Research article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Phytochemical Composition and Anti-Obesity Effect of The Methanol Extract of Ziziphous Mucronata Leaves in Wistar Rats
*Corresponding author: Idakwoji P A, Department of Biochemistry, Faculty of Natural Sciences, Kogi State University, Anyigba, Nigeria.
Received: July 11, 2022; Published:July 22, 2022
DOI: 10.34297/AJBSR.2022.16.002278
Abstract
The incidence of obesity is increasing tremendously, and it has been revealed that about 500 million adults are obese worldwide. This study evaluated the anti-obesity potentials of methanol extract of Ziziphous mucronata leaves (MEZM) in male albino rats. The extract obtained by standard methods was screened phytochemically and subjected to acute toxicity study using Lorkes method. Obesity was induced in rats by daily feeding with high fat diet for 5 weeks. Only rats with an abdominal circumference (AC) of 20 cm, thoracic circumference (TC) of 17 cm and body mass index (BMI) of 0.68 g/cm2 and above respectively were considered obese and used for the study. The obese rats were assigned into 5 groups (II- VI) of 12 rats per group replicated three times. Whereas the rats in the normal control (group I) received normal growers mash diet and distilled water, the obese negative control (group II) received the high fat diet and distilled water only, while the positive control (group III) received high fat diet, distilled water and 60 mg/kg b.wt of Orlistat. Graded doses of the extract (200, 400 and 800 mg/kg b.wt) were administered orally to groups IV, V and VI respectively for 28 days. During the treatment, AC, TC, BW and BMI were measured weekly. Following the 28-day treatment regimen, Rats were euthanized, and blood samples collected for serum lipid profile {Total cholesterol (Tchol), Triglycerides (TAG), High density lipoprotein (HDL) and Low-density lipoprotein (LDL)} analyses. Phytochemical analysis revealed the presence of tannins, saponins, phenols, steroids, flavonoids, and alkaloids in varying proportions. Acute toxicity results showed that the extract had no toxic effect on the rats and the oral LD50 was estimated to be greater than 5000 mg/kg. The extract showed dose-dependent anti-obesity effects as there was a significant (p< 0.05) decrease in AC, TC, BW and BMI of treated rats. The extract also significantly (p< 0.05) reduced serum concentrations of Tchol, TAG and LDL but with a corresponding significant (p< 0.05) increase in HDL concentration. It was concluded that Ziziphous mucronata extract possesses anti-obesity activity and could also play a significant role in the management of obesity-related complications such as cardiovascular diseases.
Keywords: Anti-obesity, Phytochemical, Ziziphous Mucronate; Dyslipidaemia; Wistar Rats
Abbreviations: MEZM: Methanol Extract of Ziziphous Mucronata Leaves; AC: Abdominal Circumference; TC: Thoracic Conference; BW: Body Weight; BMI- Body Mass Index; Tchol: Total Cholesterol; TAG: Triacylglyceride; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein.
Introduction
The study of the use of extracts of natural origin as medicines or health-promoting agents commonly known as phototherapy; is a famous ancient type of therapeutics. Different parts of plant have all been used for remedial purposes. Several studies from different global regions recorded the increase in the use of plants to cure various human diseases [1]. Phytochemical substances with therapeutic properties come from medicinal plants. These properties rely upon the presence of different secondary metabolites like alkaloids, steroids, tannins, flavonoids, and phenol, which are synthesized and deposited in specific parts of these plants [2]. ver the past few years, obesity has garnered considerable attention due to its effects on public health and correlation with serious noncommunicable diseases, some of which are leading causes of death globally, including diabetes mellitus, cardiovascular conditions, musculoskeletal disorders, and a number of cancers, including liver, gallbladder, kidney, colon, prostate, and breast cancer [3].
The incidence of obesity is increasing tremendously, and it has been revealed that about 500 million adults are obese worldwide [4]. Any imbalance in pancreatic lipase and lipoprotein lipase activity can easily influence obesity. A crucial enzyme, pancreatic lipase, which is released by the pancreas, breaks down 50-70% of fat into monoglycerides and free fatty acids for enterocyte absorption. Fat builds up in adipose tissue is often reduced by inhibition of fat digestion and absorption [5]. Inhibition of pancreatic lipase is therefore one of the key targets for anti-obesity drugs [6]. However, the triglyceride-rich lipoproteins, chylomicrons, and very low-density lipoproteins (VLDL) are hydrolyzed by the ratelimiting enzyme lipoprotein lipase, which results in the release of monoacylglycerol and non-esterified fatty acids (NEFA). These fatty acids and monoacylglycerol are both re-esterified into triglycerides (TG) and stored in adipose tissue as neutral lipids, or they are utilized by the muscles for metabolic energy. Any disturbance in lipoprotein lipase activity has an impact on how TG is distributed between muscle and adipose tissue, which in turn impacts obesity [7].
Obesity is associated with a state of excessive oxidative stress that leads to escalation in oxidative damage and oxidative stress markers which are associated with several diseases such as obesity, diabetes, other neurodegenerative diseases, cancer, and atherosclerosis [8,9]. It is crucial to note that any measures that can lower oxidative stress would be advantageous from a therapeutic standpoint [10]. Scientists are looking for natural bioactive molecules that are safe and effective at combating both obesity and oxidative stress because of the negative effects of some anti-obesity medications hence the present study aimed to evaluate anti-obesity effects of Ziziphous mucronata in Wistar rats and to determine the bioactive compounds that may be responsible for the bioactivities measured. Ziziphous mucronata is an important multi-purpose medicinal plant species used for various ailments in Africa [11].
Materials and Methods
Collection and Identification of Plant Material
The leaves of Ziziphous mucronata were collected fresh from Okpella, Edo State and identified by Mr. Akanni Gbenga of Biological Science Department, Federal University, Lokoja, Kogi State.
Chemicals and Drugs
Methanol (BDH, England) and Serum lipid profile kits were purchased from the country representative of Sigma Chemical, St. Louis USA. Drugs were purchased from a pharmacy store (Health Seal Pharmacy) in Lokoja Local Government Area, Kogi State. All other chemicals used were of analytical grade and obtained commercially.
Preparation of Methanol Leaves Extract of Ziziphous mucronata
The leaves of the plant were pulverized to coarse powder in an electric hammer mill. The plant powdered material was extracted with methanol by cold maceration with occasional shaking for 72h. The mixture was filtered using Whatmann filter paper (No 1) to obtain the filtrate. The filtrate was concentrated and evaporated to dryness on a hot water bath at 45OC to obtain the methanol extract. Henceforth, the extract would be referred to as MEZM (methanol extract of Ziziphous mucronata leaves).
Phytochemical Screening
The phytochemical composition of the extract was determined using the method of Sofowara [12].
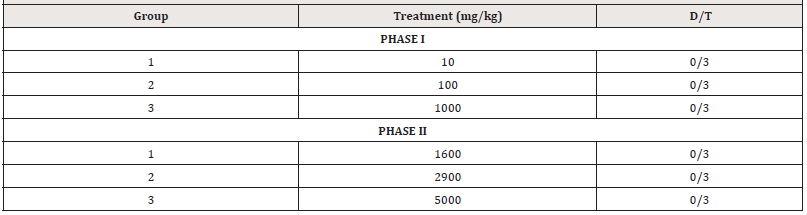
Acute Toxicity Study
The oral median lethal dose (LD50) of the extract was determined in rats according to the method described by Lorke [13]. The study was carried out in two phases. In the first phase, nine rats were randomized into three groups of three rats which were given MEZM at doses of 10, 100, and 1000mg/kg body weight. The rats were kept under the same conditions and observed for signs of toxicity which included but were not limited to paw- licking, stretching, respiratory distress and mortality for 24 h. Based on the results of the initial phase, the following MEZM doses- 1600, 2900 and 5000mg/kg body weight were administered to another set of three groups of three rats in the second phase. These rats were also monitored closely for 24 h after treatment for signs of toxicity and/or mortality. The results obtained in the second phase were used to calculate the LD50. The LD50 was calculated as the geometric means of the maximum dose producing 0 % mortality (D0) and the minimum dose that produced 100 % mortality (D100) and mathematically expressed as:

Induction of Obesity
Obesity was induced in rats in groups 2 - 6 with high fat diet (HFD) (comprising of fat (46%), carbohydrates (24%), proteins (20.3%), fibre (5%), salt mixture (3.5%), and vitamin mixture (1%). Obesity was confirmed in the animals after five weeks by the measurement of the anthropometrical parameters. Animals with abdominal circumference of 20 cm, thoracic circumference of 17cm and a body mass index (BMI) of about 0.68 g/cm2, were selected as described by [14].
Experimental Protocol
A total of 72 adult albino rats was used for the experiment and were kept in rat cages with lid and bottom. The animals were divided into six groups (I - VI) of 12 animals each with three replicates comprising of four rats each. Group I (normal control) received normal growers mash diet and distilled water, group II (obese, negative control) received high fat diet and distilled water, group III (positive control) received high fat diet, distilled water and 60 mg/ kg of body weight of orlistat, while groups IV, V and VI received high fat diet, distilled water and graded doses of 200, 400 and 800 mg/ kg respectively of MEZM. All treatments were administered using plastic syringes attached to metal oropharyngeal cannula. The rats were differentially marked for easy identification. The animals were fed once daily while their water was changed anytime in the day when the need arose. Blood samples were collected from randomly sampled rats at each group on days 0, 7, 14, 21 and 28 following the method described by [15].
Determination of Morpometric Variables
The abdominal circumference (AC) (immediately anterior to
the forelimb) was measured using a tape rule in all rats at weekly
intervals [14]. Thoracic circumference (TC) (immediately behind
the fore limb) was measured weekly intervals [14]. The body weight
(g) was measured before the start of treatments and on sample
collection days using Metler sensitive weighing balance. The rats
were anaesthetized prior to all measurement by administering 0.1
ml 1% sodium barbiturate intraperitoneally. The body weight and
body length were measured and used to determine the body mass
index (BMI) of animals using:
Body mass index (BMI) = Body weight (Kg)/ Length (cm) [14].
Estimation of Serum Lipid Profile
On the 28th day of the experiment, all the rats were euthanized by chloroform inhalation and blood samples were collected by cardiac puncture. The blood was collected into plain serum tubes, allowed to clot and centrifuged for 10 minutes at 3500 rpm. Total cholesterol (TC), triacylglycerol (TAG) and high-density lipoprotein (HDL) concentrations will be determined according to the method of [16] while low density lipoprotein (LDL) levels will be calculated using Fried Wald equation [17].
Statistical Analysis
All data were expressed as Mean± SD and statistical differences between means were determined by one- way ANOVA followed by Duncan post-hoc test for multiple comparison tests using SPSS. Values were considered significant at p≤0.05.
Acute Toxicity Study
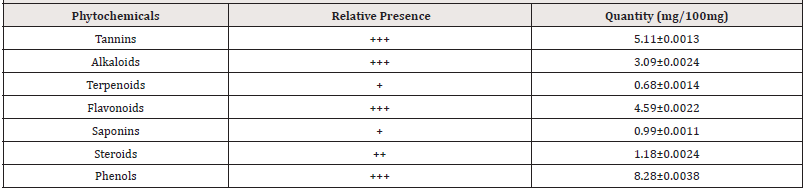
Table 1: Qualitative and Quantitative phytochemical composition of the methanol extract of Ziziphous mucronata leaves (MEZM).
Key: + slightly detected, ++ moderately detected, +++ highly detected, ND- Not detected
In both the first and second phases of the experiment, the methanol extract of Ziziphous mucronata leaves did not show any sign of toxicity or mortality during the monitoring period at the doses administered orally (Table 2). Hence the oral median lethal dose (LD50) of the extract was therefore estimated to be greater than 5000 mg/kg. (Table 2).
Effect of the methanol extract of Ziziphous mucronata leaves (MEZM) on the thoracic circumference (TC) of obese Rats
The effects of MEZM on the abdominal circumference of obese rats are presented in (Table 3). The AC of the negative control and treated groups were significantly higher (P < 0.05) than the AC of normal control in weeks 0, 1, 2, 3 and 4. At weeks 0 and 1, there was no significant (P> 0.05) difference between the AC of the treated groups compared to negative and positive controls. At week 2, only MEZM at 400 mg/kg significantly decreased (P < 0.05) AC compared with the negative control. However, at weeks 3 and 4, a dose-dependent significant (P < 0.05) decrease in AC of rats was observed compared to the negative control with the 800 mg/kg MEZM producing effects that are comparable to that of the positive control (Table 3).
Effect of the methanol extract of Ziziphous mucronata leaves (MEZM) on the thoracic circumference (TC) of obese Rats
The effects of the various doses of MEZM on the thoracic circumference (TC) of obese rats are presented in (Table 4). The AC of the negative control and treated groups were significantly higher (P < 0.05) than the TC of normal control in weeks 0, 1, 2, 3 and 4 just like as observed with TC. At weeks 0, only MEZM at 800 mg/dg produced a significant (P< 0.05) difference in TC among the treated groups compared to negative control. At weeks 1 and 2, MEZM at 200, 400 and 800mg/kg in a dose- dependent manner significantly decreased (P < 0.05) TC compared with the negative control with no significant (P > 0.05) difference between the effects of MEZM at 800 mg/kg compared to positive control. At weeks 3 and 4, a dose-dependent significant (P < 0.05) decrease in TC of rats was observed compared to the negative control. There was no significant (P > 0.05) difference between the effects of MEZM at 200, 400 and 800 mg/kg compared to positive control. (Table 4).
D/T: Number of deaths/ number of rats treated.
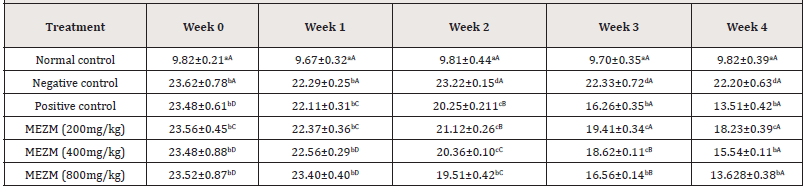
Table 3: Qualitative and Quantitative phytochemical composition of the methanol extract of Ziziphous mucronata leaves (MEZM).
Data is represented as mean ± S.D. Mean values having different lowercase letters down the columns and uppercase letters across the row as superscripts are considered significant (p< 0.05).
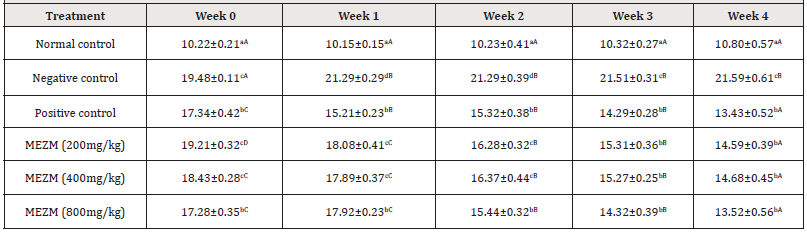
Table 4: Qualitative and Quantitative phytochemical composition of the methanol extract of Ziziphous mucronata leaves (MEZM).
Data is represented as mean ± S.D. Mean values having different lowercase letters down the columns and uppercase letters across the row as superscripts are considered significant (p< 0.05).
Effect of the methanol extract of Ziziphous mucronata leaves (MEZM) on the body weights (BW) of Obese Rats
Table 5 shows the effects of the various doses of MEZM on the body weights (BW) of obese rats. Like AC and TC, the BW of the negative control and treated groups were significantly higher (P < 0.05) than the BW of normal control in weeks 0, 1, 2, 3 and 4. At weeks 0, 1, 2 and 3, only MEZM at 800 mg/dg produced a significant (P< 0.05) decrease in BW compared to negative and positive controls. At week 4, MEZM at 800mg/kg significantly decreased (P < 0.05) BW compared with the negative control but with no significant (P > 0.05) difference when compared to positive control. (Table 5).
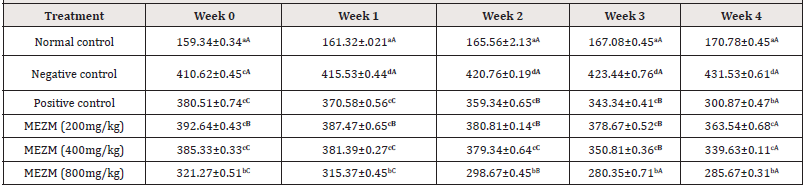
Table 5:Effect of the methanol extract of Ziziphous mucronata leaves (MEZM) on the body weights (BW) (g) of Obese Rats.
Data is represented as mean ± S.D. Mean values having different lowercase letters down the columns and uppercase letters across the row as superscripts are considered significant (p< 0.05).
Effect of the methanol extract of Ziziphous mucronata leaves (MEZM) on the body mass index (BMI) of Obese Rats
Table 6 shows the effects of the graded doses of MEZM on the BMI of obese rats. The BMI of the negative control and treated groups (except 800 mg/ kg MEZM at week 4) were significantly higher (P < 0.05) than the BMI of normal control in weeks 0, 1, 2, 3 and 4. At weeks 0 and 1, only MEZM at 400 and 800 mg/dg produced a significant (P< 0.05) decrease in BMI among the treated groups compared to negative control. At weeks 2 and 3, MEZM at 200, 400 and 800mg/kg in a dose- dependent manner significantly decreased (P < 0.05) BMI compared with the negative control with no significant (P > 0.05) difference between the effects of MEZM at 400 and 800 mg/kg compared to positive control. At week 4, a dose-dependent significant (P < 0.05) decrease in BMI of rats was observed compared to the negative control. There was no significant (P > 0.05) difference between the effects of MEZM at 800 mg/kg compared to normal and positive controls. (Table 6).
Effect of the methanol extract of Ziziphous mucronata leaves (MEZM) on the serum lipid profile of Obese Rats
The effects of MEZM on the serum lipid profile of obese rats are presented in Table 7. The mean concentrations of the total cholesterol (Tchol), triacylglyceride (TAG), high density lipoprotein (HDL) and low-density lipoprotein (LDL) of the negative control and treated were significantly higher (P < 0.05) than those of the normal control. MEZM dose-dependently and significantly decreased (P < 0.05) serum concentrations of Tchol., TAG and LDL with a corresponding increase in HDL compared to negative control. (Table 7).
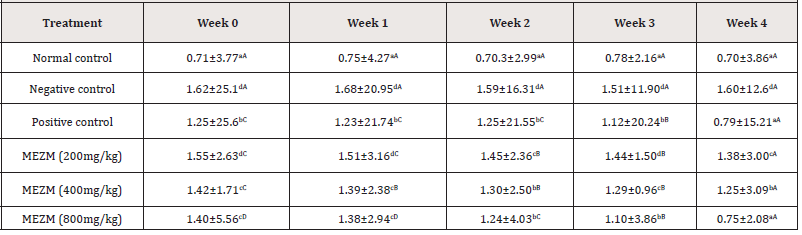
Table 6:Effect of the methanol extract of Ziziphous mucronata leaves (MEZM) on the Body Mass Index (BMI) (g/cm2) of Obese Rats.
Data is represented as mean ± S.D. Mean values having different lowercase letters down the columns and uppercase letters across the row as superscripts are considered significant (p< 0.05).
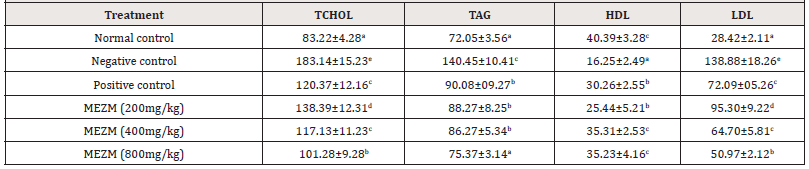
Table 7:Effect of the methanol extract of Ziziphous mucronata leaves (MEZM) on the serum lipid profile of Obese Rats.
Data is represented as mean ± S.D. Mean values having different lowercase letters down the columns as superscripts are considered significant (p< 0.05).
Discussion
Phytochemical Screening
Many herbal extracts have been reported for their anti-obesity activities and are being used for the treatment of obesity. This study investigated the phytochemical composition and anti-obesity potentials of the methanol extract of Ziziphous mucronata leaves in Wistar rats. The phytochemical analysis of the extract revealed the presence of tannins, alkaloids, saponins, terpenoids, steroids, and flavonoids in varying proportions (Table 1). These secondary metabolites have been reported to be bioactive anti-obesity agents [12]. The acute toxicity study of the extract (10-5000 mg/kg) produced no significant physical signs of toxicity such as writhing, weakness, anorexia, gasping, and palpitation, reduction in body weight, decreased respiratory rate or death within 24 hours and 2 weeks of administration for phase 1 and 2 of the study respectively (Table 2). Hence the oral median lethal dose (LD50) of the extract was therefore estimated to be greater than 5000mg/kg. The result of this study is shows that MEZM is relatively safe for human consumption.
Abdominal and thoracic circumferences were used as markers of obesity in this study (Tables 3 & 4). The extract dose- dependently reduced the increased abdominal and thoracic circumferences observed among the high fat diet fed rats. However, past researchers suggested that AC and TC are not good enough to serve as markers of obesity in rats14. We therefore report that the TC/AC increase could be as a result of fat deposited due to variation between the energy intake and energy expenditure in the obese rats. 800 mg/kg treatment of MEZM was most effective in reducing the body weight and body mass index of the obese rats (Tables 5 & 6). This result is in consonant with [14,18] who concluded after researching on “Anthropometrical Parameters and Markers of Obesity in Rats” that body mass index showed the effectiveness of serving as a threshold in dictating an abnormal increase in body weight/ obesity in rats. In view of the above findings, we therefore report that the methanol extract of Ziziphous mucronata leaves proved to possess the potential of reversing obesity.
Cardiovascular disease (CVD) is a major complication of obesity and cause of death in the world mainly due to atherosclerosis (hardening of arteries). Abnormal lipids are risk factors for CVD. Studies have illustrated the beneficial effects of saponins on blood cholesterol levels. Saponins cause a depletion of body cholesterol by preventing its reabsorption, thus increasing its excretion, in a similar way as other cholesterol-lowering drugs, such as cholestyramine. Therefore, the significant decrease in serum cholesterol produced by the extract might be due to its high saponins content. Although the role of high triglycerides as an independent factor in the development of CVD remains controversial, data from several respective studies suggest that triglycerides are probably an important risk factor. Serum triglyceride in this study was significantly reduced following treatment with the extract. This further highlights the beneficial effect of the extract in dyslipidaemia. The extract also significantly decreased LDL levels and this may be due to the significant increase in HDL concentration as HDL is reported to enhance the excretion of cholesterol from the body and thus reducing its concentration [19].
Conclusion
This study revealed that Ziziphous mucronata extract possesses anti-obesity activity and could also play a significant role in the management of obesity-related complications such as cardiovascular diseases.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Van Wyk B E, Wink M (2018) Medicinal plants of the world. England CABI.
- Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161(5): 839-851.
- George A Bray, Gema Frühbeck, Donna H Ryan, John P H Wilding (2016) Management of obesity. Lancet 387(10031):1947-1956.
- Arianne N Sweeting, Samantha L Hocking, Tania P Markovic (2015) Pharmacotherapy for the Treatment of Obesity. Mol Cell Endocrinol 418(2): 173-183.
- M Hanefeld, G Sachse (2002) The Effects of Orlistat on Bodyweight and Glycaemic Control in Overweight Patients with Type 2 Diabetes: A Randomized, Placebo-Controlled Trial. Diabetes Obes Metab 4(6): 415-423.
- Chakrabarti R (2009) Pharmacotherapy of Obesity: Emerging Drugs and Targets. Expert Opin Ther Targets 13(2): 195-207.
- Masataka Kusunoki, Kazuhiko Tsutsumi, Chen Tana, Daisuke Sato, Takao Nakamura, et al. (2013) Lipoprotein Lipase Activation Improves the Cachexia and Obesity. Journal of Obesity and Weight Loss Therapy, 3, Article ID 1000177.
- Heno F Lopes, Kelley L Martin, Khaled Nashar, Jason D Morrow, Theodore L Goodfriend, et al. (2003) DASH Diet Lowers Blood Pressure and Lipid-Induced Oxidative Stress in Obesity. Hypertension 41(3): 422-430.
- Shigetada Furukawa, Takuya Fujita, Michio Shimabukuro, Masanori Iwaki, Yukio Yamada, et al. (2004) Increased Oxidative Stress in Obesity and its Impact on Metabolic Syndrome. Journal of Clinical Investigations 114(12): 1752-1761.
- Halliwell B (2002) Role of Free Radicals in the Neurodegenerative Diseases: Therapeutic Implications for Antioxidant Treatment. Drugs Aging 18(9): 685-716.
- Neo C Mokgolodi, Yan Hu, Ling-Ling Shi, Yu Jun Liu (2011) Ziziphus mucronata: an underutilised medicinal plant in Africa For Stud China 1393): 163-172.
- Sofowara A (2006) Medical plants and traditional medicine in Africa.Reprint edition, Spectrum Books Ltd. Ibadan.
- Lorke D (1983) A new Approach to Practical Acute Toxicity Testing. Arch Toxicol 54(4): 275-287.
- E L B Novelli, Y S Diniz, C M Galhardi, G M X Ebaid, H G Rodrigues, et al. (2007) Anthropometrical parameters and markers of obesity in rats. Lab Anim 41(4):111-119.
- Hoff J (2000) Methods of blood collection in the mouse. Laboratory Animal 29(10): 50-51.
- K M Wasan, S Najafi, J Wong, M Kwong, P H Pritchard, et al. (2001) Assessing plasma lipid levels, body weight, and hepatic and renal toxicity following chronic oral administration of a water soluble phytostanol compound FM-VP4, to gerbils. J Pharm Science 4(3): 228-234.
- Crook M A (2006) Clinical Chemistry and Metabolic Medicine. 7th Edition. Hodder Arnold London pp 426.
- Dong K K, Ekpo B A, Bala D N (2012) Antiobesity effect of ethanolic extract of C. pinnatifida and C. unshiu. African Journal of Pharmaceutical Research Development 2: 63-66.
- M S Brown, J L Goldstein (1984) How LDL receptors influence cholesterol and atherosclerosis. Sci Am 251(5): 58-56.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.