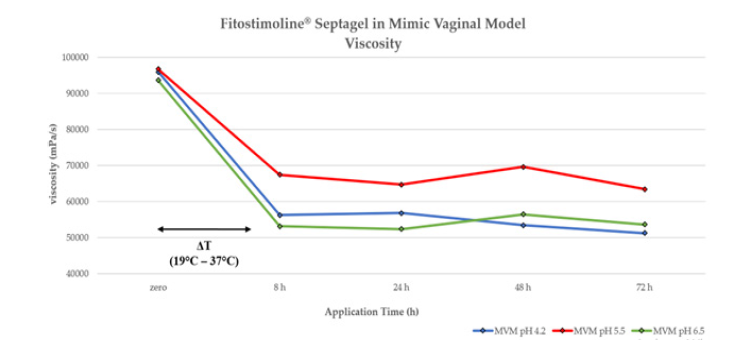
Figure 2: Evaluation of Fitostimoline® Septagel application on viscosity at different contact times. The blue line indicates an application in a mimic vaginal model at pH 4.2, the red line at pH 5.5 and the green line at pH 6.5.
In Figure 3, the swelling response of Fitostimoline® Septagel in SVF shows an upward trend. At 8 hours the hydrogel matrix of Fitostimoline® Septagel starts to absorb the vaginal fluid. This trend increases till a plateau at 48 hours and then it starts to decrease, but 1at 72 hours it still maintains a significative swelling index.
Microbiological Evaluation of Fitostimoline® Septagel Effect in Mimic Vaginal Model
The behavior of three pathogenic microorganisms Group B S. agalactiae (GBS), E. coli (EC) and C. albicans (CA) (two bacteria and one yeast that most often cause vaginal disorders), was tested in a simulated vaginal fluid in absence and presence of Fitostimoline® Septagel, during the recommended treatment period (one application each 72 hours).
Figure 4 shows the GBS behavior in different pH conditions. At physiological conditions (pH 4.2), at 8 hours there was a decrease in the number of CFU/mL, and after 24 hours, the number of microorganisms decreased by 1-2 Log10 CFU/mL, and remained constant for 72 hours. I.e. from zero – 72hrs.
In pathological conditions (pH 5.5 and 6.5), GBS founds the ideal environment at 72 hours increasing the number of microorganisms.
This condition was not reproducible in the presence of Fitostimoline® Septagel, since at time zero application, there was a drastic reduction in viable cells at all pH conditions. Culturable GBS levels declined below the detection limit of enumeration technique (< 2 Log10 CFU/mL) and no increase in GBS CFU/mL was detected in subsequent time points.
Figure 5 shows the E. coli (EC) trend at 48 hours, pH 4.2. The EC number decreased by 3 Log10 CFU/mL and by 6 Log10 CFU/ mL after 72 hours. This effect was not found in pathological pH conditions.
At time zero, in presence of Fitostimoline® Septagel, a bacteriostatic effect was observed only at pH 5.5, but already after 8 hours bacterial survival of EC was affected throughout the period of application.
Figure 6 describes the C. albicans (CA) growth behavior in SVF, with and without Fitostimoline® Septagel at 3 pHs. In SVF, the strain showed a similar increase in all pH. At the same time, a similar decrease was observed in the presence of Fitostimoline® Septagel. The difference between the pH values was statistically significant (p<0.05).
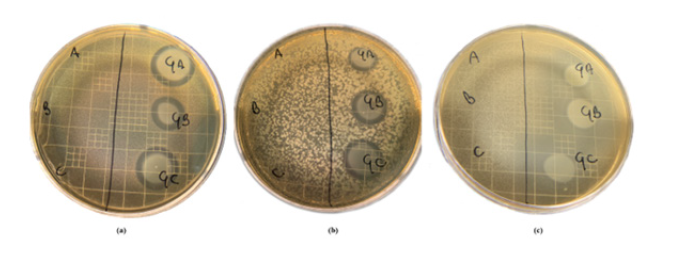
The vaginal pathogens inhibition was assessed using an agar overlay technique, running in parallel spots of SVF and SVF + Fitostimoline® Septagel at 3 different pHs (all spot samples were incubated at 37°C overnight previously and, for the gels, the proportion of 5g to 0.75 ml of SVF was respected). Figure 7 shows, in three boxes, the plates after that the respective microorganism growth conditions were met; in the areas in which Fitostimoline® Septagel was applied, clear zones of growth inhibition were observed for GBS (Figure 7a), EC (Figure 7b) and CA (Figure 7c), with a statistically significant value (p<0.05).

Figure 4: S. agalactiae trend in presence and in absence of Fitostimoline® Septagel in three different simulated vaginal models. (a) pH 4.2; (b) pH 5.5; (c) pH 6.5.
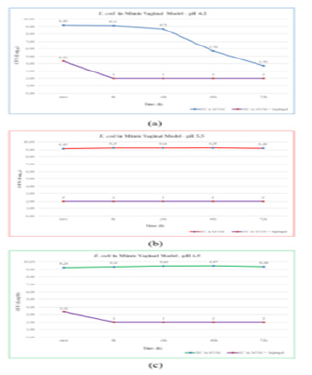
Figure 5: E. coli trend in presence and in absence of Fitostimoline® Septagel in the three simulated vaginal models. (a) pH 4.2; (b) pH 5.5; (c) pH 6.5.
Discussion
A medical device for vaginal use, applied as an adjuvant in prophylaxis and in the treatment of symptoms such as itching, burning and irritation due to the inflammation of the vaginal mucosa and infections of bacterial and/or fungal origin, must have a moisturizing, protective and refreshing action on the vaginal mucosa.
Physical and chemical properties define hydrogels as spatial structures. These structural properties are largely responsible for the hydrogel behavior and, consequently, for their final clinical application [27]. In particular, parameters such as pH and temperature can affect the adhesive capacity of the gels. Owen and Katz [19] formulated a simulated vaginal fluid (SVF), in order to evaluate its effects in vitro on the properties of topical products. This formulation was designed to simulate mainly the pH and the osmolarity of the vaginal fluid, which are properties that can affect the effectiveness of intravaginal gels. This medium was also employed by other researchers to characterize bio adhesive gels for the local therapy of vaginitis [16,29,30].
The physicochemical behavior of the formulation in the vaginal environment was then evaluated in order to guarantee the adhesion of the preparation to the vaginal mucosa with a consequent uniform and effective effect, that contributes to protecting the vaginal environment. This study focused on the medical device Fitostimoline® Septagel in its entirety, to evaluate its use in the treatment of symptoms of vaginosis and vaginitis.
Fitostimoline® Septagel was evaluated in simulated vaginal environments at 3 different pHs allowing so to observe its effect in physiological (pH 4.2) and pathological conditions (pH 5.5 and pH 6.5) after incubation at 37°C during application time, monitoring parameters such as pH, swelling ratio and viscosity (Figures 1-3).
To mimic physiological conditions, the Fitostimoline® Septagel pH remained constant and very close to 4.2, showing the formulation ability to adapt to the environment pH without disturbing it, regardless of its own pH.
This characteristic was confirmed by the intermediate pH trend which simulated conditions that differed from the normal vaginal pH. At pH 6.5, therefore in simulated pathological conditions, the activity of Fitostimoline® Septagel was to not allow further changes in pH towards favorable conditions to the proliferation of pathogenic microorganisms, returning to values closer to pH 6.0.
Swelling consist in a physical process in which a material, by absorbing liquid, increases its volume, and consequently, its mass. As the size of the analyzed materials increases, the original shape is preserved [27]. This feature makes hydrogels ideal for topical and vaginal applications.
The results showed that due to the porous nature of Fitostimoline® Septagel, the swelling index rapidly increase up to 48 hours; this means that Fitostimoline® Septagel is able to incorporate the fluid present in the vagina, removing the cause of the symptoms from the environment and allowing a return to the physiological conditions of the vaginal environment.
After 72 hours of application the gel still retained the ability to absorb fluids, even if it showed a decrease. This result was in accordance with the formation of tighter structure which is due to an increase in crosslinker concentration that shows a direct negative effect on hydrogel swelling index [31].
The results showed that the gel viscosity, once in contact with the simulated vaginal fluid at 37°C, maintains a level that continue to give to the gel a proper adhesion to the vaginal environment for the entire recommended period of administration (72 hours), regardless of the pH levels of the vaginal environment.
Genital tract infection can affect pregnant women, GBS infection is frequent in the first trimester of pregnancy. These infections cause premature birth, neonatal infection, congenital abnormalities and morbidity in the mother [32]. Caillouette et al. [20] reported an association between vaginal pH and the pathogens presence [33]. GBS recurrently colonize the vaginal tract, often in conditions of altered microbiota such as bacterial vaginosis [34]. In BV there is an increase of vaginal pH because Lactobacillus spp. are replaced by anaerobic bacteria [35,36], perhaps enabling the colonization of GBS. Thus, a rise in vaginal pH may contribute to extended survival of colonizing GBS.
pH is a critical environmental parameter for the regulation of the vaginal ecosystem. An imbalance of the ordinary vaginal microbiota usually causes vaginal pH to increase over 4.5, by creating a favorable local milieu to the growth of potentially pathogenic bacteria [37]. A vaginal pH > 4.5 is commonly found in patients affected by bacterial vaginosis and aerobic vaginitis and represents also a diagnostic parameter [5,8,38].
Studies of vaginal microbiota have long indicated that the presence of certain bacterial species and other microbial characteristics can be linked to the disease-free state of the genitourinary tract [39,40]; however, mounting evidence shows that the vagina can harbor uropathogenic bacteria. First and foremost, the vagina can act as a reservoir for E. coli, with multiple studies showing that women with an history of urinary tract infections more commonly have E. coli in their vaginal introitus also if compared to healthy controls [41-43].
Candida vulvovaginitis is common; it is responsible for a third of all cases of vulvovaginitis in reproductive-aged women. 70% of women report having had candida vulvovaginitis at some point in their lifetimes. About 8% of women suffer recurrent candida vulvovaginitis. The most common responsible pathogen is C. albicans (in about 90% of cases) [44].
The results of this study demonstrated that the in vitro vaginal environment at pH 5.5 and 6.5 was ideal for survival of GBS, EC and CA; the same data suggested a failure of all the strains to grow in a vaginal simulated model in presence of Fitostimoline® Septagel regardless of pH.
The strains behavior at pH 5.5 was similar; the results demonstrated that in 72 hours there were no great reductions in the number of viable cells, but in the presence of Fitostimoline® Septagel, all the strains showed a significative difference about susceptibility and survival. The survival of EC in SVF was pH dependent: after 48 hours, the acid pH 4.2 of the simulated vaginal model decreased the number of EC, this tendency towards a reduction in the number of viable cells is accentuated after staying
in a simulated vaginal environment for 72 hours. This effect was not found in pathological pH conditions, confirming pH 5.5 and pH 6.5 as favorable conditions for the proliferation of EC.At time zero of application, Fitostimoline® Septagel has a bacteriostatic effect only at pH 5.5, but already after 8 hours bacterial survival of EC was affected by Fitostimoline® Septagel in all the assessed conditions; this effect remained unchanged throughout the period of application. The overall results suggested that pH had no effect on CA proliferation: in the 3 assessment situations. The results showed an upward trend in the number of CFU. At time zero of application of Fitostimoline® Septagel, the number of microorganisms decreased by 3 Log10, the effect of a decrease in the vitality is accentuated after 8 hours of permanence and is confirmed for the entire period of application.
The data obtained from the overlay assay confirmed what has been described so far. The areas of inhibition were not measured as the purpose of this experiment was to visually demonstrate the inhibitory effect on the microbial proliferation of Fitostimoline® Septagel. Running the 3 SVFs at different pHs in parallel, in addition to acting as a positive control, has shown that the pH alone is not sufficient to counteract the proliferation of microorganisms such as EC that have previously shown a dependence on pH conditions.
Earlier data [17] documented the ability of Fitostimoline® Septagel to restore the state of well-being in patients with inflammatory-dystrophic vaginal disorders, due to the presence of Rigenase® in the formulation of this hydrogel that is the fundamental ingredient that gives the additional ability to create a favorable condition for a rapid and correct re-epithelization of the vaginal epithelium. Rigenase® is a specific aqueous extract isolated by Farmaceutici Damor from the natural source of T. vulgare that exhibits a moisturizing action and a scavenging effect toward free radicals, thus pointing to its relevant antioxidant activity [18].
Conclusions
A medical device for vaginal use should be applied to enhance the drug therapy administered and to accelerate healing. Our study demonstrates the effectiveness of Fitostimoline® Septagel in fulfilling this role. Our results show that Fitostimoline® Septagel has a positive pH effect, it maintains the hydrogel properties for the entire duration of the application (72 hours), guaranteeing adhesion to the place of application. It counteracts the proliferation of pathogens of both bacterial and fungal origin, proving to be a useful containment tool if time passes between the manifestation of annoying symptoms and diagnosis. The septagel formulation makes the product capable of removing infected fluid from the environment. Furthermore, it exerts a barrier effect of avoiding the growth of bad microorganisms that can make symptoms linger for longer. As Fitostimoline® Septagel contains Rigenase®, it could also promote a rapid vaginal re-epithelialization. The treatment schedule entailing application of the product every 72 hours makes it ideal to treat vaginosis symptoms at home.
Acknowledgments
The authors of this article are grateful to Vincenzo Maglione, Stefano Maurelli, Salvatore Gigliano and Carmine Migliuolo for their helpful support.
References
- Hainer BL, Gibson MV (2011) Vaginitis. Am Fam Physician 83(7): 807-815.
- Spiegel CA (1991) Bacterial vaginosis. Clin Microbiol Rev 4(4): 485-502.
- Donders GG (2007) Definition and classification of abnormal flora. Best Pract Res Clin Obstet Gynaecol 21(3): 355-373.
- Srinivasan S, Fredricks DN (2008) The human vaginal bacterial biota and bacterial vaginosis. Interdiscip Perspect Infect Dis 2008: 750479.
- Donders GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G (2002) Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. Br J Obstet Gynaecol 109(1): 34-43.
- Tempera G, Furneri PM (2010) Management of aerobic vaginitis. Gynecol Obstet Invest 70(4): 244-249.
- Tempera G, Bonfiglio G, Cammarata E, Corsello S, Cianci A (2004) Microbiological/clinical characteristics and validation of topical therapy with kanamycin in aerobic vaginitis: a pilot study. Int J Antimicrob Agents 24(1): 85-88.
- Marianelli C, Petrucci P, Comelli MC, Calderini G (2014) Silver sucrose octasulfate (IASOS™) as a valid active ingredient into a novel vaginal gel against human vaginal pathogens: in vitro antimicrobial activity assessment. PLoS One 9(6): e97791.
- Coudray MS, Madhivanan P (2020) Bacterial vaginosis-A brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol 245: 143-148.
- Corsello S, Spinillo A, Osnengo G, Carlo Penna, Secondo Guaschino, Anna Beltrame, et al. (2003) An epidemiological survey of vulvovaginal candidiasis in Italy. Eur J Obstet Gynecol Reprod Biol 110(1): 66-72.
- Kent HL (1991) Epidemiology of vaginitis. Am J Obstet Gy-necol 165(4 Pt 2): 1168-1176.
- Sobel JD (2007) Vulvovaginal candidosis. Lancet 369(9577): 1961-1971.
- Jacob S, Nair AB, Shah J, Sreeharsha N, Gupta S, et al. (2021) Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 13(3): 357.
- Caló E, Khutoryanskiy VV (2015) Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J 65: 252-267.
- Geshnizgani AM, Onderdonk AB (1992) Defined medium simulating genital tract secretions for growth of vaginal microflora Journal of Clinical Microbiology 30(5): 1323-1326.
- María Silvina Juárez Tomás, María Elena Nader-Macías (2007) Effect of a medium simulating vaginal fluid on the growth and expression of beneficial characteristics of potentially probiotic lactobacilli. Communicating Current Research and Educational Topics and Trends in Applied Microbiology A. Méndez-Vilas, et al. [Ed’s.,].
- Papa R, Troncone MG, Altruda F, Rullo V, Saponati G (2017) Clinical Evaluation of the Efficacy and Safety of a Medical Vaginal Device Containing Rigenase® for the Treatment of Vaginosis: A Randomized Study. J Clin Gynecol Obstet 6(1): 6-11.
- Antonucci I, Fiorentino G, Contursi P, Minale M, Riccio R, et al. (2018) Antioxidant Capacity of Rigenase®, a Specific Aqueous Extract of Triticum vulgare. Antioxidants (Basel) 7(5): 67.
- Owen DH, Katz FK (1999) A vaginal fluid simulant. Contraception 59(2): 91-95.
- Caillouette JC, Sharp CF, Zimmerman GJ, Roy S (1997) Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol 176(6): 1270-1277.
- Pavletic AJ, Hawes SE, Geske JA, Bringe K, Polack SH (2004) Experience with routine pH testing in a family practice setting. Infect Dis Obstet Gynecol 12(2): 63-68.
- Anumolu SS, Anupa R Menjoge, Manjeet Deshmukh, Donald Gerecke, Stanley Stein, et al. (2010) Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries. Biomaterials p. 1-14.
- Borges SF, Silva JG, Teixeira PC (2011) Survival and biofilm formation of Listeria monocytogenes in simulated vaginal fluid: influence of pH and strain origin. FEMS Immunol Med Microbiol 62(3): 315-320.
- N'Guessan Gnaman KC, Bouttier S, Yeo A, Aka Any-Grah AAS, Geiger S, et al. (2020) Characterization and in vitro evaluation of a vaginal gel containing Lactobacillus crispatus for the prevention of gonorrhea. Int J Pharm 588: 119733.
- Kemme M, Heinzel-Wieland R (2018) Quantitative Assessment of Antimicrobial Activity of PLGA Films Loaded with 4-Hexylresorcinol. J. Funct. Biomater 9(1): 4.
- Barnhart KT, Pretorius ES, Timber K, Shera D, Shabbout M, et al. (2004) In vivo distribution of a vaginal gel: MRI evaluation of the effects of gel volume, time and simulated intercourse. Contraception 70: 498-505.
- Chyzy A, Plonska-Brzezinska ME (2020) Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering. Molecules 25(24): 5795.
- Zhao X, Lang Q, Yildirimer L, Lin ZY, Cui W, Annabi N, et al. (2016) Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater 5: 108-118.
- Pavelic Z, Skalko-Basnet N, Filipovic-Grcic J, Martinac A, Jalsenjak I (2005) Development and in vitro evaluation of a liposomal vaginal delivery system for acyclovir. Journal of Controlled Release 106(1-2): 34-43.
- Pavelic Z, Skalko-Basnet N, Jalsenjak I (2005) Characterisation and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitis. International Journal of Pharmaceutics 301(1-2): 140-148.
- Rozaini MZ, Zuki ABZ, Noordin MM, Norimah Y, Abdullah MNH (2004) The effect of topical application of Malaysian honey on burn wound healing. JVM 16(1&2): 47-50.
- Hay P (2005) Genito-urinary infections in pregnancy. Women’s Health Med 2(2): 47-50.
- Borges S, Silva J, Teixeira P (2012) Survival and biofilm formation by Group B streptococci in simulated vaginal fluid at different pHs. Antonie Van Leeuwenhoek 101(3): 677-682.
- Locksmith G, Duff P (2001) Infection, antibiotics, and preterm delivery. Semin Perinatol 25(5): 295-309.
- Moodley P, Sturm AW (2000) Sexually transmitted infections, adverse pregnancy outcome and neonatal infection. Semin Neonatol 5(3): 255-269.
- Simhan HN, Caritis SN, Kronh MA, Hillier SL (2003) Elevated vaginal pH and neutrophils are associated strongly with early spontaneous preterm birth. Am J Obstet Gynecol 189(4): 1150-1154.
- Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, et al. (1989) Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol 27(2): 251-256.
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, et al. (1983) Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74(1): 14-22.
- Ravel J, Brotman RM (2016) Translating the vaginal microbiome: Gaps and challenges. Genome Med 8(1): 35.
- Stapleton AE (2016) The Vaginal Microbiota and Urinary Tract Infection. Microbiol. Spectr 4: 79-86.
- Lewis AL, Gilbert NM (2020) Roles of the vagina and the vaginal microbiota in urinary tract infection: Evidence from clinical correlations and experimental models. GMS Infect Dis 8: Doc02.
- Navas-Nacher EL, Dardick F, Venegas MF, Anderson BE, Schaeffer AJ, et al. (2001) Relatedness of Escherichia coli colonizing women longitudinally. Mol Urol 5: 31-36.
- Meštrović T, Matijašić M, Perić M, Čipčić Paljetak H, Barešić A, et al. (2020) The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics (Basel) 11(1): 7.
- Jeanmonod R, Jeanmonod D (2022) Vaginal Candidiasis.






 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.