Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Arnold-Chiari Anomaly: A Review of Literature
*Corresponding author: Dzmitry Valchkevich, Associate Professor, Normal Anatomy Department, Grodno State Medical University, Grodno, Belarus.
Received: April 08, 2022; Published: April 20, 2022
DOI: 10.34297/AJBSR.2022.16.002193
Abstract
Arnold-Chiari malformations refer to abnormalities of the hindbrain originally described by the pathologist Hans Chiari from Austria in the 1890s. The essence of the Arnold-Chiari anomaly is the descent of the cerebellar tonsils into the foramen magnum with compression of the medulla oblongata and the development of corresponding neurological symptoms. The Arnold-Chiari anomaly can manifest itself at any age after the action of a provoking factor. This article describes the modern etiology, classification, clinical manifestations, as well as diagnostic and treatment methods of Arnold-Chiari anomaly.
Keywords: Human Anatomy, Anomaly, Arnold-Chiari Anomaly
Introduction
Arnold-Chiari anomaly is a group of congenital anomalies in the development of the brain, in which the main disorders are associated with the functions of the cerebellum and medulla oblongata (the part of the brain, where such vital centers as respiratory, vasomotor are located) [1]. At the same time anomaly is accompanied with displacement of these structures into the proximal portion of vertebral canal. The essence of the Arnold- Chiari anomaly is the descent of the cerebellar tonsils into the foramen magnum with compression of the medulla oblongata and the development of corresponding neurological symptoms. During that inclination of cerebellar tonsils, the circulation of the cerebrospinal fluid is difficult with a disorder of its outflow and development of hydrocephalus [2,3]. The Arnold-Chiari anomaly can manifest itself at any age after the action of a provoking factor (infection, intoxication, trauma, strong emotional stress). The main method of treatment is surgical, which is aimed at equalizing the hydrodynamic pressure of the cerebrospinal fluid at the level of the craniospinal junction, creating a large occipital cistern and eliminating compression of the brain stem [4,5].
Reasoning
Etiology
At the moment, there are the following main reasons leading to
the development of the Arnold-Chiari anomaly:
1. Violation of the embryonic development of the central nervous
system (CNS) in combination with anomaly of the skull
and spine [6]. This factor is of primary importance for the
formation of Chiari malformation of types II and III.
2. Craniosynostosis or insufficient growth of the skull, especially
the posterior fossa, can cause it to narrow. This is believed to
cause compression of the cerebellum, which is pushed down
through the foramen magnum [6,7].
3. Spinal cord injury is one of the mechanisms of Chiari
malformation type II. It occurs due to a congenital spinal
hernia, usually of the lumbosacral region. During longitudinal
growth of the spine, the associated spinal cord stretches, which
in turn displaces the neural structures in the posterior fossa
downward through the foramen magnum [8].
4. Violation of CSF dynamics [7,9,10].
Classification
In 1891–1894, J. Arnold and N. Chiari described a malformation of the brain, characterized by a caudal displacement of the cerebellum and brain stem and causing certain changes in the cervical spinal cord. Later this disease was called “Arnold-Chiari anomaly” (ACA) and was attributed to congenital malformations that are hereditary in nature [2,7]. The traditional division of ACA into 4 types is based on quantitative determination of the dislocation (displacement) of the cerebellar tonsils into the foramen magnum below the Chamberlain’s line (Figure 1). This line connects the posterior edge of the hard palate and the position (the middle of the posterior edge of the foramen magnum) [11].
Chiari malformations are classified based on their morphology
and anatomical defects:
i. Chiari I is characterized by one or both pointed cerebellar
tonsils, which protrude 5 mm below the foramen magnum
(Figure 2).
ii. Chiari II – caudal dislocation of the lower parts of the cerebellar
vermis, medulla oblongata and fourth ventricle.
iii. Chiari III – caudal displacement of all structures of the posterior
cranial fossa with the formation of suboccipital or high cervical
encephalomeningocele.
iv. Chiari IV – cerebellar hypoplasia with ectopia of the medulla
oblongata [2,3,7,8,12].
In clinical practice, type 1 AСA is more common, types 2, 3
and 4 refer to rare severe (often incompatible with life) congenital
malformations [5,11-13].

Figure 2: The comparison of the anatomical structures of posterior cranial fossa in normal condition (a) and in type I ACA (b).
Diagnostics
Diagnosis of anomalies of the craniovertebral junction includes
a number of research methods:
1. Clinical neurological examination and clinical and genealogical
analysis
2. Magnetic resonance imaging of the craniovertebral junction,
brain and spinal cord in sagittal and axial projections in T1 and
T2 modes; MR-angiography is added as needed
3. Computed tomography of the craniovertebral junction if it is
impossible to perform MRI, if it is necessary to assess the bone
structures of this area
4. X-ray examination of the skull and cervical spine if CT or MRI of
the craniovertebral region is impossible
5. Examination of the fundus
6. Otoneurologic examination
7. Prenatal echography is performed according to indications
8. Transcranial Doppler sonography, evoked stem potentials are
performed according to indications [13-16].
Magnetic resonance imaging (MRI) is the most informative and widely used method for the diagnosis of ACA [4,6,8,15,16]. With MRI, in addition to detecting the characteristic dislocation of the tonsils, we can get useful information about the presence or absence of pathology (syringomyelia, hydrocephalus). Computed tomography (CT) with myelography/cisternography can be performed for patients who cannot undergo MRI (if there are contraindications to this diagnostic method). However, in this case, the use of high-speed (for example, 64-slice) spiral CT (MSCT) will be optimal, which makes it possible to diagnose ACA without the use of a contrast agent, while non-contrast multislice computed tomography (MSCT) with sagittal reconstruction eliminates the need for myelography. At the same time, the use of phase contrast MRI helps to investigate the flow of cerebrospinal fluid (CSF) at the level of the foramen magnum and to distinguish symptomatic ACA types 0 and 1 from asymptomatic cerebellar ectopia, as well as to clarify the indications for surgical decompression and predict the outcome of surgical treatment [11,17].
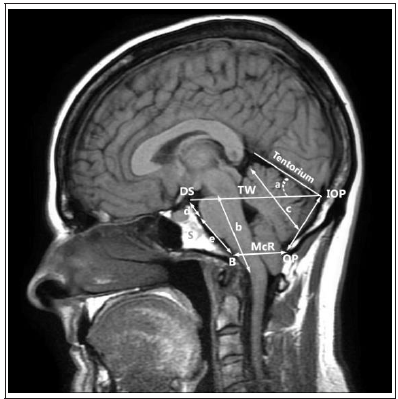
When diagnosing Arnold-Chiari anomaly using MRI, the
landmarks shown in Figure 3 are used:
1. d + e – length of clivus.
2. S – spheno-occipital synchondrosis
3. D – the length of the base of the sphenoidal plate from the back
of the sella turcica and spheno-occipital synchondrosis to the
clivus
4. e – the distance between synchondrosis and basion (the lowest
point of the anterior edge of the foramen magnum along the
midline)
5. b – the length of the brainstem between the junction of the
midbrain and the pons and the junction the medulla oblongata
with the spinal cord
6. a – the angle of the tentorium cerebelli in relation to the
Twining’s line (the line connecting the tuberculum sellae and
the confluens of the sinuses)
7. c – the length of the cerebellar hemisphere
8. DS – the superior margin of the dorsum sellae
9. IOP – internal occipital protuberance
10. OP – opisthion
11. B – basion
12. TW – Twining line
McR (from B to OP) (McRae’s line) – the line of entry into the foramen magnum. It connects the basion and position (Figure 1) [8,18,19].
A number of studies have shown differences in the structure of the posterior cranial fossa in patients with ACA syndrome compared with patients in whom this anomaly was not observed [16,20-22] (Table 1).
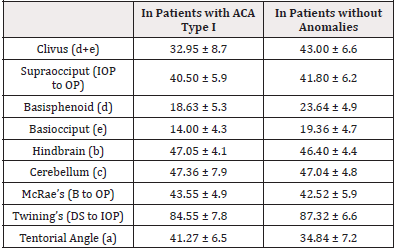
Table 1: Anatomy of the posterior cranial fossa in patients with ACA compared with ones in whom there is no anomaly (in mm).
Сlinical Symptoms of ACA
The Arnold-Chiari anomaly may be manifested with the
following neurological syndromes [1,2,7,10]:
a. Hypertensive-hydrocephalic
b. Cerebellar
c. Bulbar-pyramidal
d. Radicular
e. Vertebrobasilar insufficiency
f. Syring myelitis
Hypertensive-hydrocephalic syndrome develops as a result of impaired circulation of cerebrospinal fluid (CSF). Normally, the cerebrospinal fluid flows freely from the subarachnoid space of the brain into the subarachnoid space of the spinal cord. The drooping lower part of the cerebellar tonsils blocks this flowing, like a bottle cork. The formation of cerebrospinal fluid in the vascular plexuses of the brain continues, and it has nowhere to drain (apart from the natural mechanisms of absorption, which in this case are not enough). CSF accumulates in the brain, causing an increase in intracranial pressure (intracranial hypertension) and expansion of liquor-containing spaces (hydrocephalus). This manifests itself as a bursting headache, which is aggravated by coughing, sneezing, laughing, straining. Pain is felt in the back of the head, neck area, possible neck muscle tension. There may be episodes of sudden vomiting, in no way associated with food intake [1,15,23]. Cerebellar syndrome manifests itself as a violation of the coordination of movements, a “drunken” gait, a passing fall when performing purposeful movements.
Patients are worried about dizziness. Tremors may appear in the limbs. Speech may be impaired (it becomes divided into separate syllables, chanting). Quite a specific symptom is “downward nystagmus”. These are involuntary twitching of the eyeballs, directed, in this case, downward. Patients may complain of double vision due to nystagmus [1,17]. Bulbar pyramidal syndrome is named after the structures that are compressed. Bulbus is the name of the medulla oblongata, therefore bulbar syndrome means signs of damage to the medulla oblongata. And the pyramids are anatomical structures of the medulla oblongata, which are bundles of nerve fibers that carry impulses from the cerebral cortex to the anterior horns of the spinal cord. The pyramids are responsible for voluntary movements in the limbs and trunk. According to the above, bulbar pyramidal syndrome clinically manifests as muscle weakness in the limbs, numbness and loss of pain and temperature sensitivity (fibers pass through the medulla oblongata).
Compression of the nuclei of the cranial nerves, located in the brain stem, causes visual and hearing disorders, speech (due to impaired movement of the tongue), nasal voice, choking when eating, difficulty breathing. Possible short-term loss of consciousness or loss of muscle tone with preserved consciousness [1,20,23,24]. Radicular syndrome in the case of Arnold-Chiari anomaly consists in the appearance of signs of dysfunction of the cranial nerves. These can be impaired mobility of the tongue, a nasal or hoarse voice, impaired swallowing of food, hearing defects (including tinnitus), impaired sensitivity on the face [1,15].
Vertebrobasilar insufficiency syndrome is associated with impaired blood supply in the corresponding blood pool. Because of this, there are attacks of dizziness, loss of consciousness or muscle tone, vision problems. As you can see, most of the symptoms of Arnold-Chiari malformation arise not as a result of one immediate cause, but due to the combined influence of various factors. So, attacks of loss of consciousness are caused both by compression of specific centers of the medulla oblongata and by impaired blood supply in the vertebrobasilar basin. A similar situation occurs with visual impairment, hearing impairment, dizziness and so on [15,24,25]. Syring myelitis syndrome does not always occur, but only in cases of a combination of Arnold-Chiari malformation with cystic changes in the spinal cord. These situations are manifested by dissociated sensory disturbances (when temperature, pain and tactile sensitivity is isolated in isolation, and deep (position of the limb in space) remains intact, numbness and muscle weakness in some limbs, dysfunction of the pelvic organs (urinary and fecal incontinence) [1,2,7,11,15,25,26].
Treatment
Treatment depends on the presence of symptoms of the disease. If the defect was detected by chance (that is, it does not have clinical manifestations and does not bother the patient) during magnetic resonance imaging, then the treatment is not carried out. The patient is under dynamic observation so as not to miss the moment of the appearance of the first clinical symptoms of brain compression.
If the anomaly manifests itself as a slightly pronounced
hypertensive-hydrocephalic syndrome, then attempts are made to
conservative treatment.
I. Dehydration drugs (diuretics). They reduce the amount of
cerebrospinal fluid, helping to reduce pain
II. Nonsteroidal anti-inflammatory drugs to reduce pain
III. Muscle relaxants in the presence of muscle tension in the
cervical region [1,2,5, 8].
If there is no effect or the patient shows signs of other neurological syndromes (muscle weakness, loss of sensitivity, signs of dysfunction of the cranial nerves, periodic attacks of loss of consciousness and so on), then they resort to surgical treatment. The main treatment for Chiari malformation is surgery to restore cerebrospinal fluid flow through the craniovertebral junction and relieve pressure on the cerebellum and hindbrain by decompressing the posterior fossa [27]. Surgery is recommended for patients with persistent symptoms and confirmed tonsillar hernia. For asymptomatic tonsil hernias, follow-up is recommended if symptoms do not develop. The best surgical results are seen when surgery is performed within 2 years of the onset of symptoms [27- 29].
The standard surgical technique for Chiari I is decompression of the posterior fossa [28,30]. This is achieved by suboccipital craniectomy with foramen magnum enlargement, often in conjunction with C1 and possibly C2 laminectomy. The dura mater may or may not open, followed by dissection of the arachnoid adhesions, if any. Depending on the available expansion of the dura and the size of the posterior fossa, duraplasty may be required. For duraplasty, an autograft, such as the occipital fascia, as well as an artificial dura can be used. More recently, minimally invasive techniques similar to those used in the spine have been described. This allows for smaller incisions, less damage to soft tissue, less manipulation of the dura mater (in this case, there are fewer complications).
The initial surgical correction of Chiari II is the correction of the myelomeningocele, usually within the first 48 hours. It can also be done in the womb with a hysterotomy. Closure of spinal dysraphism can be accomplished in various ways: by primary closure of the skin, a musculocutaneous flap, or a fascial skin flap (depending on the severity, the layers involved and the available adjacent tissue). The vast majority will eventually need a ventricular shunt to drain the cerebrospinal fluid in case of hydrocephalus. Posterior decompression is performed later if necessary to allow suboccipital expansion. In Chiari III anomaly, the occipital/high cervical encephalocele is corrected with resection of the hernial contents (since they are usually nonviable), followed by closure of the dura mater and cranioplasty [12].
Conclusion
Arnold-Chiari malformation is one of the developmental anomalies of the brain. It may be asymptomatic, or it may manifest itself from the first days of life. The clinical manifestations of the disease are very diverse; diagnostics is carried out using magnetic resonance imaging. The therapeutic approaches are different: from the absence of any intervention to surgical methods.
Conflicts of Interests
The authors declare that there is no conflict of interest.
References
- Zaharova ES Sindrom, AV Vorob'jova (Anomalija) (2019) Arnol'da-Kiari kak projavlenie vrozhdjonnogo zabolevanija v praktike vracha-pediatra. Vestnik novyh medicinskih tehnologij [Jelektronnoe izdanie] 3: 34-37.
- Avramenko TV, AA Shevchenko, IJu Gordienko (2014) Mal'formacija Arnol'da-Kiari. Prenatal'nye i klinicheskie nabljudenija. Pediatrija [Jelektronnoe izdanie] 87-88.
- Raynor RB (1986) The Arnold-Chiari malformation. Spine (Phila Pa 1976) 11(4): 343-344.
- Bikmullin TA, JeR Bariev, VI Anisimov (2015) Sravnitel'nyj analiz razlichnyh metodov hirurgicheskogo lechenija anomalii Arnol'da-Kiari. Innovacionnye tehnologii v medicine. Tom 1 [Jelektronnoe izdanie] 4: 28-29.
- Rhoton AL Jr (1976) Microsurgery of Arnold-Chiari malformation in adults with and without hydromyelia. J Neurosurg 45(5): 473-483.
- Mancarella C, Delfini R, Landi A (2019) Chiari Malformations. Acta Neurochir 125: 89-95.
- Schijman E (2004) History, anatomic forms, and pathogenesis of Chiari I malformations. Childs Nerv Syst 20(5): 323-328.
- Ringstad Geir, Per Kristian Eide (2019) Chiari malformation type 1 - diagnosis and treatment. Tidsskr Nor Laegeforen 139(10).
- Krupina NE (2016) Formirovanie Mal'formacii Kiari. Vestnik Ural'skoj medicinskoj akademicheskoj nauki 1: 91.
- Milhorat TH, Nishikawa M, Kula RW, Dlugacz YD (2010) Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir (Wien) 152(7): 1117-1127.
- Kantimirova EA, NA Shnajder, MM Petrova, IG Strockaja, NE Dutova, et al. (2011) Vstrechaemost' anomalii Arnol'da-Kiari v praktike nevrologa. Mezhdunarodnyj nevrologicheskij zhurnal 7: 6-7.
- Joaquin A Hidalgo, Craig A Tork, Matthew Varacallo (2021) Arnold Chiari Malformation.
- Ovsova OV, OA Lvova (2010) Anomalii kraniovertebral'noj oblasti. Klinicheskaja medicina 4(10): 44-48.
- Barashnev JuI, M Triada H (2001) Perinatal'naja nevrologija 640.
- Mozhaev CB (2012) Osobennosti patogeneza, kliniki i diagnostiki anomalii Kiari 1 tipa Nejrohirurgija 3: 13-19.
- Raymond F Sekula, Peter J Jannetta, Kenneth F Casey, Edward M Marchan, L Kathleen Sekula, et al. (2005) Dimensions of the posterior fossa in patients symptomatic for Chiari I malformation but without cerebellar tonsillar descent. Cerebrospinal Fluid Res 2:11.
- Milhorat TH, Nishikawa M, Kula RW, Dlugacz YD (2010) Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir (Wien) 152(7): 1117-1127.
- Susman J, Jones C, Wheatley D (1989) Arnold-Chiari malformation: a diagnostic challenge. Am Fam Physician 39(3): 207-211.
- MacManus D, Bartlett P (1986) The role of nuclear magnetic resonance imaging in the diagnosis of Arnold-Chiari malformation. Radiography 52(606): 275-280.
- Jörg Klekamp (2015) Chiari I malformation with and without basilar invagination: a comparative study. Neurosurg Focus 38(4): E12.
- Roller LA, Beau B, Saindane AM (2015) Demographic Confounders in Volumetric MRI Analysis: Is the Posterior Fossa Really Small in the Adult Chiari 1 Malformation?. AJR Am J Roentgenol 204(4): 835-841.
- Aboulezz AO, Sartor K, Geyer CA, Gado MH (1985) Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: a quantitative approach with MR imaging. J Comput Assist Tomogr 9(6): 1033-1036.
- Wall M (2010) Idiopathic intracranial hypertension. Neurol Clin 28(3): 593-617.
- Smith JS, Shaffrey CI, Abel MF, Arnold H Menezes (2010) Menezes AH Basilar invagination. Neurosurgery 66(3): 39-47.
- Menezes AH (2012) Craniovertebral junction abnormalities with hindbrain herniation and syringomyelia: regression of syringomyelia after removal of ventral craniovertebral junction compression. J Neurosurg 116(2): 301-309.
- Greitz D (2006) Unraveling the riddle of syringomyelia. Neurosurg Rev 29(4): 251-263.
- Goel A, Kaswa A, Shah A (2019) Atlantoaxial Fixation for Treatment of Chiari Formation and Syringomyelia with No Craniovertebral Bone Anomaly: Report of an Experience with 57 Cases. Acta Neurochir Suppl 125: 101-110.
- Abd El Barr MM, Strong CI, Groff MW (2014) Chiari malformations: diagnosis, treatments and failures. J Neurosurg Sci 58(4): 215-221.
- Tubbs RS, Lyerly MJ, Loukas M, Shoja MM, Oakes WJ (2007) The pediatric Chiari I malformation: a review. Childs Nerv Syst 23(11): 1239-1250.
- Nash J, Cheng JS, Meyer GA, Remler BF (2002) Chiari type I malformation: overview of diagnosis and treatment. WMJ 101(8): 35-40.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.