Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Antihyperglycaemic Constituents from Cleistopholis Patens and Sansevieria Liberica as Justification of their Antidiabetic Ethnomedicinal Claims
*Corresponding author: Antihyperglycaemia, β-sitosterol, β-stigmasterol, Diabetes mellitus, medicinal plants.
Received: November 29, 2022; Published: December 09, 2022
DOI: 10.34297/AJBSR.2022.17.002376
Abstract
The antihyperglycaemic activity of the methanol leaf and rhizome extracts of Cleistopholis patens and Sanseviera liberica, respectively has been reported and their dichloromethane and n-hexane partitioned fractions, respectively were found to be active. This study therefore evaluated the antihyperglycaemic activity of the chromatographic column fractions of the dichloromethane and n- hexane partitioned fractions of Cleistopholis patens and Sanseviera liberica, respectively. The glucose lowering constituents of the active subfractions were also isolated thereby further justifying their antidiabetic ethnomedicinal usage in Nigeria. The active dichloromethane and n-hexane fractions of methanol leaf and rhizome extracts of Cleistopholis patens and Sanseviera liberica, respectively were subjected to column chromatography. The subfractions obtained were assayed for antihyperglycaemic effect using glucose-induced hyperglycaemic model with glibenclamide (5 mg/kg) and 1 % Tween 80 in normal saline as positive and negative controls, respectively. The most active column fractions of the two plants were further purified by various chromatographic separations which led to the isolation of their antihyperglycaemic constituents. The results of the study showed that subfractions C2, C4, C5, and C8 of C. patens at 400 mg/kg gave comparable antihyperglyceamic activity to glibenclamide (5 mg/kg). Also, subfractions C2, C4, C8 and C9 of the n-hexane fraction of S. liberica were comparable in activity and gave comparable and similar profile of activity to glibenclamide (5 mg/kg). The structural elucidation of the isolated β-sitosterol from C. patens and β-stigmasterol from S. liberica that were carried out using 1D and 2D NMR spectra and their NMR data compared well with literature. The study concluded that the β- sitosterol from Cleistopholis patens and β-stigmasterol from Sanseviera liberica were some of the antihyperglycaemic constituents of the plants and thus justified their folkloric antidiabetic usage in Nigeria.
Keywords: Cyanobacteria; Bioactive Compounds; Biological Activities; Drug Applications
Introduction
Diabetes mellitus is a non-communicable disease characterized by deficiencies in the metabolism of fats, carbohydrates and protein that result in persistent hyperglycaemia due to insulin deficiency or inadequate insulin secretion [1,2]. Global epidemiology showed that about 422 million people are diabetic with the majority of the patients living in low- and middle-income countries. Type 1 and type 2 diabetes mellitus contribute 5-10 % and 90-95 %, respectively to the population of diabetic subjects worldwide [3] while over 1.5 million deaths occur because of diabetes every year [4]. It is presented with excess weight loss, stroke, blindness, and other severe complications [5]. Over the years, various
kinds of synthetic hypoglycaemic drugs have been developed for the management of diabetes. However, the drugs have been variously reported to have significant deficiencies such as, serious side effects and lack of potency in diabetic subjects [6,7]. Hence, increased focus on exploring plants and other natural sources of antidiabetic agents is important.
Cleistopholis patens (Benth.) Engl. & Diels (Annonaceae) also called ‘Apako’ and ‘Ojo’ among the Yorubas and Igbos in Nigeria, respectively, is a tree reaching to 30 m high, occurring from Sierra Leone to Uganda and Zaire [8]. Ethnomedicinally, it is used in the treatment of jaundice, infective hepatitis, stomach disorders as well as in the management of diabetes [9-12]. It has been reported for antiplasmodial, insecticidal and anthelminthic activities [13,14]. Some isolated compounds from the plant include β-stigmasterol, β-sitosterol, campesterol which are terpenoids [15]. Others are β-hydroxysampangine, bornyl-p-trans-coumarate and bornyl-p-cis-coumarate, α-copaene, δ-cadinene, β- caryophyllene [13,14]. In 2017, Ayoola and his coworkers reported the antihyperglycaemic and antioxidant activities of the extract of Cleistopholis patens and its most active dichloromethane partitioned fractions [12]. However, the compounds responsible for its glucose lowering effect were not identified.
Sansevieria liberica Gerome and Labroy (Agavaceae) is known as ‘‘Ida orisa’’ in the Western Nigeria and is folklorically used for the treatment of asthma, diabetes, abdominal pains, hypertension, menorrhagia, piles, sexual weakness [16,17]. Antihypertensive, anticancer, diuretic, antioxidant; hepatoprotective, hypoglycaemic and antihyperglycaemic activities have been reported [18-23]. Some isolated compounds from its stem bark and leaf include, pavetannin, aplysamine-2, abscisic acid, α-conidendinin and quercetin-3-O-α-L-arabinofuranoside [24]. Terpenoids such as β-stigmasterol, β- sitosterol, campesterol have also been isolated from the plant [18]. In a recent study, Ayoola and his team reported a significant and consistent antihyperglycaemic and antioxidant activities of the extract, partition and column fractions of its rhizome [23]. The n-hexane fraction of the extract was found to give a promising and comparable activity with glibenclamide, the positive control [23]. Similar to that of C. patens, the compounds responsible for the antihyperglycaemic activity of S. liberica are yet to be identified. This study was therefore designed to further purify the active dichloromethane and n-hexane partition fractions of C. patens and S. liberica, respectively with the aim of isolating the antihyperglycaemic constituent(s) of the two plants with a view to further justifying their antidiabetic ethnomedicinal claims.
Materials and Methods
Chemicals, Equipment and Instrumentation
UV Spectrophotometer (Model M107, SpectronicCamspec Ltd, U.K.), Accu-chek TM Glucometer (model GB
11558973, Roche, Germany) with Accu-chek TM test strips (Roche, Germany), column chromatographic
(Dimension: 60 × 4 cm, silica gel mesh 70–230) apparatuses were used. Others were aluminium plated thin-layer chromatographic (silica gel 60 F254, 0.25 mm) and glass plated preparative thin-layer chromatographic (silica gel 60 F254, 0.25, 0.5, 1, 2 mm, Whatman Inc., U.S.A.), silica gel (70-230 mesh, Merck & Co., Inc., U.S.A.). Nuclear magnetic resonance (NMR) spectra (400, 500, and 600 MHz) were obtained with Bruker AMX 400, Varian Nova 500, and Varian Unity Plus 600 NMR instruments. All solvents used were of analytical grade.
Animals
Healthy male and female albino Wistar rats (120-180 g) bred under standard conditions (temp. 27 ± 3 °C, relative humidity 65%) at the animal house, Department of Pharmacology, Faculty of Pharmacy, O.A.U., Ile- Ife, Nigeria were used for the study. They were fed on a standard commercial rat pellet diet (Bendel Feeds, Nigeria) and water was given as required. Groups of five rats were fasted for 18 h before administration of glucose, column fractions, drug or vehicle [12,23]. All animal experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the National Academies Press (Committee for the update of the guide for the care and use of laboratory animals) [25].
Plant Materials, Extraction and Partition Fractions
Cleistopholis patens leaf and Sansevieria liberica rhizome were separately collected from the medicinal plant garden, Department of Pharmacognosy, Faculty of Pharmacy, Obafemi Awolowo University (OAU), Ile-Ife, Nigeria. They were identified, authenticated and voucher specimens, IFE 16472 and FPI 2176, respectively were deposited at the Pharmacy Herbarium, Department of Pharmacognosy, Faculty of Pharmacy and IFE Herbarium, Department of Botany, O.A.U, Ile-Ife. Cleistopholis patens leaf were air-dried, powdered and extracted with methanol at room temperature, filtered and concentrated in-vacuo to give 10.8 % w/w yield. The methanol extract was suspended in water, successively partitioned with n-hexane and ethylacetate to obtain their corresponding n-hexane, ethylacetate and aqueous partition fractions that were concentrated in-vacuo. The rhizome of Sansevieria liberica was washed with water, chopped into small pieces, oven-dried at 60 ᵒC, powered and 4 kg of the powdered material was extracted with methanol at room temperature and concentrated in-vacuo to give 11.0 % w/w yield. The methanol extract was suspended in water, successively partitioned with n-hexane and ethyl acetate and concentrated in vacuo to obtain their corresponding fractions.
Column chromatography (CC) of patens and S. liberica fractions
The active antihyperglycaemic dichloromethane fraction (24 g) of the methanol leaf extract of C. patens was adsorbed on silica gel and subjected to column chromatography using gradient solvent systems of increasing polarity comprising of n-hexane, dichloromethane, ethylacetate and methanol. The collected eluates were bulked into subfractions CP1-CP15 based on their TLC profile. Similarly, n-hexane fraction (15.0 g) of the methanol rhizome of S. liberica extract was subjected to column chromatography using gradient solvent systems of increasing polarity of n-hexane, dichloromethane, ethylacetate and methanol. The resulting eluates were bulked into CP1-CP9 based on TLC analysis.
Antihyperglycaemic Assay of Column Fractions
Overnight fasted normal rats were orally administered with glucose (10 g/kg) and those with blood glucose level ≥ 7.0 mmol/L (126 mg/dL) after 0.5 h (T0) were considered hyperglycaemic which were selected and divided into groups of five rats. Each group of rats was separately orally administered with 1% Tween 80 in normal saline (negative control), column fractions and glibenclamide at 5 mg/kg (positive control). A drop of blood was taken from the tip of the tail of each rat at 0.0, 0.5, 1.0, 2.0 and 4.0 h and the glucose level were measured using a glucometer and strip. The blood glucose levels at 0.0 h (T0) were recorded as 100 % while the others were expressed as percentage of the T0 values [26-29].
Isolation of Compound 1 from the Dichloromethane Fraction of patens
Subfraction CP4 (2.0 g) with comparable antihyperglycaemic activity with glibenclamide and fewer TLC spots was adsorbed on silica gel, subjected to CC, eluted with gradient mixtures of solvents and the resulting fractions were bulked by TLC into 20 bulked subfractions coded CP4A- CP4T. Subfraction CP4G (39.0 mg) was subjected to PTLC (0.5 mm, CHCl3: MEOH; 1:1). The resulting bands gave rise to crystalline white powder, A (12 mg), B (2.0 mg) and C (19.0 mg). Isolate A was adjudged pure based on its TLC profile and labelled as compound 1.
Isolation of Compound 2 from the Active n-Hexane Fraction of liberica
Antihyperglycaemic CC fraction, C4 (0.65 g) of S. liberica was adsorbed on silica gel, eluted with solvent systems and the resulting gradient fractions were bulked into D1-D10 based on their TLC profile. Subfraction D5 (0.15g) was further chromatographed on a silica gel, gradiently eluted with solvent systems and bulked into seven subfractions, E1-E7 monitored by TLC. Subfractions E3 and E4 were purified by PTLC (0.5 mm) (N-hex: EtOAc; 9:1) and (N-hex: EtOAc; 8.5:1.5), respectively to yield compounds B (20.0 mg) and C (18 mg), respectively.
Identification of the isolates
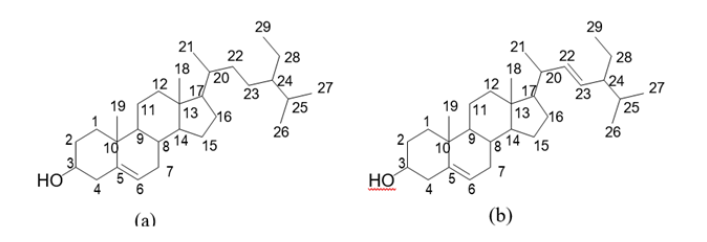
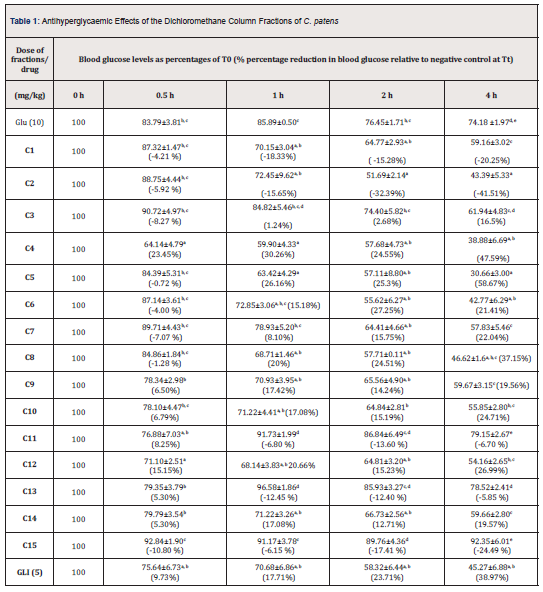
1H/1H-NMR, Homonuclear Correlated Spectroscopy (COSY), 1H/13C-NMR- Heteronuclear Multiple Bond Correlation (HMBC), Heteronuclear Single Quantum Correlation (HSQC), Total Correlation Spectroscopy (TOCSY), and Electro-Spray Ionisation Mass Spectrometry (ESIMS) data of the isolate A from Cleistopholis patens were compared with information in the literature and its identity was confirmed to be, β-sitosterol, while isolates B and C from Sansevieria liberica were one and the same compound and labelled as compound 2 which was found to be, β-stigmasterol [30-32] (Figure 1).
Statistical Analysis
The results obtained from the study were expressed as the mean ± SEM for the number (n=6) of animals in the groups. One Way Analysis of Variance (ANOVA) was used followed by Bonferroni t-test or Student Newman-Keuls post hoc tests to determine the source of significant differences. (p<0.05) was taken as statistically significant.
Results and Discussion
(Table 1) Data show the mean ± SEM blood glucose levels at the different time points expressed as percentage of levels at 0 h (T0), n = 5. Values with different superscripts within columns are significantly different (p<0.05) while values with similar superscript are comparable (p>0.05). GLU (10 g/kg): glucose with 1% Tween 80 in normal saline (negative control); C1–C15: Bulked column fractions of n-hexane fraction of C. patens; GLI: Glibenclamide (positive control).
Glibenclamide, a sulphonylurea, that was used as the antidiabetic standard drug in this study has been reported to be working through minor early extra-pancreatic and major late insulin stimulation [33]. This has made it possible to understand the possible extra-pancreatic and insulin stimulating mechanisms of action of plant extracts/fractions/test agents in glucose-loaded and drug-induced hyperglycaemic rat’s models. This happens when the test agents show similar activity profile to that of glibenclamide or other insulin stimulatory drugs as positive controls [34,35]. Also, it has been reported that results of the glucose loaded rats can be extrapolated to the type 2 diabetic state in humans [36]. Hence this model was employed in this study. The group of rats that received normal saline (negative control) showed a time dependent decrease in blood glucose level up to the fourth hour. This observation could be explained by the homeostatic regulatory mechanism in the normal animals, and it established the healthy state of the rats’ pancreases [29,34,37]. The antihyperglycaemic activity demonstrated by the fifteen column fractions of the active dichloromethane partitioned fraction of C. patens in this work could be grouped into three. The first group consisted of subfractions C11, C13 and C15 that lacked activity from 0.5-4 h of the
experiment which indicated that the antihyperglycaemic constituents of the chromatographic column fraction were not present in these subfractions. The second group were subfractions C1, C3, C6, C7, C9, C10, C12 and C14 that showed moderate and comparable (p>0.05) activity at 4 h of which only the activity of C3 and C7 was time dependent. They were however significantly (p < 0.05) less active than the positive control. Their moderate elicited antihyperglycaemic effect indicated that they contained some of the extra-pancreatic and insulin stimulating compounds in the plant. The third group, however, are subfractions C2, C4, C5, and C8 that gave high antihyperglyceamic activity that was comparable to glibenclamide (Table 1). This showed that the compounds responsible for the hyperglycaemia lowering activity were mostly concentrated in these subfractions. Similar profile of activity of subfractions C2, C5, and C8 with that of glibenclamide may indicate that their mechanism of action may be majorly through insulin stimulation [29,33,37]. Subfraction CP4 with comparable antihyperglycaemic activity with glibenclamide and fewer TLC spots was therefore chosen for further purification for the isolation of its antihyperglycaemic constituent (Table 2).
Data show the mean ± SEM blood glucose levels at the different time points expressed as percentage of levels at 0 h (T0), n = 5. Values with different superscripts within columns are significantly different (p<0.05) while values with similar superscript are comparable (p>0.05). GLU (10 g/kg): glucose with 1% Tween 80 in normal saline (negative control); C1–C9: Bulked column fractions of n-hexane fraction of S. liberica; GLI: Glibenclamide (positive control).
The results of the antihyperglycaemic activity of the column fractions of the active n-hexane fraction of S. liberica showed that subfractions C1 and C3 were devoid of activity at 0.5-4 h which indicated that they lacked the antihyperglycaemic constituents of the partitioned fraction of the plant extract. Subfractions C5, C6 and C7 lacked activity at 0.5-2 h but showed minor activity at 4 h which showed that they lacked the compounds for extrapancreatic activity with some insulin stimulating constituents. However, subfractions C2, C4, C8 and C9 gave a time dependent glucose lowering effect up to the fourth hour that was comparable to glibenclamide (5 mg/kg) at all the time points. This indicated that these subfractions had similar minor extrapancreatic and major insulinotropic mechanisms of action of glibenclamide and the compounds for these effects were majorly concentrated in these subfractions [29,33,37] (Table 2). Subfraction CP4 comparable to glibenclamide (5 mg/kg) at all the time points, relatively good weight and few TLC spots was further purified to obtain its active compound.
Compound 1 that was identified as β-sitosterol and compound 2, β-stigmasterol in this study were isolated from the most active column fractions of Cleistopholis patens (CP4) and Sansevieria liberica, (C4), respectively indicated that they constituted part of the compounds that were responsible for the antihyperglycaemic effect of the plants (Tables 1 and 2). In a similar work, β-sitosterol, isolated from Solanum surattence and Aristolochia indica has been reported to have a promising antidiabetic effect in drug-induced hyperglycaemia and has been recommended for clinical studies for drug development [38,39]. Similarly, β- stigmasterol isolated from Bacopa monnieri, Senecio biafrae and Gelidium spinosus has been established to elicit antidiabetic activities both in in vitro and in vivo studies [40-42]. Also, β- stigmasterol has been reported to be working through both extrapancreatic and insulin stimulating mechanism of actions [40,41] which was similarly confirmed by the result of this study (Table 2). Though β-sitosterol and β-stigmasterol have been isolated from both Cleistopholis patens and Sansevieria liberica in the earlier studies on this plant [14,18], they have not been identified as the antihyperglycaemic constituents of the plants. This work has therefore shown that these compounds constitute some of the antihyperglycaemic constituents of the plants.
Conclusion
The results of this study concluded that β-sitosterol and β-stigmasterol isolated from the methanolic leaf extract of Cleistopholis patens and rhizome of Sansevieria liberica, respectively are some of the antihyperglycaemic constituents of the plants may be working through extrapancreatic and insulin stimulating mechanism of actions which justified their use in the management of diabetes mellitus.
Acknowledgements
The authors are grateful to Mr. Ogunlowo Ifeoluwa of the Department of Pharmacognosy, Obafemi Awolowo University, Ile-Ife, Nigeria for his assistance in the collection and authentication of the plant materials.
Conflict of Interest
There was no conflict of interests among the co-authors or any other persons in the course of this work.
References
- Piero MN, Nzaro GM, Njagi JM (2015) Diabetes mellitus-a devastating metabolic disorder. Asian Journal of Biomedical and Pharmaceutical Sciences 5(40): 1-7.
- Chaudhury A, Duvoor C, Reddy Dendi V S, Kraleti S, Chada A A, et al. (2017) Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Frontiers in Endocrinology 8: 6.
- Madhumathi R, Gowdaiah PK, Dudhwewala A, Chaithra AN, Dande, et al. (2014) Echocardiographic evaluation of diastolic dysfunction in asymptomatic type 2 diabetes mellitus patients. Diabetes 8: 200-209.
- (2021) World Health Organization. The Global Diabetes Compact. 1-7.
- Le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DC, et al. (2017) 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. The Lancet 389(10077): 1399-1409.
- Zhou Z, Zhang, J Zhang, W Zhang, Bai Y, et al. (2011) Rapid screening for synthetic antidiabetic drug adulteration in herbal dietary supplements using direct analysis in real time mass spectrometry. Analyst 136(12): 2613-2618.
- Quianzon CC, Cheikh IE (2012) History of current non-insulin medications for diabetes mellitus. Journal of Community Hospital Internal Medicine Perspectives 2(3): 1-5.
- Burkill HM (1985) The useful Plants of West Tropical Africa, 2nd edition, Royal Botanic gardens, Britain 109-110.
- Olagunju JA, Fagbohunka BS, Oyedapo OO, Abdul AIA (2006) Effects of an ethanol root extract of Plumbago zeylanica Linn. on some serum parameters of the rats. Phytotherapy Research 13: 346-348.
- Odugbemi T (2008) Outlines and Pictures of Medicinal Plants from Nigeria. 2nd edition, University of Lagos press, Nigeria 95-138.
- Gbolade AA (2009) Inventory of antidiabetic plants in selected Districts of Lagos State, Nigeria. Journal of Ethnopharmacology 121: 135-139.
- Ayoola MD, Adebajo CA, Obuotor EM, Oladapo TO, Fleischer TC, et al. (2017) Antihyperglycaemic and Antioxidant Activities of Five Nigerian Antidiabetic Plants. Journal of Science and Technology 37(2): 71-84.
- Boyom FF, Ngouana V, Kemgne EA, Menut, PH Amvam Z, Gut J, et al. (2011) Antiplasmodial volatile extracts from Cleistopholis patens Engler & Diels and Uvariastrum pierreanum (Engl. & Diels) (Annonaceae) growing in Cameroon. Parasitology Research 108(5): 1211-1217.
- Akendengue B, Champy P, NzambaJ, Roblot F, Loiseau PM, et al. (2009) Antifungal and anthelmintic activities of Cleistopholis patens (Annonaceae). Planta Medica 10(75): 1143-1145.
- Oguntimein BO, Erhun WO (1985) The terpenoids of Annonaceae: I: The stem bark of Cleistopholis patens. Nigerian Journal of Pharmaceutical Sciences 1: 25-30.
- Gill LS (1992) Ethnomedical Uses of Plants in Nigeria.University of Benin Press, Benin City, Nigeria 209.
- Osabohien, E, Egboh SH (2008) Utilization of Bowstring Hemp fiber as a filler in Natural Rubber Compounds. Journal of Applied Polymer Science 107: 210-214.
- Ikewuchi JC, Ikewuchi CC (2011) Hypoglycaemic, hypocholesterolemic, anti-anaemic and ocular-protective effects of an aqueous extract of the rhizomes of Sansevieria liberica Gérôme and Labroy (Agavaceae) on alloxan induced diabetic Wistar rats. Asian Journal of Pharmacy and Technology 1(4): 137-148.
- Ikewuchi CC, AyaloguE O, Onyeike EN, Ikewuchi JC (2012) Effect of aqueous extract of the leaves of Sansevieria liberica Gérôme and Labroy on blood pressure indices and pulse rates of sub-chronic saltloaded rats. Journal of Natural Remedies 12(1): 30-38.
- Amao O (2015) Hypoglycaemic activity studies on root extracts of Sanseviera liberica root in streptozotocin-induced diabetic rats. World Academy of Science, Engineering and Technology International Journal of Medical and Health Sciences 9(2): 1.
- Abidemi JA, Zahoor AW, Sadhana S, Girish M, Naresh K S, et al. (2015) In vitro and in vivo anticancer activity of root extracts of Sansevieria liberica Gerome and Labroy (Agavaceae). Evidence- Based Complementary and Alternative Medicine 20(15): 55-66.
- Omodamiro OD, Jimoh MA (2017) Evaluation of in-vitro antioxidant, anti-inflammatory and diuretic potential of an ethanol extract of Sansevieria liberica leaves on Wistar albino rats. The Pharmaceutical and Chemical Journal 4(1): 16-24.
- Ajileye AJ, Ayoola, MD, Elujoba AA, Akinwunmi KF (2020) Antihyperglycaemic and antioxidant activities of Sansevieria liberica as justification for its antidiabetic claims. African Journal of Pharmacy and Pharmacology 14(3): 59-66.
- Eze CC, Inya Agha SI, Ezugwu CO, Ezea SE (2017) Evaluation of anti-inflammatory property of the leaves of Sansevieria liberica And Labr. (Dracaenaceae). Asian Pacific Journal of Tropical Medicine 4(10): 791-795.
- (2011) Committee for the update of the guide for the care and use of laboratory animals, institute for laboratory animal research, division on earth and life studies, national research council of the national academies. Guide for the Care and Use of Laboratory Animals, 8th ed. The National Academies Press, Washington DC.
- Adebajo AC, Iwalewa EO, Obuotor EM, Ibikunle GF, Omisore NO, et al. (2009) Pharmacological properties of the extract and some isolated compounds of Clausena lansium stem bark: antitrichomonal, anti-diabetic, anti-inflammatory, hepatoprotective and antioxidant effects. Journal of Ethnopharmacology 122(1): 10-19.
- Adebajo AC, Ayoola MD, Obagbemi OR, Obuotor EM, Ogunsina MO, et al. (2013) Antihyperglycaemic and antioxidant activities of Eugenia uniflora leaf: evaluation of ethnomedical claims IV. Ife Journal of Science and Technology 1: 1-18.
- Adebajo AC, Ayoola, MD, Odediran SA, Aladesanmi AJ, Schmidt TJ, et al. (2013) Evaluation of ethnomedical claims III: antihyperglycaemic activities of Gongronema latifolium root and stem. Journal of Diabetes 5(3): 336-343.
- Akinwunmi KF, Ayoola MD (2018) Antihyperglycaemic, anti-inflammatory and antioxidant activities of Carica papaya and Citrus lanatus seeds. Ife Journal of Science 20(2): 207-217.
- Pateh UU, Haruna AK, Garba M, Iliya I, Sule IM, et al. (2009) Isolation of stigmasterol, β- sitosterol and 2-hydroxyhexadecanoic acid methyl ester from the rhizomes of Stylochiton lancifolius Pyer & Kotchy (Araceae). Nigeria Journal of Pharmaceutical Sciences 8(1): 19-25.
- Kamboj A, Saluja AK (2011) Isolation of stigmasterol and β-sitosterol from petroleum ether extract of aerial parts of Ageratum conyzoides (Asteraceae). International Journal of Pharmaceutical Sciences 3(1): 94-96.
- Pierre LL, Moses MN (2015) Isolation and characterisation of stigmasterol and β-sitosterol from Odontone mastrictum (Acanthaceae). Journal of Innovations and Pharmaceuticals Biological Sciences 2(1): 88-95.
- Luzi L, Pozza G (1997) Glibenclamide: an old drug with a novel mechanism of action? Acta Diabetologica 34: 239-244.
- Kar A, Choudhary BK, Bandyopadhyay NG (1999) Preliminary studies on the inorganic constituents of some indigenous hypoglycaemic herbs on oral glucose tolerance test. Journal of Ethnopharmacology 64(2): 179-184.
- Rang HP, Dale MM, Ritter JM, Flower RJ (2007) Rang and Dale’s Pharmacology. 6th ed. Churchill Livingstone: London 844.
- Verspohl EJ (2002) Recommended Testing in Diabetes Research. Planta Medica 68: 581-590.
- Ayoola MD, Oriola AO, Faloye KO, Aladesanmi AJ (2020) Justifying the Antidiabetic Ethnomedicinal Claim of Massularia acuminata through its Antihyperglycaemic Activity. American Journal of Biomedical Science and Research 8(2): 76-81.
- Gupta R, Sharma AK, Dobhal MP, Sharma MC, Gupta RS, et al. (2011) Antidiabetic and antioxidant potential of sitosterol in streptozotocin induced experimental hyperglycaemia. Journal of diabetes 3(1): 29-37.
- Karan SK, Mishran SK, Pal D, Mondal A (2012) Isolation of sitosterol and evaluation of antidiabetic activity of Aristolochia indica in alloxan-induced diabetic mice with reference to in vitro antioxidant activity. Journal of Medicinal Plant Research 6(7): 1219-1223.
- Ghosh T, Ghosh S, Maity TK (2014) Antihyperglycaemic activity of stigmasterol isolated from Bacopa monnieri Aerial parts against alloxan induced diabetic rats. International Journal Natural Products Res 4: 40-46.
- Ayoola MD, Adebajo AC, Zotor FB, Pinkoane MG (2019) Justifying Antidiabetic Ethnomedicinal Claim of Senecio biafrae through Its Antihyperglycemic and Antioxidant Activities. Annals of Complementary and Alternative Medicine 1(2): 1-8.
- Poulose N, Sajaran A, Ravindran A, Chandran A, Priyadharshini GB, et al. (2021) Antidiabetic potential of a stigmasterol from the Seaweed, Gelidium spinosus and its application in the formulation of nano emulsion conjugate for development of functional buiscuits. Frontiers in Nutrition 8: 694362.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.