Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Antibacterial and Antiuroletiasis Activities of Cissus Rotandifolia Extract on Urolithiasis Rats Induced by Ethylene Glycol and its Mechanism as Antiurolithiasis Remedy.
*Corresponding author: Hussein S Gumaih, Associate Prof. of Animal Physiology, Biology Department, Faculty of Science, Sana’a University, Yemen and Faculty of Science and Arts, Najran University, Saudi Arabia.
Received: March 22, 2022; Published: April 01, 2022
DOI: 10.34297/AJBSR.2022.16.002184
Abstract
Cissus rotandifolia (CR) is a medicinal plant widely used in the southern region of Saudi Arabia and Yemen. The present study aimed to evaluate the antibacterial and antiurolithiasis effect of CR and its mechanism. Thirty rats divided into five groups: Group A (negative control), Group B were treated with EG and ammonium chloride (AC) and served as positive group. Group C and D served as treated groups and treated as B group with 200 mg/kg and 400 mg/kg of CR respectively for 28 days and group E fed as group B for 28 days and then treated for28 days by 1000 mg CR extract and served as curative group. Another 10 rats divided into two groups: group F took normal diet and served as negative control group and group G took normal diet with 500 mg/kg of CR to investigate the mechanism of CR as antiurolithiatic substance.
At the end of the experiment blood samples were collected from rats. Kidney rats were removed to histopathological examined.
We found a significant decrease in serum urea, creatinine, and MDA of CR groups. Also the kidneys of CR treated group appeared mostly to be calculi-free compared to positive control. A significant increase in water intake, urine volume, urinary magnesium, citrate and urinary pH of CR treated rats when compared to negative control. These results proved the antibacterial activity of CR
The present study emphasized the safe herbal remedies of CR as antioxidants, antibacterial, nephroprotective as well as its antiurolithiatic role. We recommended to use it in pharmaceutical forms as it is safe and effective as antiurolithiasis is remedy.
Keywords: Cissus Rotandifolia; Antiuroletiasis; Antibacterial; Urolithiasis
Introduction
Kidneys have a critical function of the urinary system. They are involved in the excretion of metabolic waste products and chemicals, are responsible for the production of certain hormones and vitamins, and have a key role in blood pressure regulation. They are the maintenance of normal composition and volume of body fluid. This is accomplished by glomerular filtration, tubular reabsorption, and tubular secretion [1].
Urolithiasis (UL) means the accretion of a solid, hard mass of nonmetallic minerals inside the urinary tract. Stone formation is culmination of a series of physicochemical events like supersaturation, nucleation, growth and aggregation of the crystal [2]. Nearly 4-15% of the human populations suffer from urinary stone problem all over the globe [3].
Urolithiasis is generally composed of calcium as calcium oxalate (CaOx) (75-80%), magnesium as ammonium magnesium phosphate (struvite) (10%), uric acid (5-10%), and 0.5-1% is composed of cystin [4].
Oxalate induce injury of renal epithelial cell lines through its cytotoxic effects mediated by apoptosis, necrosis, release of cellular enzymes and membrane lipid peroxidation [5].
Common mechanisms of urolithiasis are superstauration, crystallization, change of urinary pH, metabolic alterations such as hypercalciuria and hyperuricosuria and deficiency of stoneinhibiting factors like citrate and magnesium [6].
There are a number of practices for treatment of urinary calculi, including surgery, endoscopic procedures such as ureter-scopy, extracorporeal shock wave lithotripsy (ESWL) and synthetic drugs. Medical management of urolithiasis is still challenging for modern medical practice. ESWL causes complications like decreased renal function, subcapsular hematomas, inflammation, ischemia, renal fibrosis, hemorrhage, hypertension and steinstrasse “multiple small stones blocking ureter” [7], while surgical methods are costly and require longer recovery times [8].
Although, some drugs used to prevent and treat urolithiasis, the overuse of synthetic drugs, results in higher incidence of adverse drug reactions and not completely solve the problem. Therefore, alternative treatments using natural resources showing antiurolithiatic activity are important. Medicinal plants are used worldwide, and there is increasing interest in treating kidney stones using medicinal plants [9].
Herbal medicines have many phytoconstituents which may exert their beneficial effect in kidney stone treatment. Plant extracts contain phytochemicals that inhibit stone formation by inhibiting synthesis and agglomeration of crystals [10]. Wild edible plants are species of plants that grow freely in the wild habitat without any agricultural treatments and can be consumed as a food [11]. Cissus rotundifolia (CR; Halas) is a wild edible plant grows extensively in the southern region of Saudi Arabia and Yemen, their leaves are widely consumed after cooking by local people as leafy vegetables [12]. Halas is used traditionally in Yemen for the treatment of gastrointestinal troubles [13],
Several studies suggested that CR extracts are beneficial as antiinflammatory and hepato-protective [14,15]. Most of the remedies are very useful, but their mechanisms of action remain unclear and need more investigations.
Materials And Methods
Experimental Animals
Male albino rats Rattus rattus (Rattus norvegicus albinus) each weighing about 200 - 250g were used in this study. The rats were reared in the animal house of Biology Department, Faculty of Science at Sana’a University. They were housed in a standard metallic cages under the same environmental conditions with an alternate 12 h light-dark cycle at room temperature (20±2οC). The animals had ad libitum access to a commercial diet and water. The bedding of the animal cages was changed every 48hrs. Animals were left seven days prior to the experiment for adaptation.
Animals were fed on a diet with the formula which was kindly supplied by the department of animal production, Faculty of Agriculture, Sana’a University.
Leaves of CR were collected from Taiz governorate, Yemen and were identified and authenticated at Botany Department, Faculty of Science, Sana`a University. Plant was carefully washed with tap water, rinsed with distilled water, chopped into small pieces and shade dried at room temperature, and then they were grinding into fine powder. The extraction of bioactive material from the powder was carried out with 70% methanol and 30% distilled water using Soxhlet apparatus. The extract was concentrated by a rotary evaporator and subjected to freeze drying in a freezer [16]. The percentage yield was found to be 10.50%. The extract was preserved in refrigerator until further use.
Experimental Design
Stone Induction: In this study, hyperoxaluria was induced by administration of ethylene glycol(EG)v/v (0.75%) in drinking water for 21 days and1% ammonium chloride (AC) v/w. 1% of AC was given only for the first 7 days, as administration of for more than 7 days lead to death of the rats [17,18].
Dose Preparation:The methanolic extract of CR was dissolved in distilled water at a dose mg/kg body weight and shacked until completely dissolved.
Experimental Animals:Thirty male rats were randomly divided into five groups, each of six rats. Group-A: fed with normal diet and serve as negative control. GroupB: took normal diet with EG (0.75%) and AC (1%) for 28 days and serve as a positive control. Group-C and D: took the same substances as groupB with 200 mg/ kg and 400 mg/kg of CR respectively for 28 days and reserve as prophylactic and group E as group B, took normal diet with EG (0.75%) and AC (1%) for 28 days and then treated for28 days by 1000 mg CR extract and served as curative group.
Assessment of Antiurolithiatic Activity
Collection and Analysis of Blood:In the last day of the experimental period, all animals were fasted overnight and blood was collected from orbital veins. Serum was separated by centrifugation at 3,000 r.p.m for 15 mins and analyzed. Blood plasma was separated by centrifugation at 3,000 r.p.m for 15 mins. After centrifugation.
Estimation of Lipid Peroxidation (LPO):Malondialdehyde (MDA) was determined according to the method of [19].
Biochemical Analysis:The serum levels of creatinine and urea were measured by kinetic UV assay colorimetric methods using kits supplied by Roche diagnosis attached with Roche/Hitachi analyzer machine according the method obtained by [20].
Histopathological Study:Kidneys, liver and heart were weighted, fixed in formalin 10% and processes through graded alcohol series and xylene. Then embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin for histopathological examination under light microscope.
Investigates the Mechanism of Antiurolithiasis of CR
Additional 10 male rats were randomly divided into 2 groups, each of 5 animals were used to investigate the mechanism of CR as antiurolithiatic substance. Group F: were fed with normal diet and left as negative control group whereas, group G: took normal diet with 500 mg/kg of CR and allowed free access to food and drinking water [21].
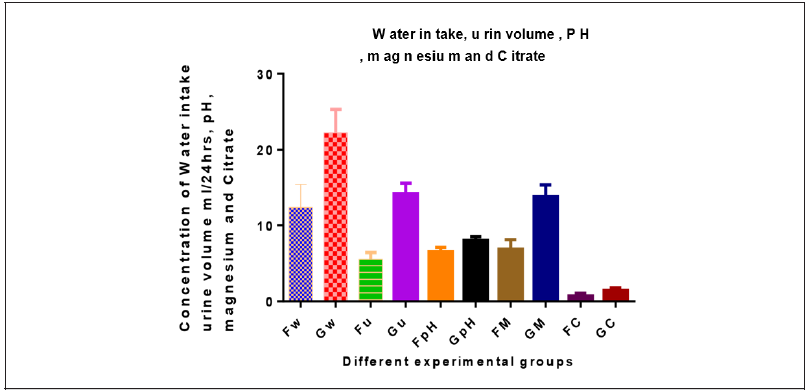
Determination of Water Intake, Urine Volume and PH:rats were kept separately in metabolic cages. Water intake was measured and 24hs and urine samples were collected. A drop of concentrated hydrochloric acid was added to the urine prior to storage at 4 °C, to measure its urine volume. pH of the fresh urine samples from all rats was measured with the help of a calibrated pH meter (Model: WTW-Series pH-720) [22].
Determination of Urine Magnesium and Citrate:The determination of the magnesium concentrations and citrate in urine samples by colorimetric procedure was determined according [23].
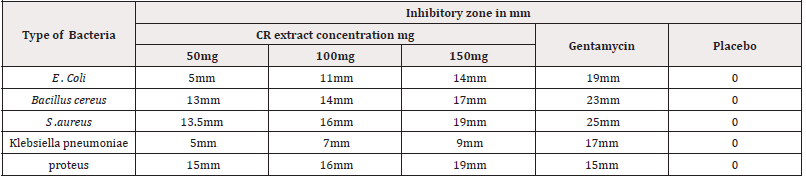
Antibacterial Activity:Five types of bacteria were used for this study. Gram-positive bacteria included Bacillus cereus, staphylococcus aerus and Gram negative bacteria included Escherichia coli, Proteus and Kelibsella pneumoniae. All the tested strains were local isolates and were obtained from Department of Biology, Division of Microbiology, Faculty of Science, Sana’a University. These bacteria served as test pathogens for antibacterial activity assay. Three different concentrations of each extract of selected plants (50, 100 and 150 mg/ml) were dissolved in 10% dimethyl-sulfoxide (DMSO) in purified water to be used in antibacterial activity test. Extract solutions were prepared just before carrying out the test. Antibacterial activity of the extracts was determined by agar well diffusion method.
The bacterial suspensions containing 106 CFU/ml of bacteria were spread on petri dishes plates with a sterile swab moistened with the bacterial suspension. In each of these plates, five wells were cut out using a standard corn borer (7mm). About 60μl of each extract was added into different wells (duplicate each concentration), DMSO was used as a negative control. Positive control antibiotic wells were placed in the plate.
All the plates were incubated for 24hs at 37 °C. After incubation, bioactivity was evaluated by measuring the zone of inhibition. The experiment was performed in two of antibiotics standard Gentamycin (10 mcg) and Neomycin was used as reference to determine the sensitivity of each bacterial species tested and used as control positive. The antibacterial activity of CR extract was determined by agar diffusion method according to Ma et al., (2018).
Statistical Analysis
The data were collected and expressed as a mean±SE/. The statistical significances between groups were analyzed using oneway analysis of variance (ANOVA). A p value < 0.05 considered significant.
Results
Effect of CR extract on the plasma level of MDA
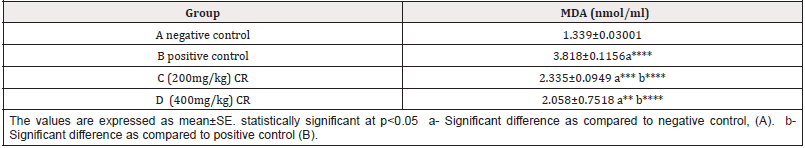
The mean levels of MDA in different groups were: 1.339±0.03001, 3.818±0.1156, 2.335±0.0949 and, 2.058±0.7518 for groups A, B,C&D respectively (Table 1 & Figure 1).
Effect of CR extract on the serum level of creatinine and urea
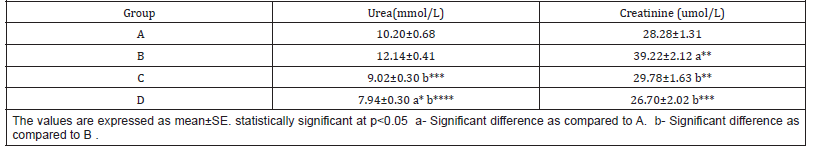
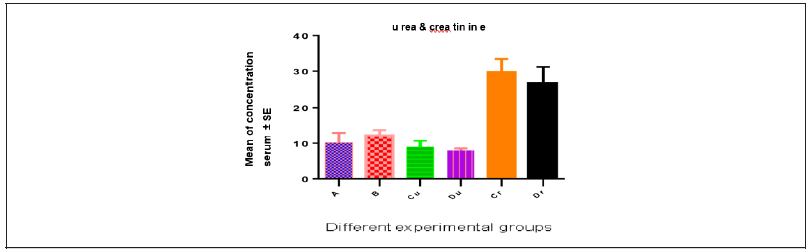
The mean values of urea were 10.20±0.68, 12.14±0.41, 9.02±0.30 and 7.94±0.30; the mean values for creatinine were 28.28±1.31, 39.22±2.12, 29.78±1.63 and 26.70±2.02 for groups A,B,C and D respectively (Table 2 & Figure 2).
There is significant increased in water intake, urine volume, pH, urinary Mg and citrate in group G compared to group F (Table 3 &Figure 3).
The pathogenicity mechanisms of Aerococcus urinae include biofilm formation [2,14,15], human platelet aggregation [2,15] and cytotoxicity to human urothelial cells [15]. In a mouse model described by Gilbert et al. [16], Aerococcus urinae exhibits tropism for the kidney and causes histological inflammation and neutrophil recruitment to the kidney. These findings may help understand better its potential to act as an uropathogen.
Effect of methanolic extract of CR as antibacterial
CR extract has antibacterial activity and this is dose dependent in all bacteria tested. This activity is better than gentamycin for proteus (Table 4 & Figure 4).
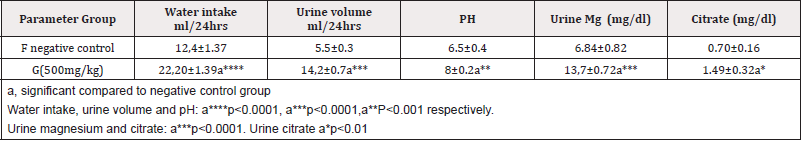
Table 3:Effect of methanolic extract of CR on Water intake, urine volume and PH, urinary Mg and citrate.
Pathological Study
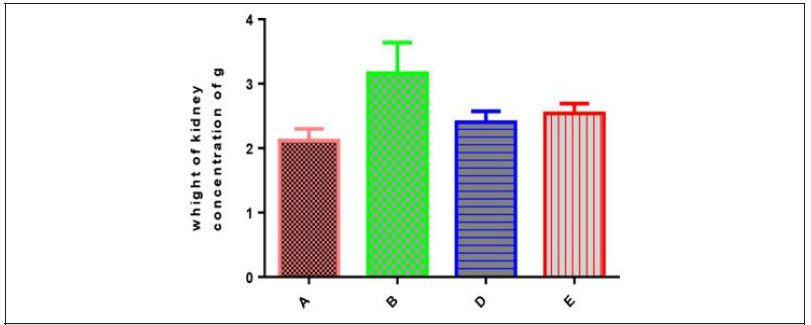
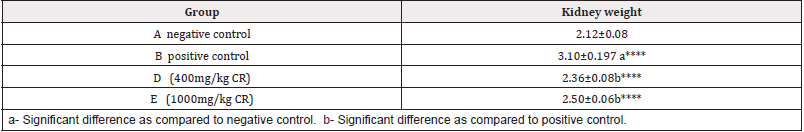
change in kidney weight in different groups:The mean kidney weights in different groups were: 2.12±0.08, 3.10±0.197, 2.46±0.08 and 2.54±0.06 in groups A, B. C and D respectively (Table 5 & Figure 5).
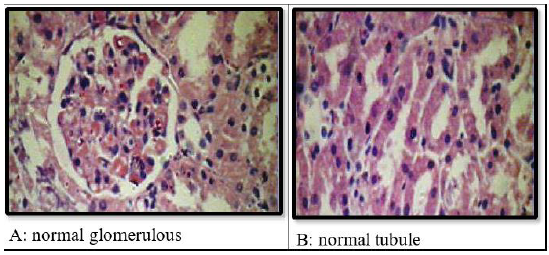
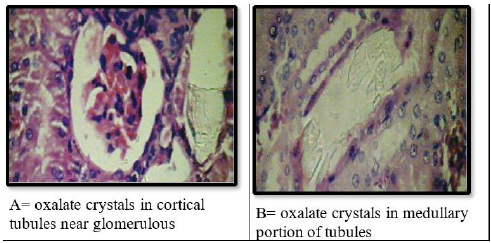
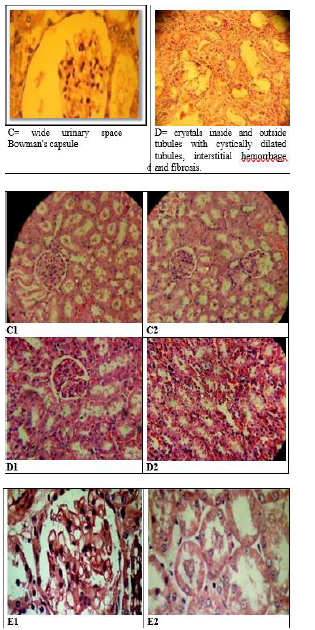
Effect of methanolic extract of CR oncalcium oxalate density and integrity of kidney:Histological examination of kidneys of different groups revealed compete absence of oxalate crystals in groups A, C,D and E. The positive control group (group B) is the only group shows crystals. This group also show some adaptive responses in their kidneys like massive dilation of Bowmans space, hyperceleularity of glomeruli and dilatation of renal tubules. The latter are blocked by oxalate crystals. The casts were seen intratubular and extratubular with interstitial hemorrhage (Figure 6 & 7).
Discussion
Noted conditions that predispose to Aerococcus urinae urinary tract infections are underlying comorbidities and seniority. The continuous progress of diagnostic methods allowed the misidentification obstacle to be overcome, thus leading to an increased detection of Aerococcus urinae infections and to the realization that this is a microbe of non-negligible clinical interest in geriatric, multimorbid patients.
The present study investigated the impact of CR experimentally induced urolithiasis in rats and the associated biochemical changes. This study found statistically significant changes in all measured parameters when compared to the normal group. Ethyline glyocle (EG) and ammonium chloride (AC) combination is a widely used experimentally for inducing hyperoxaluria by CaOx stones [17,25].
EG induced CaOx crystals in urinary tubules can produce damages in ECs by many mechanisms like production of free radicals, hence disruption of renal cellular membrane integrity probably by inducing lipid peroxidation (LPO), hence renal epithelial injury that increases the areas available for crystal attachment and eventual retention within the kidney [26-28].
Medicinal plants have a wide variety of phenolic compounds such as flavonoids and alkaloids that act potentially as antioxidants scavenge reactive oxygen species (ROS) and inhibit LPO [29]. Moreover, the increase of LPO and decrease of antioxidant potential have been reported in the kidneys of rats supplemented with a calculiproducing diet. In this context, oxalate has been reported to induce LPO and to cause renal tissue damage by reacting with polyunsaturated fatty acids in the cell membrane [30].
The current work revealed a significant increase in plasma TBARS levels in urolithiasis rats group indicates the pathological changes in tissues which increase the production and liberation of LPO into the circulation. This result is agreed with [31]. On the contrary, the decreased levels of plasma TBARS observed in the treatment group by CR indicates its strong antioxidant activity and this is consistent with others [32].
EG poisoning can lead to acute renal failure which is characterized by proximal tubular necrosis and an accumulation of (CaOx) monohydrate crystals in the urine and kidney tissues [33]. The estimation of serum urea and creatinine gives idea about the extend of affection of renal damage function. Our study showed significant change in serum urea and creatinine and these indicating that there is a deterioration renal function in group B compared to negative control and treated groups (A,C&D). The deterioration in renal function in group B may be through direct toxicity by EG or by OxCa or both, consistent with others [30,33]. Furthermore, the significant decrease in urea and creatinine in CR treated group (D) indicating the protective effect of CR against EG/ CaOx induced renal damage, hence preservation of normal renal function. The protective effect of CR may be due to prevention of crystal deposition in renal tubules, and prevention of CaOx induced injury on renal tubules, and this is consistent with others [34,35].
The protective effect of CR against stone formation was obvious in our study. Kidneys of treated (curative and preventive) groups (C ,D&E) was free of crystals either intratubular or interstitial compared with positive control group (B). These findings are agreed with [36]. The exact mechanism of this protection unclear. However, the following are possible mechanisms: 1- CR methanol extract reduces the LPO level thus preventing CaOx crystal attachment and subsequent development of kidney stones. 2- saponins and flavonoids present in CR prevent calcium and oxalate deposition [37]. 3- the large amount of vitamin E present in CR [38] has also, shown to decrease the urinary excretion of oxalate and calcium and restore antioxidant ability [39].
The urolithiolytic activity of CR was promising in our study as kidneys of treated group (group E) were also free of crystals compared to the positive control group. The mechanism of such urolithiolytic effect of CR also investigated in our study. There is a significant increase of water intake, urine volume, urinary pH, urinary Mg and citrate in group G compared to group F. The increase in water intake, hence urine volume reduce supersaturaion of urine by CaOX and this is consistent with others [40]. In addition, the diuretic effect of CR may be due to inhibition of aldosterone [32].
Urinary pH is a major determinant for kidney stone formation suggested that a urine pH approximately near 6 on the pH scale reduces the risk of kidney stone formation, however, the risk of uric acid and calcium stone formation increases progressively at urinary pH<5.5 [41]. Furthermore, the slight decrease in pH lead to increase urine calcium excretion mediated by a decrease in renal tubular calcium reabsorption. In addition, the increase in systemic acidity leads to a decrease in urinary citrate excretion [42]. So, if urinary pH rises, renal citrate production does as well, thus producing a decrease in tubular citrate reabsorption, and increased citrate excretion [43]
Alterations in urinary pH might be due to genetic variants or mutations in transport pathways, lifestyle habits such as specific diets or metabolic diseases, and infections. Inappropriately acidic or alkaline urine affects the solubility of various metabolites and salts. Furthermore, alkaline urine reduces the solubility of calcium phosphate products, and promotes the formation calcium phosphate stones [44].
Mg combines with oxalate, potentially reducing oxalate absorption in the gastrointestinal tract (GIT) and decreasing CaOx supersaturation in urine [45]. Moreover, Mg has been shown to lower urinary supersaturation of CaOx and increase urinary citrate [46].
Finally, the antibacterial activity of CR was also investigated and we found acceptable antibacterial effect against some bacteria that may be a predisposing factor for urolithiasis. This antibacterial activity is concentration (dose) depended in most bacteria. However, it is effective in low concentration in some bacteria like proteus.
In conclusion, CR is good for urolithiasis as protective and curative remedy. It has diuretic activity, rising urinary pH, increase urinary citrate and magnesium. Additionally, it has antibacterial activity, renal tissue protective activity both structurally and functionally.
References
- Stumpers S, Thomson N (2013) Review of kidney disease among Indigenous people. Australian Indigenous Health Bulletin 13(2): 1-22.
- Yashir F, Waqar M (2011) Effect of indigenious plant extracts on calcium oxalate crystallization having a role in urolithiasis. Urol. Res 39(5): 345-350.
- Khare P, Mishra VK, Arun K, Bais N, Singh R (2014) Study on in vitro anti-lithiatic activity of phyllanthusniruri Linn. Leaves by homogenous precipitation and turbiditory method. Inter J Pharma Pharmaceu Sci 6(4): 124-127.
- Jayaraman UC, Gurusamy A (2018): Review on Uro-Lithiasis Pathophysiology and Aesculapian Discussion. IOSR Journal Of Pharmacy 8(2): 30-42.
- Aggarwal A, Tandon S, Singla S, Tandon C (2010): Diminution of oxalate induced renal tubular epithelial cell injury and inhibition of calcium oxalate crystallization in vitro by aqueous extract of Tribulusterrestris. International Brazilian Journal of Urology 36(4): 480-489.
- Ferraro PM, Taylor EN, Gambaro G, Curhan GC (2017) Dietary and lifestyle risk factors associated with incident kidney stones in men and women. J Urol 198(4): 858-863.
- Pearle MS (2012) Shock-wave lithotripsy for renal calculi. Engl. J. Med 367: 50-57.
- Goldfarb DS, Coe RL (1999) Prevention of Recurrent Nephrolithiasis. Am Fam Physician 60(8): 2269-2276.
- Rathod NR, Biswas D, Chitme HR, Ratna S, Muchandi IS, et al. (2012) Urolithiatic effects of Punicagranatum in male rats.J. Ethnopharmacol . 140(2): 234-238.
- Bhatta CA, Shashi SC, Aswath A (2012) Phytochemical and ethno-pharmacological profile of Cratraevanurvala Buch-Hum (Varuna): A review. Asian Pac J Trop Biomed 45: 1162 -1170.
- Beluhan S, Ranogajec A (2010) Chemical composition and nonvolatile components of Croatian wild edible mushrooms. Food Chem 124(3): 1076-1082.
- Korish M (2016) Nutritional evaluation of wild plant Cissus rotundifolia. Ital J Food Sci 28: 43-49.
- Geissler PW, Harris SA, Prince R, Olsen A, Odhiambo R, et al. (2002) Medicinal plants used by Luo mothers and children in Bondo district, Kenya. J Ethnopharmacol 83: 39-54.
- Al-Mehdar AA, Al-Battah AM (2016) Evaluation of Hypoglycemic Activity of Boswelliacarterii and Cissus rotundifoliain Streptozotocin/Nicotinamide-Induced Diabetic Rats." Yemeni J Med Sci 10: 1-9.
- Mohammed WMA, Abbas AAY, Mohmmed H, Qasem MA, Saleem HAM, et al. (2019) Antidiabetic activity of Cissus routndifolia leaves supplement. World J. Pharmaceut. Res p.8.
- Jimoh A, Tanko Y, Mohammed A (2013) Anti-diabetic effect of methanolic leaf extract of Cissus cornifoliaon alloxan-induced hyperglycemic in Wister rats. Ann Biol Res 4(3): 46-54.
- Fan J, Glass MA, Chandhoke PS (1999) impact of ammonium chloride administration on a rat ethylene glycol urolithiasis model. Sca. Microsc. Int 13(2-3): 299-306.
- Khan A, Bashir S, Khan SR, Gilani AH (2011) Antiurolithic activity of Origanumvulgareis mediated through multiple pathways. BMC Complement Altern Med. 11(96): 2-16.
- Buege JA, Aust SD (1978) Microsomal lipid peroxidationmethods enzymology. PMID 52: 302-310.
- Rock RC, Walker WG, Jennings CD (1987) Nitrogen metabolites and renal function. In: Tietz NW, et al. [ed’s]. Fundamentals of Clinical Chemistry. 3rd Philadelphia. WB. Saunders. pp. 669-704.
- Hullatti KK, Sharada MS, Kuppasth IJ (2011) Studies on diuretic activity of three plants from Menispermaceae family. Pelagia Research Library 2(1): 129-134.
- Ntchapda F, Bonabe C, Romain D, Azambou K, Talla E, et al. (2016) Diuretic and antioxidant activities of the aqueous extract of leaves of Veprisheterophylla (Engl.) R. Let (Rutaceae) in rats. BMC Complement Alternative Med 16(516): 1-10.
- Dagley ST (1974) In Methoden der enzymatischen Analysis (Bergmeyer) HU Hrsg) Bd 2 S 1607-1611.
- Ma YL, Zhu DY, Thakur K, Wang CH, Wang H, et al. (2018) Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion (Allium cepaL.). Int J Biol Macromol 111: 92-101.
- Christina AJ, Muthumani P (2013) phytochemical investigation and anti lithiatic activity of Abelmoschusmoschatusmedikus. Int J Pharm Pharmace Sci 5(1): 108-113.
- Cruzan G, Corley RA, Hard GC, Mertens JJ, McMartin KE, et al. (2004) Subchronic toxicity of ethylene glycol in Wistar and F344 rats related to metabolism and clearance of metabolites. Toxicol Sci 81(2): 502-511.
- Khan SR (2011a) Crystal/cell interaction and nephrolithiasis. Arch Ital Urol Androl 83(1): 1-5.
- Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urology 189(3): 803-811.v
- Naczk M, Shahidi F (2006) Phenolic in cereals, fruits and vegetables: Occurrence, extraction and analysis. J Pharm Bioml Analysis. 41: 1523-1542.v
- Karadi RV, Gadge NB, Alagawadi KR, Savadi RV (2006) Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharma 105(1-2): 306-311.
- Aggarwal A, singla SK, Tandon C (2014) Urolithiasis phytotherapy as an adjunct therapy. Indian J Exp Biol 52(2):103-111.
- Gumaih HS, Salamah EN, Almadiy AA, Maktari MA, Alasbahy A (2020) Therapeutic Efficacy of Cissus rotundifolia as Antiurolithiasis and Antihypertensive Agent in Albino Rats. Int J Nanotechnology and Allied Sci 4(2): 30-40.
- Martin K (2009) Are calcium oxalate crystals involved in the mechanism of acute renal failure in ethylene glycol poisoning. Clin Toxicol 47(9): 859-869.
- Makasanaa A, Ranpariyab V, Desaia D, Mendparaa J, Parekha V (2014) Evaluation for the anti-urolithiatic activity of Launae aprocumbens against ethylene glycol-induced renal calculi in rats. Toxicol Reports 1: 46-52.
- Gumaih H, Al-Yousofy F, Ibrahim H, Ali S, Alasbahy A (2017) Evaluation of ethanolic seed extract of parsley on ethylene glycol induced calcium oxalate, experimental model. Int J Sci Res 6(3): 16831688.
- Moram GS (2016) Evaluation of anti-urolithiatic effect of aqueous extract of parsley (petroselinum sativum) using ethylene glycol-induced renal calculi. World J Pharmace Res 5(2): 1721-1735.
- Mubarak AY, Maktari MA, Gumaih H, Alasbahy A (2021) Phytochemical and Bioactive Evaluation of Cissus rotundifolia and Maydis stigma Cultivated in Taiz, Yemen. Biol Res 6(3): 66-75.
- Shalabi AA (2017) Chemical and Biological assessment of Cissus rotundifolia growing in Yemen. PHD thesis. Faculty of Pharmacy, Cairo University, Egypt.
- Sumitra K, PragasamV, Sakthivel R, Kalaiselvi P, Varalakshmi P (2005) “Beneficial effect of vitamin E supplementation onthe biochemical and kinetic properties of Tamm-Hors fallglycoproteinin hypertensive and hyperoxaluric patients . Nephrology. Dialysis. Transplant 20(7): 1407-1415.
- Pak CYC, Sakhaee K, Crowther C, Brinkley l (1980) Evidence justifying a high fluid intake in treatment of nephrolithiasis. Ann Int Med 93(1): 36-39.
- Sakhaee K (2007) Urinary pH as a Risk Factor for Stone Type Physicochemical and Pathophysiologic Bases of Elevated Urine pH. Ame Inst Phys 74-81.
- Bushinsky DA, Grynpas MD, Asplin JR (2001) Effect of acidosis on urine supersaturation and stone formation in genetic hypercalciuric stone-forming rats. Kidney. Int 59(4): 1415-1423.
- Suarez M, Youssef RF (2015) Potassium Citrate: Treatment and Prevention of Recurrent Calcium Nephrolithiasis .J. Clin .Nephro. Res 2(1): 1015-1011.
- Wagner CA, Mohebbi N (2010) Urinary pH and stone formation. J Nephron 23(16): 165-169.
- Liebman M, Costa G (2000) Effects of calcium and magnesium on urinary oxalate excrtion after oxalate loads. J Urol 163: 1565-1569.
- Riley JM, Kim H, Averch TD, Kim HJ (2013) Effect of Magnesium on Calcium and Oxalate Ion Binding. J Endouro 27(12): 1487-1492.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.