Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Enzyme Engineering of The Powderization of Plant Tissues Caused by Enzymatic Degradation of Structural Substrates
*Corresponding author: Xuejun Zhang, Department of Enzyme biochemistry and natural product chemistry, Guizhou University, Guiyang, China..
Received: November 21, 2022; Published: November 28, 2022
DOI: 10.34297/AJBSR.2022.17.002366
Abstract
Eucommia Ulmoides Gum (Eu-Gum), or gutta-percha, is a basic and strategic material. However, the inner dark brown hard shell within Eu fruit shell is very difficult to remove, being the bottleneck of high purification of fruit shell Eu-Gum. From the experimental phenomenon of pulverization of Eu plant tissue, the author realized the mechanism of that enzyme degraded substrates to pulverize plant tissue and thus putted forward a theory of plant tissue pow erization by enzymatic degradation, “the enzymatic degradation of structural substrates causes the collapse of plant structure and the pow erization of tissue” to guide this study of the purification of Eu fruit shell crude Gum. It was found that the xylan residues in xylose hemicellulose were structural substrates in plant tissue..
The alkaline xylanase pulverized the outermost pericarp tissue and the acid xylanase disintegrated structure of and pulverized tissue of the inner dark brown hard shell by degrading substrate, and soft green, gray whit Eu-Gum capsule pieces were obtained. The surfactant assisted ultrasonic removed residual detritus and pigment from the Gum filament to obtain 98.94% pure white primary floc Eu-Gum. The fruit yield of the new orchard zed Eucommia ulmoides varieties cultivated in recent year is 31~40 times higher than that of the wild varieties, being the highest among all Eucommia ulmoides organs. The theory of enzymatic degradation and pow erization has been proved to have practical guiding effect on the production of high purity Eu fruit shell Gum.
Keywords: Structural substrate, Xylose hemicellulose, Xylanase, Tissue powderization, Inner hard shell, Theory of powderization by enzymatic degradation, White floc eu gum
Introduction
Eucommia ulmoides Gum, Eucommia trans-rubber or Duzhong (Chinese Name) Gum, could be applied to human body materials in vivo because of its non-toxic, and has good physiological compatibility and blood compatibility, so could be made to fill the root of the deciduous tooth gum tip for Eucommia ulmoides; It’s also possible to make materials for different purposes in different parts of the adult body. Eucommia Ulmoides Oliver (Eu) is an economic rubber tree in subtropical and warm temperate zones [1]. China’s forest area accounts for 95% of the global total. Eucommia transrubber and Hevea Brasiliense’s cis rubber are isomers of the same compound with complementary structure. Eucommia rubber could be a substitute rubber source of Hevea Brasiliense’s which main become extinct soon. Natural biological resources must rely on biological enzymolysis to extract, to keep the nature of biological essence of the resources stable and unchanged. Biological enzymes are non-toxic, harmless catalysts, with high efficiency and low energy consumption. They have been used for extraction of natural products from plants for more than 80 years. At the first International Conference on Enzyme Engineering in 1971, enzyme biochemistry was named “Enzyme-engineering” [2].
Enzyme-engineering extraction of natural products is efficient and pollution-free. Eu-Gum is one of the few biological materials in the world with duality of rubber and plastic and a variety of unique functions, an important basic material and strategic material. The application of Eu-Gum lies in its high purity with unique natural physical and chemical properties without impurities and solvents. Eu bark, leaf and fruit are the organs with the highest content of Gum. The Gum content is 10%~12% in fruit (samara) (shell ≥17%), 6%~10% in stem bark and 1%~3% in leaf [1,3-4]. Professor DU, et al. [1] pointed out: the order of Eu-Gum content in each organ was fruit>stem bark>leaf. The Gum content in fruit of different variation types was 4.34~4.89 times that of leaves, 3.79~5.47 times that of leaf in different producing areas and 2.98~6.75 times that of leaf in different clones. Fruit has the highest utilization rate of comprehensive resources and the largest economic benefit. Products, edible oil (27%~33%) [5], pharmaceutical ingredients and Eu-Gum, all have high economic value. Wild Eucommia ulmoides has long had a bad reputation for low fruit yield. The fruit yield of the improved and orcharized Eucommia varieties developed by Professor DU’s team was 31 to 40 times [6] that of the wild trees.
The Disintegration and Powderization of Plant Tissues by Acid Protease
The content of Eu-Gum in pure shell after kernel removal was greatly increased. In the first stage of enzymolysis extraction, natural medicine components and crude Eu-Gum could be obtained simultaneously. The drug components were dissolved in the enzymolysis solution, while EU Gum silk remained in solid form [7,8] (Table 1) (Figure 1).

Figure 1: Pictures of the hydrolyzation of structural substrate by acid protease, which resulted in the disintegration of structure and powderization of organ tissue.
Table 1 showed that the extraction rate of acidic protease was 2~13 times that of pectinase and cellulase. The photos of corresponding enzymatic hydrolysis phenomenon in Figure 1 showed that the high extraction rate of acidic protease was due to the accompanied disintegration and pulverization of plant tissue. After dried at 60℃, all of powder obtained from the enzymolysis muds passed through a 30-mesh sieve, particle size was less than 0.55mm. The contact area between powdery tissue and solution increased greatly. The dissolution rate, amount and types of drug components increased exponentially as well. The structure collapse and tissue powdery phenomenon of acidic protease consistently presented in all acid protease experiments. The metabolic enzymes in plants remained near the situ as structural proteins during senescence and degeneration of plant tissues. Enzymatic erosion of structural proteins led to tissue disintegration and powderization.
The old enzymolysis extraction theory believed that enzymolysis extraction of plant natural products was based on enzymatic degradation of cell wall tissue to extract products inside the cell. And yet, the experimental phenomenon in this study showed that the degradation of structural substrates by enzymes accompanied by tissue disintegration and powderization was the key to the high yield of natural products. According to (Table 1) and (Figure 1), the tissue powder phenomenon and high extraction rate always appeared in the experiments on the biochemical properties of acid protease, suggesting that the tissue powdery phenomenon of enzymatic hydrolysis of plants originated from the erosion of structural substrates. Accordingly, the powderization theory of enzymatic hydrolysis substrates: “the enzymatic degradation of structural substrates causes the collapse of plant structure and the powderization of tissue” was put forward to guide the study of enzymatic degradation and powderization of the inner shell tissue inside Eucommia fruit.
To clarify the real process and results of experiments more directly and transparently, more photos were adopted in this paper.
Bottleneck of Enzymatic Degradation and Purification of Fruit Shell Gum
(Figure 2) There were two stages in the enzymatic hydrolysis of plant organs containing Eu-Gum to extract natural medicines and Eu-Gum comprehensively. The first stage was to extract drug ingredients, but the Eu-Gum and plant detritus in solid form were retained. The second stage was to purify Eu crude Gum, the enzyme disintegrated the structure of residual tissue debris and purified Eu-Gum by pulverized tissue. Fruit-shell Eu-Gum was the net-like plane pocket capsule layer as shown in Figure 7. The Eu- Gum layer divided the fruit shell tissue into two tissue layers, the outer pericarp, and the inner shell. The inner shell consisted of two layers of tissue, namely, the intermeddle tissue and the innermost tissue. The innermost tissue was the dark brown hard shell wrapped in the oily kernel. Meanwhile, the intermeddle tissue was the tissue embedded with Gum filaments see Figure 5B that was located between the innermost hard shell and Eu-Gum layer. In the purification stage of Eu-Gum, acidic protease eroded the structural proteins in the intermeddle tissue layer to disintegrate the structure of the layer and powdered its tissue. Thus, the adhesion of the dark brown innermost layer hard fruit shell and gum capsule is removed, as shown in Figure 2. The dark brown innermost hard shell is the bottleneck of high purification of fruit shell Eu-Gum.

Figure 2: The inner dark brown hard shell that wrapped the kernel. The inner dark brown hard shell before and after the hydrolysis by acidic protease.
The state of the innermost dark brown hard shell attached to
the Eu fruit capsules in the Eu fruit shell crude gum Figure11A
showed as Figure 2. The inner shell was naturally contained within
the EU Gum capsule. The big size of the fragment as shown in Figure
2 cannot escape from the opening (≤4.5mm) of the kernel removed
from the capsule in Figure 7. The above plant tissue pulverization
theory pointed out this research direction of corroding innermost
dark brown hard fruit shells:
a) Identification of structural substrates in inner fruit shell
b) Screening enzymes with degradation function.
Materials and Methods
Materials
Raw material: Fruit Shell Crude Gum (content ≤80%), donated by the Bei-Long Eucommia Biological Engineering Co., LTD, Qingzhou City, Shandong Provence.
Enzymes and biochemical reagent: Enzyme preparation, 537- Acid protease (50,000 U/g), Alkaline xylanase (42,000 U/g) and Acid xylanase (200,000 U/g) were purchased from the Shandong Sukehan (Weifang) Bio-technology Co., LTD. Glycine standard (A111465), ≥99.0% was purchased from Shanghai Aladdin Biochemical Technology Co. LTD. Xylose standard was purchased from Sigma-Aldrich (Shanghai) Trading Co. LTD. Citric acid and Sodium citrate (food additive, purity 99%) were purchased from Weifang Ensign Industrial Co., LTD.
Chemical reagent: Distilled water: Laboratory homemade by SZ-96A quartz-distiller. 3,5-Dinitrolated salicylic acid and ninhydrin were purchased from the Shanghai Kefeng Industrial Co. LTD. Sodium hydroxide, phenol, sodium sulfite, sodium potassium tartrate, sodium acetate, glacial acetic acid, anhydrous ethanol, etc., all were analytically pure and purchased from Tianjin Kermeo Chemical Reagent Co., LTD.
Instruments and Equipment
SHA-CA constant temperature water bath oscillator with digital display, manufactured by Changzhou Putian Instrument Manufacturing Co., Ltd. AL04 electronic balance with 1/10,000th accuracy, purchased from Mettler-Toledo Instrument Co., Ltd., Germany. UV754N UV-visible Spectrophotometer and pHS-3E pH meter, manufactured by Shanghai INESA Scientific Instrument Co., Ltd. The SB-400DTY multi-channel ultrasonic sweep cleaner from the Ningbo Xinzhi Biological Technology Co., Ltd. The B-260 water bath, manufactured by Shanghai Yarong Biochemical Instrument Factory; The 500 electronic platform scale with 1/100th accuracy from Bangyi Precision Measuring Instrument (Shanghai) Co., Ltd. Thermos Heraeus Multifuge X3R Universal Benchtop Centrifuge, XW-80A vortex mixer and Thermos Scientific™ variable volume pipette and tip from Thermos Fisher Scientific (China) Co., Ltd. The stainless-steel standard sieve was purchased from the Shang-Yusheng- chao Instruments Co., Ltd. Shaoxing, Zhejiang province.
Methods
Enzymatic degradation
a) The disintegration and powderization of “the outermost
brown pericarp” The outermost brown pericarp 50.0 g of air-dried
Fruit Shell Crude Gum was added in a 1000-mL conical flask. 600
mL of citric acid-sodium buffer (pH 7.2) was divided into three
portions to mix with 15 g of alkaline xylanase preparation in
turn and elute the active enzyme by stirring in a 250-mL beaker.
The upper enzyme-containing liquid was centrifugally collected
and combined in the erlenmeyer flask, which was shaken on a
water bath shaker at 50℃ for 48 hours. The hydrolysate and solid
residues were separated with a 30-mesh sieve. The hydrolysate
solution was ready for testing and the solid residues with Eu-Gum
were returned to the erlenmeyer flask for a repeated enzymolysis
under the same conditions. After 40 h’s shaking, the hydrolysate
solution and solid residue were separated by the sieve, and the
solid residue was washed twice with clear water and returned
to the original erlenmeyer flask for next enzymolysis. The first
hydrolysate solution was infused into six 100-mL centrifuge tubes,
which were balanced on the platform scale and centrifuged at 5000
rpm for 5 minutes. The supernatant was stored for testing.
b) The disintegration and powderization of “the intermeddle
tissue embedded with Gum filaments” The tissue layer located
between Gum capsule layer and dark brown inner hard shell is
shown as Figures 5B & 6B. 600 mL of citric acid-sodium citrate
(pH 3.2) buffer was divided into three parts to mix with 18 g acid
protease preparation, stirred to elute the active enzyme in a 250-
mL beaker in turn. Three parts of enzyme-containing solutions
were centrifugally collected in the erlenmeyer flask with solid
residues, which was shaken at a moderate speed for 48.0 hours
on a water bath shaker at 55°C. Then the hydrolysate and the solid
Eu-Gum with plant residue were separated into a 30-mesh sieve.
The solid residues were returned to the erlenmeyer flask to repeat
the disintegration under the same conditions. After 40 h’s repeated
disintegration, the hydrolysate and the solid residue were separated
with the 30-mesh sieve and the solid residues were washed three
times with clean water in the 30-mesh sieve and returned to the
original triangular flask. Divided the first hydrolysate solution
into six 100 ml centrifuge tubes and then balanced. Caps tighten
centrifuge tubes were centrifuged at 5000 rpm for 5 min. The
supernatant should be stored in a refrigerator in the dark for
testing.
c) The disintegration and powderization of “ The innermost
tissue, the dark brown hard inner shell” 600 mL of citric acidsodium
citrate buffer (pH 4.8) was divided into three parts to mix
with 16 g of acid xylanase preparation in a 250 mL beaker for
elution of active xylanase in turn, which were centrifuged at 3600
rpm for 4 min. Three parts of supernatants with active enzyme
were combined with solid residues degraded by acid protease in
the erlenmeyer flask to shake quickly on a water bath shaker at
50°C for 72 hours. The enzymatic solution containing tissue powder
was separated with a 30-mesh sieve. The solid residues in the sieve
were washed two times to collect tissue powder with clean water
and returned to the original Erlenmeyer flask for the secondary
enzymatic hydrolysis. The repeated acid xylanase powderization
was carried out under the same condition as the previous for 70 h,
and 30 mesh sieve was used to separated solution and elutriated
the solid residues with clean water five times to collect all tissue
powder. Solutions containing the tissue powder were centrifuged
at 6000 rpm for 10 minutes to collect the solid fine powder and
air-dry. The first hydrolysate solution was infused into 6 centrifuge
tubes to centrifuge at 4500 rpm for 8 minutes. The supernatant was
stored in the refrigerator for testing, and the sediment fine powder
was air-dried and photographed.
Processing of hydrolysate solution: Each of the three hydrolysates that had been prepared for test was parallelly absorbed for three 80.0mL using pipettes. 3×3=9 samples were prepared in nine beakers. 14g activated carbon was added to each beaker for decolorization, stirring, heating to a boil for 4minutes. Then beakers were cooled with tap water and cooled samples were centrifuged to collect the supernatant. The settled activated carbons were washed with 20mL hot water, centrifuged to collect the supernatant. The washing solutions were combined separately, then 9 supernatants were carefully infused into nine 100mL volumetric bottles, adding distilled water to the bottle mark, and shaking well. Nine samples shall not be mixed or cross-operated.
Determination of Hydrolyzed Amino Acids [10]
Determination of amino acid by ninhydrin colorimetry:
a. Principle: Free amino acids are heated with ninhydrin in
a sodium acetate-acetic acid buffer (pH 6.8) to generate blue-violet
compounds (except proline). Its color is proportional to the amino
acid content. The absorbance value is measured at 568nm. Its
sensitivity could reach 2×10-10-5×10-10mol·L-1, and the derivatives
are stable and reliable.
b. Amino acid standard solution: Accurately weighed 0.200g
of the glycine standard stored in a desiccator and dissolved with a
small amount of water. The solution was quantitatively transfer to
a 100mL volumetric flask, adding water to the graduation line and
got the standard solution of 2.0mg·mL-1 concentration. 10.0 mL of
this standard solution was pipetted into another 100mL volumetric
flask, adding distilled water to the mark, and shaking upside down
to obtain the glycine standard solution of 200μg·mL-1 for use.
c. Ninhydrin solution: Weighed 1.50 g of ninhydrin in a 250-
mL beaker and added a little absolute ethanol to dissolve it. After
all dissolved, the solution was quantitatively transferred into a 100-
mL brown volumetric flask, adding absolute ethanol to the mark.
The ninhydrin chromogenic solution was stored in dark cooler for
spare. If the ninhydrin is reddish, the red oxidized material part
needs to be removed, see the following Note.
d. Sodium acetate-acetic acid buffer (pH 6.8) solution:
Prepared 0.2 mol·L–1 sodium acetate solution and 0.3 mol·L–1 acetic
acid solution, mixed two solutions at a volume ratio of 8.6:1.4, and
fine-tuned the pH to 6.80 with a pH meter.
e. Potassium iodate solution: Accurately weighed 0.10 g
potassium iodate into a 250 mL beaker, added 30 mL of distilled
water to dissolve, then added 20 mL of absolute ethanol and mixed
them evenly, then stored in a grinding triangle bottle with stopper.
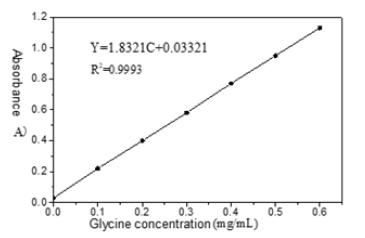
Standard curve drawing: Accurately pipetted 0.0, 0.50, 1.00, 1.50, 2.00, 2.50, 3.00mL (equivalent to 0, 0.10, 0.20, 0.30, 0.40, 0.50, 0.60mg glycine) from 200μg·mL-1 glycine standard solution with an adjustable pipette gun into seven 10 mL colorimetric tubes with stopper, and added distilled water until the volume of each tube was exactly 3.0 mL, then added 1.0mL sodium acetate-acetic acid buffer (pH=6.8) and 1.0mL ninhydrin solution (concentration 15g·L-1). Then we tightly covered the tube with cap and vortexed to mix well, heated in a boiling water bath for 15 min, took out rapidly and cooled with tap water to room temperature. 5.0 mL potassium iodate solution was added to each tube, making up distilled water to the mark (ethanol may volatilization), with capped cover and vortex. Tubes stood for 15min and were measured for their absorbance A at 568nm with reagent blank as a reference. The concentration range of hydrolyzed amino acid has a good linear relationship with absorbance.
Determination of samples: Accurately pipetted 3.00mL of the treated centrifuged enzyme hydrolysate liquid, followed the standard curve preparation steps, and measured the absorbance A of chromogenic samples under the same conditions and the same operation. The corresponding amino acid microgram mass concentration could be found according to the absorbance A on the standard curve.
1) Note:Ninhydrin is oxidized to light red or dark red under the influence of light, air, temperature, and humidity. Purification is required before use. 5 g ninhydrin is dissolved in 20 mL hot distilled water, and 0.25g activated carbon is added and gently stirred for 1 min. The solution is heated for 30min and taken a hot filtering with filter paper. The filtrate is placed in refrigerator overnight. The next day, the solution with crystals was extracted and filtered to obtain light yellow crystals, which were washed with 1.0mL cold water and dried in a glass desiccator. The crystals were stored in a brown glass bottle for later use.
Methodological investigation of amino acid ninhydrin:
a) Accuracy:Took 1.50 mL of the glycine standard into 6 colorimetric tubes of 10-mL. The operation was carried out according to the method and conditions in “2.4.2” to determine the absorbance at 568nm, and the RSD was 1.03%. (N=6).
b) Repeatability:Took 2.50 mL of the centrifugal supernatant of enzymatic hydrolysate into 6 colorimetric tubes of 10-mL. The operation was carried out according to the method and conditions in “2.4.2” to determine the absorbance at 568nm, and the RSD was 0.95% (n=6).
c) Stability:Took 1.50 mL of glycine control standard solution into three 10mL colorimetric tubes. The operation was according to the conditions and methods of “2.4.2” to measure every 2 h for a total of 1 0 h. The RSD is 2.44% (N=3) and the result was stable within 6.5 h.
d) Recovery:Drew 1.50 mL enzymatic hydrolysates of amino acid content measured-to-known into 6 colorimetric tubes of 10mL, added standard solution 1.20 mL to each tube, pressed perform the same operation under the conditions of “2.4.2” to measure the absorbance value. The average recovery rate was 98.36%, and the RSD was 0.85% (n=6). The ninhydrin spectrophotometric method in this experiment was accurate and reliable.
Determination of Hydrolyzed Xylan and Other Monosaccharides [11]
Determination of xylan by DNS colorimetry:
a) Principle:DNS is the dinitro salicylic acid. That is, under alkaline conditions, the REDOX reaction of dinitro salicylic acid with reducing sugar yields 3-amino-5-nitro salicylic acid, which shows brownish red under boiling condition and has absorption at 540 nm. Its color depth is proportional to the sugar content reducing within a certain concentration range. The colorimetric method can also be used to determine the content of reducing xylose when drawing the standard working curve with standard xylose.
b) DNS chromogenic agent solution:Measured 750 mL of distilled water with graduated cylinder into a 1000-mL beaker and added 10.0 g of 3,5-dinitrosalicylic acid, 16.0 g of NaOH, 5.0 g of phenol, 5.00 g of sodium sulfite and 300.0 g of potassium sodium tartrate when the solution was heated to slight heat. After dissolving, the solution was added distilled water to volume scale line in a 1000-mL brown volumetric flask, which was then placed in a dark place at room temperature for a week before use [12].
c) Xylose standard solution:Accurately weighed 1.0 g of anhydrous xylose (baked it to constant weight in an oven at 105°C in advance). After dissolving in distilled water, the standard solution volume was diluted to volume mark in a 1000-mL volumetric flask, and a standard solution with a concentration of 1 mg·mL–1 was obtained.
The standard curve and the methodological investigation:Added xylose standard solution 0, 0.20, 0.40, 0.60, 0.80, 1.00mL in 6 colorimetric tubes of 25mL with distilled water to exactly 1.00 mL each respectively. Then 3.0mL DNS color developer was added into each colorimetric tube, mixing well, and reacting in boiling water for 5.0min. After being cooled with tap water to room temperature, distilled water was added in tubes to the 25mL mark and well shaken. The measurement of absorbance at 540nm was made to draw a standard curve for xylose with reagent blank as a reference. The concentration range of hydrolyzed xylose has a good linear relationship with absorbance and regression coefficient (R2).
a) Accuracy:Took 0.40mL and 0.60mL xylose standard solutions and the operating was carried out according to the method and conditions in “2.5.2” to determine OD540 and to calculate the recovery rate = measured content/actual xylose content×100% and deviation=recovery rate-100%.
b) Stability:After the DNS reagent reacted with the xylose standard solution, the absorbance value was measured every 30minutes, and the measurement was performed 5times repetitively to determine the stable period.
c) Precision:Took 0.6mL xylose standard solution into 6colorimetric tubes of 25mL to be tested sample and the above standard curve as the accompanying control, measured 6 parallels, calculated the average and standard deviation and the precision of the measurement results.
Results and Discussion
The Degradation of Structural Substrate Proteins
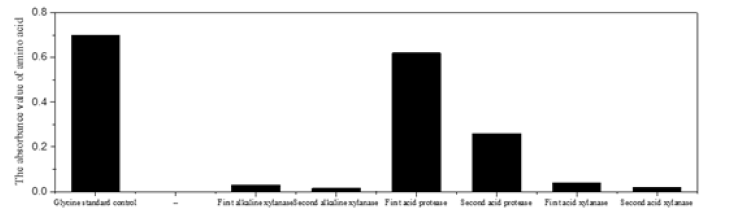
(Figure 3,4) (Table 2) Figure 4 showed the content of amino acid from the same batch of fruit shell crude Gum degraded by alkaline xylanase, acid protease, and acid xylanase in sequence. The high content of amino acids in the enzymatic hydrolysate indicated the presence of structural proteins in the crude Gum, but it didn’t indicate the hydrolyzed product amino acid was from which layer in Figure 5A that showed the structure from inside to outside of the shell. The bottom layer is the reticulated Eu-Gum layer, and the intermeddle tissue layer is the site of polymerization of Eu-Gum, embedding gum silks as being Figure 5B & 6, and the surface (or innermost) layer is the smooth dark brown hard-shell layer that closely wrapped the kernel. To judge the relationship between hydrolyzed protein and inner fruit shell tissues, the fruit crude Gum as Figure 11A was degraded and eroded by acidic protease.
Results showed that: the intermeddle tissue layer embedded with Eu-Gum filament collapsed and powdered since there was polymerase within the tissue, leading to the inner dark brown hard shell being separated from Eu-Gum capsule as shown in Figure 2 (Figure 5).
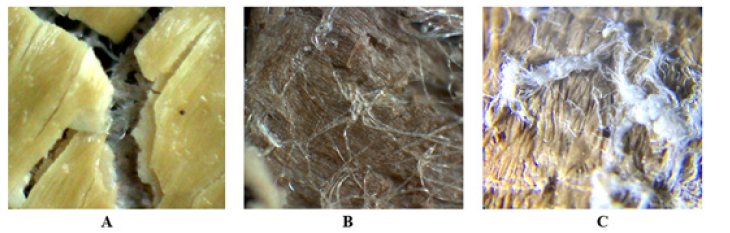
Figure 5: Structure of inner fruit shell. Note*: A is the structure of the three layers of inner fruit shell, B and C: the intermeddle tissue layer embedded with Gum silk.
The amino acids hydrolyzed by protease in the colour reaction of ninhydrin come from the intermeddle tissue embedded with Gum silk as shown above. The erosion of the structure protein, iso-pentadiene Eu-Gum-polymerizing enzyme, contained in this tissue layer resulted in powderization of the tissue layer located between the capsule layer and the dark brown hard inner fruit shell. All of these were due to that the polymerized Eu-Gum within this intermeddle tissue layer was interwoven and piled on the tissue layer, forming a planar network of Eu-Gum capsules wrapped this tissue layer. This physiological process of Eu-Gun formation reasonably explained the phenomenon of Eu-Gum silk embedded in the tissue layer and the outer pericarp and inner shell separated by Gun capsule.
However, in the first stage, the intact water-repellent Gum capsule layer blocked the contact between the acid protease and the inner shell tissues, so the inner fruit shell with Eu-Gum was retained as crude Gum. In the second stage, as the capsule was opened, the exposed intermeddle tissue layer was directly eroded and degraded by the acid protease. Meanwhile, the structure of intermeddle layer collapsed, and tissue powdered, so the Gum capsule layer and dark brown hard inner shell were separated from each other. The degradation and erosion of structural proteins was accompanied by the disappearance of the intermeddle tissue, remaining the innermost dark brown hard fruit shell-- the bottleneck tissue in the hyper purification process of Eu fruit crude Gum.
Structural Substrate in the Innermost Dark Brown Hard Fruit Shell
Definition of structural substrate: a widely distributed and ubiquitous biodegradable substrate in a structural tissue. Plant tissue is a unit with the same structure and function composed of one or several types of cells with similar morphology and structure and the same function, which is the basic structural unit of plant organs [13]. The erosion of the structural substrate caused extensive defects and fractures of the entire structure, causing structural disorder and confusion, which resulted in the powderization of the inner dark brown hard fruit shell. Th degree of freedom of the powdered tissue in aqueous solution increased, and powder discharge rate and diffusion capacity from the opening of Eu-Gum capsule increased. In the tissue of Eu organs, there were two main types of structural substrates: protein and hemicellulose.
Structural protein, an erodible protein substrate by acid protease. Enzymes for life metabolism of plant exist widely in plant organs. In 1926, JB Sumner, an American scientist, prepared urease crystals from the bean for the first time [14] and confirmed that they were proteins. Enzymes are protein, a biological macromolecule. The old tissue degenerates and the enzyme proteins remain near the site as structural proteins.
Structural hemicellulose, an erodible polyxylose hemicellulose substrate by xylanase. Plant is composed of protective, transport, nutritional mechanical, and meristematic tissues, which all are composed of cells [15]. German botanist Matthias Schleiden’s cell theory [16] pointed out that all animal and plant organisms are composed of cells. A cell is a ubiquitous structural tissue, composed of protoplasts and cell wall. Cell wall is a structural tissue unit, whose composition are cellulose (30%-45%), hemicellulose (20%-35%) and lignin (10%-30%), three major polymers [17]. Hemicellulose is a universal plant structure tissue. Although cellulose is the most widely distributed in plants, cellulose has a high degree of crystallinity and is insoluble in water and difficult to hydrolyze. Hemicellulose is a highly hydrophilic heterogeneous polysaccharide polymer, which has three types: polyxylose, polygraph-mannose and polygalactylate-grape-mannose. Xylan is commonly known as hemicellulose due to its high proportion, 15% to 35% of the dry weight of plant cells.
It has the following characteristics [17]:
a) Short molecular chains intersperse between the cellulose
filaments.
b) With lignin to form lignin-carbohydrate, bonding
cellulose, and lignin together.
c) The amount of polyxylose hemicellulose accounts for
50% of the total lignin-cellulosic tissue and binds to the surface of
cellulose microfibers.
d) Xylose hemicellulose is β-D-Xylol pyranose, linked to
other carbohydrate residues by 1→4 glycoside bonds.
Xylanase Eroded Structural Xylan Residues
The xylanase (EC3.2.1.8) used in this study was expressed by Trichoderma fungi, which has two kinds: one can degrade xylan in acidic condition and the other degrade xylan in alkaline condition [18,19] to corrode xylan residues in different plant tissue environments. In the enzymatic hydrolysis and purification of crude Eu-Gum, the acid protease eroded and powdered the intermediate layer tissue, and the innermost dark brown hard shell and the dark brown outer pericarp remained, as shown in Figure 6 outer pericarp and Figure 2 inner hard shell. Figure 7 showed that the amount of xylose hydrolyzed by alkaline xylanase was not high because of the small physical volume of outermost pericarp. The outermost dark brown pericarp on white Eu-Gum capsule was powdered and eliminated by alkaline xylanase as Figure 10A, but the innermost dark brown hard fruit shell did not disintegrate (Figure 6).
Although acidic xylanase could not disintegrate the outmost fruit pericarp, it was able to powder the dark brown inner hard fruit shell as shown in Figure 9. Both acidic and alkali xylanases were multiple enzyme system. But under acidic or alkaline conditions, the conformations of their respective secondary and tertiary peptide chains revealed different active centers corresponding to the spatial conformations of the association groups in different substrates. The xylan hemicellulose either in innermost dark brown hard shell or in outermost pericarp were degraded depending on their accurate association of the corresponding groups between the substrate and the enzyme.
The DNS method is a classic method for the determination of reducing monosaccharides proposed by [20]. Wang Jun Li, et al.’s colour reagent method for determination of xylose [11] had a high accuracy, a small parallel deviation and the colour product had a long stabilization time, which was superior to other DNS reagent formulation method. β-D-1,4 endo-xylanase first released xylose oligosaccharides by acting on β-D-1,4-xylanoside bonds of the main xylan chain [21]. The sensitivity of oligosaccharides was low because only the end groups reacted with DNS. The oligosaccharides were further hydrolyzed by β-D-1,4 exon-xylanase to xylose, which could react with DNS quantitatively and had a highest RF value of thin layer. In the acidic xylanase system, there were sidechain glycoside hydrolases, β-1,4-endo-xylanase, β-xylanase, α-Larabinose, α-D-glucosidase, acetyl-xylanase, and phenolic esterase, which acted on the glucoside bond between xylose and side chain substituent in coordination with main chain hydrolase to erode xylan an. The powderization of innermost dark brown hard fruit shell was enhanced. The determination results of hydrolyzed xylose in Figure 7 confirmed that the erosion of structural xylan was indeed accompanied by the powderization of outermost pericarp and innermost hard fruit shell. The physical volume of innermost hard-shell tissue was much larger than that of outer pericarp, and the amount of hydrolyzed xylose was much larger than that of alkaline xylanase. The powder in Figure 9 showed the appearance phenomenon of that acid xylanase eroded xylose residues in hemicellulose in Figure 7, indicating that the inner hard-shell tissue was powdered when acid xylanase eroded the structural xylan within the inner hard-shell tissue (Figures 7,8).
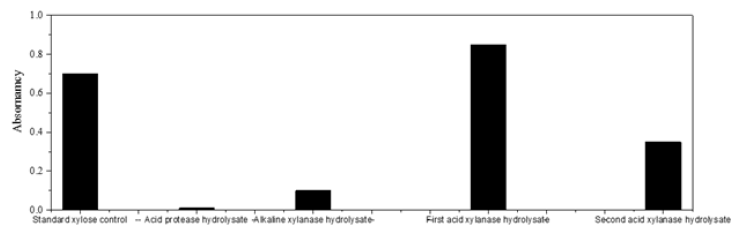
Figure 7: Determination and control of hydrolyzed xylose. The enzymatic degradation of xylan hemicellulose by acid xylanase and alkaline xylanase.

Figure 8: Separation of Eu-Gum silk and inner hard shell. Note*: A: Eu Gum silk and inner shell separated from each other after erosion of intermeddle tissue embedded with Eu-Gum silk. B: sunken the exposed innermost dark brown hard shell.
After the Eu fruit shell crude Gum was hydrolyzed by acid protease and the intermeddle tissue layer embedded with Eu-Gum silk was powdered, Fruit shell capsule layer and the innermost hard shell were separated from each other shown as Fig. 8A. The white edge was the free Eu-Gum silk, and the center was concentrated innermost dark brown hard shell. As the Eu-Gum silk had a density 0.91g/cm3 lass than that of water, it floated in the upper part of the solution and the separated bare dark brown inner hard shells sank to the bottom of the solution shown as Figure 8B.
The Erosion of Xylan and the Powderization of Dark Brown Hard Inner Fruit Shells
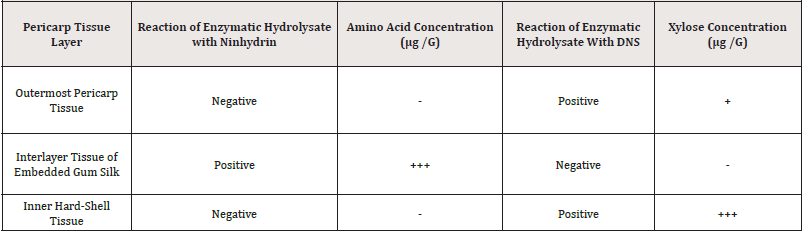
The ninhydrin identifies amino acids and dinitro salicylic acid (DNS) identifies reducing xylose are the classic qualitative and quantitative methods. Their coloration confirmed the presence of amino acid and xylose in hydrolysate. In this study, the structure disintegration and tissue pulverization depended on the existence of corresponding structural substrates, protein, and xylose hemicellulose in the biodegradable materials. The detection experiments of amino acids and xylose, as shown in Table 3, confirmed the correctness of the previous research results, especially the detection of xylose demonstrated the existence of xylose substrate in innermost dark brown hard shell. It is because there were Eu-Gum polymerase proteins in the intermeddle layer of inlay filaments that remain near the original site, while there were no structural proteins in the outermost pericarp and innermost hard shell. However, the xylose hemicellulose that was presented in the innermost dark brown hard shell resulted in the detection of mono-xylose that reacted with DNS, see the table below. The erosion of xylose caused the disintegration of the innermost hard fruit shell layer and the powderization of the tissue (Table 3).
The cell wall specialization of mature cells is characteristic for plant tissues. The lignification of the cell wall that is filled with and attaches to lignin enhances the hardness and mechanical strength of the cell wall. Therefore, a complex enzyme of xylanase and laccase was used in enzymolysis and purification of fruit shell crud Eu-Gum. Laccase hydrolyzed the lignin located in the outer layer of the cell wall, so xylanase was able to approach and erode xylan residues in cell wall tissue, causing the powderization of the innermost dark brown shell tissue (Figure 9).
Fortunately, laccase was suitable for pH range of 4.5-6.5, operating temperature of 30-65℃ and the working temperature of the acid xylanase was 30-60℃ at the best pH=4.8, their similar enzymatic properties promoted and improved their respective enzymatic hydrolysis efficiency. At pH 4.8 and 50℃, the synergistic effect of the two enzymes could significantly enhance the efficiency of powdering innermost dark brown hard fruit shells. The unsymbolized crud Eu-Gum was washed with a sieve, and the dark brown hard fruit shell powder dispersed into water. The innermost dark brown hard-shell powder in solution was collected by centrifugation and dried at 60℃. The fine powder as shown in Figure 9 passed through a 30-mesh standard sieve with a particle size ≤0.55mm, so powder could freely diffuse into the solution and separated from Eu fruit shell Gum (Figure 10).
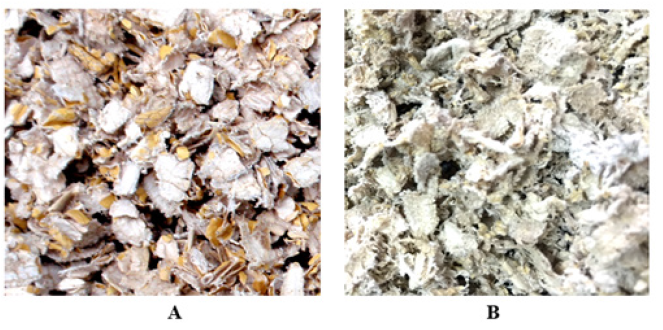
Figure 10: Eu Gum capsule slice. Note*: A: Eu fruit shell crude Gum slice. B: Flossy, loose capsule thin slice with gray and light green pigment.
In Figure 10A, the Eu-Gum capsule layer and the innermost dark brown hard fruit shell adhered together before being hydrolyzed by acid protease and acid xylanase. Due to the innermost rigid hard fruit shell, the Gum capsule presented a rigid flat state as shown in Figure 10A. After enzymatic powderization, Gum capsules without supporting from the innermost hard shell were natural flexible Eu- Gum slice as shown in Figure 10B. The state change confirmed the fact that laccase assisted the disintegration and powderization of acid xylanase to produce the innermost dark brown hard fruit shell powder with particle size ≤0.55mm. Therefore, those demonstrated that the enzymatic hydrolysis of structural substrate to powdered plant tissue theory is a real occurrence and a practical way to produce ultra-pure essential Eu-Gum.
Ultrasonic Purification of Cystic Eu Gum Slice
The fruit shell capsule slice was not pure Eu-Gum since it was attached with grey and light green plant pigments. Physical ultrasonic cleaning was a green mechanical method for stripping pigment and tissue debris. The cavitation effect of ultrasonic tore water molecules to form vacuum microbubbles and forced vibration of Eu-Gum silk and impurity debris were the main purification process [22]. These two kinds of substances were made up of different molecule substances in the primary structure, meaning their difference was essential, and they are different in the frequency of forced vibration. Differential frequency vibration caused loosening and stripping of attached fragments and pigment spots on the Eu-Gum silk. As the vacuum microbubble expanded continuously, the negative pressure in the cavity increased and the high-speed microjet was formed by internal burst collapse in an instant. The pressure of 1012-1013Pa and local high temperature were generated in the surrounding area [23]. The result was vibration fatigue loosening of debris and pigment, micro-jet impacted detritus and colour spot shedding.
Light flakes of the soft flocculent capsule in Figure 11 experienced the erosion and degradation of alkaline xylanase, acid protease, and acid xylanase, and the internal and external tissue components closely related to the Eu-Gum capsule had been powdered and removed. However, the color of the Eu-Gum capsule flakes in Figure 10 is not the original natural pure white. The reason why it is grey and light green is because the surface of the Eu-Gum filament is also covered with a small amount of grey, light green, and brown plant pigments.
Hydrophobic alkane chains of Eu-Gum were repelled and tangled by water molecules, and plant pigment was also hydrophobic and so attached to Eu-Gum silk. Surfactant reduced the surface tension between Eu-Gum and pigment plaques and water molecules, and the thermodynamic equilibrium constants dispersed of Eu-Gum and pigment in water moved toward the diffusion direction of increasing entropy. Therefore, Eu-Gum silk stretched out and spread out to increase its surface and the amount of the captured ultrasound wave was enhanced. The surface area exposed to water and the cleaned surface area of Eu-Gum silk was significantly increased (Figures 11,12).
Under the ultrasonic action assisted by surfactants, the Eu- Gum capsules slices were disassembled into natural pure white floc of single Eu-Gum filaments that were intertwined with each other. There was no y-shaped filament structure with three branches observed. The test report from the FAIR TEST science and technology (Tianjin, China) Co., LTD showed that the purity of white floc Eu-gum was 98.94%, reaching high purity.
Conclusions
Most of the enzymes that could degrade plant tissues were fungal enzymes, such as: white rot mold, soft rot mold, brown rot mold, Aspergillus Niger, wood rot mold, Trichoderma, and other saprophytic fungi. It was difficult to produce enzymes by large-scale deep submerged fermentation of parasitic fungi and symbiotic fungi to produce enzyme preparation. In the study, three enzymes with the function of powdering plant tissue were found: acidic protease that had a universal disintegrating structure and powdering tissue function, alkaline xylanase that disintegrated structure and powdered tissue of outer fruit peel, and acid xylanase that disintegrated inner hard-shell structure and powdered inner hard-shell tissue.
The theory of pulverization of plant tissue proposed in this paper successfully guided the powderization experiment of dark brown inner hard shell in this study, and meanwhile, confirmed that xylan hemicellulose was a structural substrate in plant tissue. Soft gray-green Eu-Gum capsules slices were purified by ultrasonic cleaning with surfactants to prepare native floc pure white fine EU Gum with a purity of 99%.
Acknowledgments
The study was financed by the Ministry of Science and technology, China (No. 2017YFD0600702), the National Key RESEARCH and Development Program of the 13th Five-Year plan, named “Key Technology research on Directional Cultivation and Efficient Utilization of Rubber and medicinal Eucommia ulmoides”. We thank Deputy director of Economic Forest Research and Development Center of Chinese Academy of Forestry, Eucommia ulmoides Engineering Research center of State Forestry Administration, doctoral supervisor of Central South University of Forestry and Technology, and researcher DU Hong-Yan. This research paper was to express my heartfelt tribute memory and deep memories for my supervisor and helpful friend, Prof. Chris Abell, a chemical biologist and pharmaceutical chemist in the Department of Chemistry, University of Cambridge, UK.
Author Contributions
Conceptualization of enzymatic extraction theory, XjZ. and CJ, data curation, MmZ, and XjZ funding acquisition, HT, and XjZ investigation, CJ methodology, MmZ, XjZ, and ZpZ, project administration, CyH, and HT, resources, CJ, and HT, visualization, ZpZ, and MmZ, writing-original draft, XjZ, and LH, writing—review and editing, XjZHL, and CJ All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Ministry of Science and technology, China (No. 2017YFD0600702), the National Key Research and Development Program of the 13th Five-Year plan, named “Key Technology Research on Directional Cultivation and Efficient Utilization of Rubber and Medicinal Eucommia Ulmoides”.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- DU Hong yan, DU Lan ying, FU Jian min, DU Xi shan, ZHANG Xiao zhi, et al. (2006) The Difference and Its Pertinence Analysis of the Gutta-percha Content in Organs of Eucommia ulmoides Oliv. Journal of Central South Forestry University 26(4): 1-4.
- Mo Chong wen (2007) Protein Chemistry and Technology. Beijing, Chemical Industry Press pp.20-21.
- HE Wen guang (2011) Dynamic changes of content and relative molecular mass of gutta-percha in Eucommia ulmoides Oliv. leave, Nonwood Forest Research 29(1): 46-51.
- Jinnian Peng (2007) Study on Content and Molecular Weight Distribution of Gutta-Percha from Eucommia ulmoides Leaves. Doctoral Thesis, Shenyang Pharmaceutical University.
- Zhang Yongkang, Zhou Qiang, Chen Gongxi, Shen Xuxiang, Hu Jiangyu, et al. (2015) Research Status and Progress on Comprehensive Development and Utilization of Samara of Eucommia ulmoides Oliver. Chinese Plant Resources 34(1): 53-59.
- (2016) State Forestry Bureau (under the State Council), National Industrial Development Plan for Eucommia ulmoides.
- XIE Ling, Tao Han, Zhang Xuejun, Zhang Lingli, He Yangjie, et al. (2021) Optimization of Ultrafiltration Technology of Enzymatic Hydrolysate from Eucommia ulmoides Peel. China Pharmacy 32(13): 1557-1564.
- Xuejun Zhang, Chuan Cheng, Mengmeng Zhang, Xiaoyan Lan, Qinghui Wang, et al. (2008) Effect of Alkali and Enzymatic Pretreatments of Eucommia ulmoides Leaves and Barks on the Extraction of Gutta Percha. J Agric Food Chem 56(19): 8936-8943.
- Chen Yufu, Zhang Xuejun, Wang Mingli, He Yangjie, Zhang Mengmeng, et al. (2019) Comparative study on extraction of 4 highly active ingredients such as aucubin from Eucommia ulmoides Oliv. Natural Product Research and Development 31: 10-15.
- Li Wanguang, Wang Taohua, Wang Xinwen, Ji Yishun (2017) The Comparison of Four Methods in Testing Degree of Hydrolysis. 42(5): 35-37.
- Wang Jun Li, Nie Guo Xing, Cao Xiang, Zhang Jian Xin, Zhang Peng Fei, et al. (2010) Effects of Different DNS Reagents in Determination of Xylose Content. Food Research and Development 31(7): 1-4.
- Nie Guoxing, Li Chunxi, Wang Junli (2002) Influence of Preparation Method on the Activity of Xylanase. Cereal & Feed Industry (10): 45-47.
- Qiang Sheng Ed (2006) Botany. Beijing, Higher Education Press.
- C Fajszi, T Keleti (1972) Isoenzymes of tetrameric proteins. Biopolymers.
- YU Zi ping, PENG Hong, LIN Da, RUAN Rong sheng (2011) The Structure Characteristic of Hemicellulose: A Review. POLYMER BULLETIN 6: 48-54.
- LU Shi Wan (1991) Botany. Beijing, Higher Education Press.
- Ning Xu (2012) Energy Plant Cell Wall Structures and Their Digestibility with Alkaline Pretreatments., Master’s Thesis, Huazhong Agricultural University.
- Jeffries TW (1994) Biodegradation of lignin and hemicelluloses/Biochemistry of microbial degradation. Springer Netherlands pp.233-277.
- SUN Chao, CHEN Weiping (2013) Research progress in microorganism xylanase and its application. China Brewing 32(4): 24-29.
- Gail Lorenz (1959) MILLER: Use of dinitro salicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31(3): 426-428.
- LIU Jun (2010) Research Progress of Xylanase Auxiliary Bleaching Mechanism. Paper and Paper Making 29(12): 58-60.
- Sun Zhuo, XU Feng, Wang Wei, Sun Mingzhe, Wang Cong, et al. (2015) Theoretical analysis of ultrasonic wave cleaning technology of Chinese herbal medicine. Agriculture & Technology 35(1): 72-73.
- Gibbs (2013) Essential Reading of Basic Ultrasound Physics. Peoples Military Medical Press.







 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.