Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Cell Free Fetal DNA As Biomarker for Pregnancy Loss Disorders
*Corresponding author:Tehreem Tahir, Institute of Biomedical & Genetic Engineering (IBGE) Islamabad, Pakistan.
Received: November 24, 2022; Published: December 02, 2022
DOI: 10.34297/AJBSR.2022.17.002369
Abstract
Cell free fetal DNA (cff DNA) is apoptotic fetal DNA that is shed from placenta and flows in maternal blood. This can be used as an indicative biomarker to rule out pregnancy associated complications. The objective of this study is to investigate the correlation of the concentration of cff DNA with intrauterine deaths. The RHD gene (Rh blood group, D antigen) was used as fetal DNA marker for the confirmation of fetal DNA and GAPDH gene (Glyceraldehyde-3-phosphate dehydrogenase) was used as internal control. The control group comprises of 54 healthy singleton pregnant women without any history of pregnancy associated complications or fetal chromosomal abnormalities. The control group was confirmed by follow up for the delivery of normal baby.
Case group consisted of pregnant women who were showing abnormal gynecological findings/history of pervious miscarriages or chromosomal abnormalities. Intrauterine death (IUDs) was confirmed after following up the pregnancy. T-test was applied to find out the correlation between control and case groups. We have observed significantly higher quantities of cell free fetal DNA in case group as compared to control group (P value <0.0001, r2=0.4266). The case group was further subdivided into five groups depending upon the reason of intrauterine death of fetus. A significant correlation has been observed among all the five case subgroups as compared to control group (P value <0.0001). Our study concludes there is a significant increase in the concentration of cell free fetal DNA of IUD cases before onset of the conditions.
Keywords: Cell free fetal DNA, Apoptotic fetal DNA, Intrauterine death, Oli gohydaraminos, Multiple trisomes, Miscarriages
Abbreviations IUDs: Intrauterine Death; cff DNA: Cell free fetal DNA; RHD: RH blood group,D antigen; GAPDH: Glyceraldehyde-3-Phosphate Dehydrogenase; NIPT: Non-Invasive Prenatal Testing; TOP: Termination of Pregnancy; CHD Congenital Heart Disease; mt DNA: mitochondrial DNA; FGR: Fetal Growth Restriction
Introduction
The prenatal determination of sex chromosomes was the opening of a new era to investigate the disorders in developing fetus [1,2]. Fuches and his colleagues in 1960 determined gender to rule out sex linked recessive diseases; hemophilia from amniotic fluid [3]. Advancements in the field of prenatal diagnosis starting from intrauterine detection of genetic defects [4] and first prenatal diagnosis of Down’s Syndrome [5] to preimplantation genetic diagnosis [6] a broad spectrum of prenatal genetic tests have facilitated the parents about the wellbeing of their unborn child.
Around 3% to 5% of pregnancies lead to still birth or the infants with malformation due to chromosomal abnormalities. Aneuploidy is one of the major types of chromosomal abnormality that results the cells with extra or missing chromosomes. During the normal process of meiosis, cellular division produces haploid sperm and eggs, containing one copy of chromosomes from each parent which fertilize to make a diploid zygote. Data suggested that errors during spindle fiber formation due to the less elasticity of aged maternal cells results in the fetus with aneuploidies [7,8].
According to American College of Obstetricians and Gynecologists maternal age alone should not be taken as a threshold to offer prenatal screening rather it should be available to all women. The criteria for the selection of prenatal diagnosis to the parents having increased risk of aneuplodies can be determined by advanced maternal age (35 years or above), pervious deliveries with aneuplodies, still births, spontaneous abortions and chromosomal aneuplodies/translocations/inversions in either of the parent [9].
Nowadays, a wide range of prenatal tests are available for pregnant women to assure the well-being of the unborn child which fall into two groups: the non-invasive and the invasive method. Non-invasive prenatal testing utilizes blood of pregnant women to detect cell free fetal DNA (cff DNA) to screen fetal abnormalities. This method was recognized by Lo et al in 1997 [10]. Invasive prenatal testing includes amniocentesis, chorionic villi sampling and fetal blood sampling. These tests are performed under sonographic control by specialized obstetricians who provide the specimen for genetic analysis. Importantly, all invasive techniques carry a significant procedure-related risk for an abortion. Generally, invasive procedures are arranged upon securing high risk of Non-Invasive Prenatal Testing (NIPT) of pregnant woman [10].
Due to the risk of fetal abnormalities [9] and chances of miscarriages during invasive prenatal sampling, the use of cell free fetal DNA is considered a more attractive alternative of prenatal diagnosis. Cff DNA is apoptotic fetal DNA that shed from villous trophoblast during the development of fetus [11]. In addition to the role of cff DNA in detection of chromosomal abnormalties and single gene disorders, studies have shown the association of significant increase in the level of cff DNA in maternal plasma during pathological conditions for example scleroderma, [12] preeclampsia, [13,14] trisomy 21 [15] intrauterine growth restriction [16,17] and preterm labor [18]. Hence cffDNA can be used as an alternative screening marker for the detection of pregnancy loss disorders. The objective of this study is to investigate correlation of the concentration of cff DNA with intrauterine deaths in Pakistani female population.
Material & Methods
Ethical Statement
The study was approved by the ethical review committee of Institute of Biomedical & Genetic Engineering (IBGE) Islamabad. It was conducted according to the Declaration of Helsinki. The informed patient consent was taken before the all the research activities and analysis.
Inclusion Criteria
Samples were collected from General Hospitals of Islamabad from January 2020 to December 2021 after the 10th week of gestation. The control group comprises of 54 healthy singleton pregnant women without any history of pregnancy associated complications or fetal chromosomal abnormalities who delivered healthy babies at full term (37 weeks of gestation or above). Confirmation of control group was done after the delivery of normal baby. Case group (n=18) consisted of pregnant women who have shown abnormal gynecological findings/history of pervious miscarriages or previous birth with chromosomal abnormalities.These pregnancies were followed up for the clinical outcome so intrauterine deaths (IUDs) or miscarriages were confirmed after following up the pregnancies. Some IUDs were due to unknown causes, others happened as a result of pregnancy associated disorder; oligohydramionsis or due to multiple trisomies (causes are summarized in table 1).
Abbreviations: Intrauterine death (IUDs), Cell free fetal DNA (cff DNA)
Exclusion Criterion
a) Pregnancy loss due to Injury or infection
b) Induced abortions for normal fetus (except in case of intrauterine death)
c) Fetal loss due to Diabetes or hypertension.
Sample Processing
5ml of peripheral blood was obtained in EDTA vacutainer. Plasma was extracted from maternal blood by centrifugation of blood at 2500RPM for 10 min at 4ᴼC. Re-centrifugation of the plasma was done at 16000 RPM for 10 min at 4ᴼC. The plasma extraction was performed on the same day as phlebotomy. Plasmas were kept at -70ᴼC for storage. 1ml plasma was used for cell free fetal DNA extraction though slight modification of manufacturer protocol for blood and body fluids (QIAamp DNA blood mini-Kit, Qiagen, Cat No. 51106. Extracted cff DNA was diluted in 100 µl of buffer AE (Qiagen) and kept at -70ᴼC for storage. cffDNA was quantified by Nanodrop 2000c (Thermo Scientific).
The RHD gene ( Rh blood group, D antigen) was used as fetal DNA marker for the confirmation of fetal DNA( RHD gene amplification in cell free fetal DNA extracted from negative blood group female baring Rh factor positive fetus is the confirmation of the presence of fetal DNA) and GAPDH gene (Glyceraldehyde-3-Phosphate Dehydrogenase) was used as internal control (this in-house gene will amplify both fetal & maternal DNA following the protocol of Devyser RHD (8-A060) using Slan -96P real time PCR System. For the confirmation of trisomies (trisomy 18, 21 and 13) Rapid aneuplodies Detection (Devyser Compact v3, 8-A017.3-25) was done following manufacturer instructions.
Statistical Analysis
The quantity of cell free fetal DNA of case and control group were analyzed using T-test. Statistical software GraphPad Prism version 9.3.1 was used for statistical analysis of data. The data was expressed in comparison of concentration of cell free fetal DNA in case and control groups. Concentration of cell free fetal DNA among case subgroups with control group and comparison of gestational ages and maternal ages between case and control group.
Result and Discussion
Samples were collected from General Hospitals of Islamabad from January 2020 to December 2021 after the 10th week of gestation. The control group comprises of 54 healthy singleton pregnant women without any history of pregnancy associated complications or fetal chromosomal abnormalities who delivered healthy babies at full term (37 weeks of gestation or above). Confirmation of control group was done after the delivery of normal baby. Case group (n=18) consisted of pregnant women who have shown abnormal gynecological findings/history of pervious miscarriages or previous birth with chromosomal abnormalities. These pregnancies were followed up for the clinical outcome so intrauterine deaths (IUDs) or miscarriages were confirmed after following up the pregnancies. Multiple trisomies were confirmed by QF-PCR from abortus fetus. Then our study correlated for the concentration of cell free fetal DNA in case and control group.

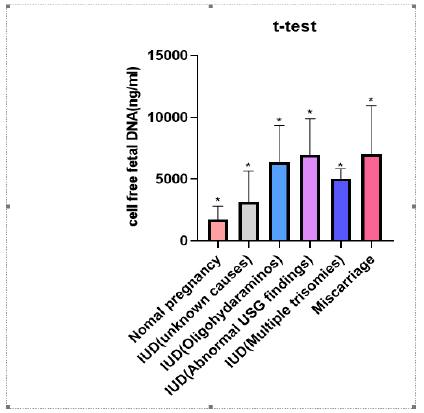
Figure 1: Significant increase in the concentration of cff DNA of complicated pregnancies as compared to the normal pregnancy.
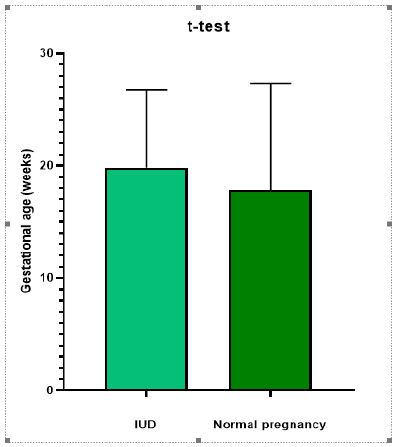
A significant correlation (P value <0.0001, r2=0.4266) has been found for the concentration of cell free fetal DNA in case group vs control group. (Figure 1). Moreover, a significant correlation has been observed among all the five case subgroups (Figure 2) as compared to control group (P value <0.0001). However, there was no significant correlation between gestational ages of case and control groups (P=0.4160, r2=0.009342) (Figure 3) and maternal ages of both the groups; p= 0.4492, r2= 0.008326 (Figure 4).
Concentrations of cell free fetal DNA were significantly high even at early gestational stages in all the pregnancies which ended up with fetal deaths due to unknown causes, IUDs, miscarriages, multiple trisomies or oli gohydramionsis. Distribution, clinical characteristics and concentration of cff DNA of case subgroups is described in Table 1. Clinical characteristics, pregnancy outcome and concentration of cff DNA of case and control group have been shown in table 2. The average gestational age and maternal age of the control group is 17.471 weeks and 30.660 years respectively. The average gestational age and maternal age of case group is 20.3 weeks and 29.6 years respectively (Table 2). The average concentration of cell free fetal DNA of control group is 1740.56 ng/ml (Table 2) whereas the average concentration of cell free fetal DNA of case group is 5161.1ng/ml (Table 2) which is many folds higher as compared to control group. Sample size is 61 to reach confidence interval of 95%.
Abbreviations: Cell free fetal DNA (cff DNA)
This is a primarily study on Pakistani population based on the evaluation of pregnancy out come by initial measurement of concentration of cell free fetal DNA. Recently transgenic Green fluorescent protein (GFP) mice were used to scrutinize that the continual increase in concentration of cell free fetal DNA till labor has not been linked maternal inflammatory response [19] Elevation in total amount of cell free fetal DNA in maternal blood was previously reported significantly high in trisomy 21 and preterm labor using Y specific markers for the confirmation of cell free fetal DNA [18-22]. We have used RHD gene amplification in cell free fetal DNA extracted from negative blood group female baring Rh factor positive fetus for the confirmation of the presence of fetal DNA as fetal marker and concluded that the elevated concentration of cffDNA can be used as an indicative biomarker to rule out any pregnancy associated complication latter in pregnancy.
This is to be noted that cell free fetal DNA concentration has increased many folds before any indication of pregnancy loss or chromosomal abnormalities in case group as compared to control group. The confirmation about the clinical outcome of pregnancies of case group and control group was done before applying statistical analysis. This is worth mentioning that the gestational age at the time of sampling is far before the onset of pregnancy loss (pregnancy losses and their reason were letter confirmed when we followed up our cases). Thus, we conclude that concentration of circulating fetal DNA has increase many folds in pathological conditions as compared to normal pregnancies the possible cause of increasing concentration of cell free fetal DNA in pathological problems is due to increase in apoptosis of placental cells.
The research implication of our study is to determine the threshold of increasing concentration of cffDNA in pregnancy loss disorders. This can be an easy approach to detect pregnancy loss disorders like IUDs miscarriages and aneuplodies. This preliminary data has the potential to be used in clinical applications if carried out on a large number of samples. This is a cost effective and attractive approach to identify pregnancy loses conditions. No doubt that prenatal diagnosis helps the parents and obstetricians for timely desion of termination, but it is not limited to Termination of Pregnancy (TOP) rather it has many other potential benefits. In higher risk cases, where a couple is having children with chromosomal abnormalities; normal result can reduce the obvious parent anxiety which is very important to relieve stressed out pregnant woman [21]. Knowledge of anomalies allows certain preinatal clinical arrangements. For example, there is a significant association between congenital heart disease (CHD) and Down’s syndrome [21]. Similarly, there are chances of delivery with diaphragmatic hernia in aneuplodies [22]. The appropriate operative interventions would be lifesaving in such cases. Awareness about delivery with chromosomal abnormality augments the parents to prepare both emotionally and financially and facilitates the parents to develop a management plan for raising a child with special needs. Prenatal testing though cell free fetal DNA has opened a new era of non-invasive diagnosis of human health condition before birth [23] Over ages, our medical history exceeded far for diagnosis more than treatment. However, the ultimate goal is to treat all medical conditions prenatally, that is not possible if we will not be able to see the whole depiction of disorder inside the womb.
Cell free fetal DNA has opened a novel approach to predict worse developmental pregnancy outcomes. Studies have reported elevated levels of cell free fetal DNA in maternal plasma [24,30] and in maternal serum cell free mitochondrial DNA (mt DNA) [25] in preeclampsia. Conversely there have been low levels of maternal serum cell free mitochondrial DNA in early gestational 11 to 12+6 weeks in preeclampsia [26] Therefore it is a need to develop consensus upon predicting diagnostic/prognostic value [27]. A plasma RNA quantification panel has been developed for detection of Fetal Growth Restriction (FGR) and preeclampsia which requires investigations for larger patient cohorts [28]. in line with our study Borna et al reported significant association between concentrations of cell free fetal DNA & pregnancy associated complications of gestational diabetes, chronic hypertension, still births, fetal trisomies, preeclampsia preterm delivery and intrauterine growth restriction [29].
Conclusion
Elevated concentration of cffDNA can be used as a biomarker to rule out any pregnancy associated complication later in pregnancy. It is to be noted that after following up of the entire pregnant woman included in our study, we correlated the elevated concentration of cell free fetal DNA in case group. This could be a cost effective and rapid screening for detection of pregnancy associated complications. Our data has shown increased concentration of fetal DNA extracted from maternal plasma in all suspected subjects before the any clinical indication of fetal demise [30]. The limitation of our study is confined number of patients which are taken from tertiary care hospitals of a confined area moreover inclusion of only willing patients is also a limiting factor. However, based on this preliminary data potential clinical applications can be developed if carried out on a large number of samples. As we included only RHD gene as fetal marker more fetal markers, for example DNA methylation markers for the confirmation of fatal origin of cell free fetal DNA [23] can be included to generalize the study. More fetal markers may imply accuracy in the utility of early detection of pregnancy related complications [31].
Acknowledgements
We would like to thank Mr Qaiser Mansoor; principal scientist, Institute of Biomedical & Genetic Engineering (IBGE) Islamabad for his guidance and support.
Author’s Contributions
Author 1: Idea/hypothesis, scientific work, data collection, statistical analysis and paper writing.
Author 2 & 3: Patients/sample submission and gynecological investigations according to the criteria of study.
Conflict of Interest
The authors declare no conflict of interest.
References
- Serr DM, Sachs L, Danon M (1955) The diagnosis of sex before birth using cells from the amniotic fluid (a preliminary report). Bulletin of the Research Council of Israel 5B(2): 137-138.
- Riis P, Fuchs F (1960) Antenatal determination of fœtal sex: in prevention of hereditary diseases. The Lancet 276(7143): 180-182.
- Jacobson CB, Barter RH (1967) Intrauterine diagnosis and management of genetic defects. American Journal of Obstetrics and Gynecology 99(6): 796-807.
- Valenti C, Schutta E, Kehaty T (1968) Prenatal diagnosis of Down's syndrome. The Lancet 292(7561): 220.
- Edwards RG, Gardner RL (1967) Sexing of live rabbit blastocysts. Nature 214(5088): 576-577.
- Ghosh S, Ghosh P (2015) Genetic Etiology of Chromosome 21 Nondisjunction and Down syndrome Birth: Aberrant Recombination and Beyond. J Down Syndr Chromosom Abnorm 1(10.4172): 2472-1115.
- Jameel T, Rashid Y, Anwar M, Sultana N, Waqar MA, et al. (1999) Down's syndrome: prospects for prevention by antenatal diagnosis. JOURNAL-PAKISTAN MEDICAL ASSOCIATION 49(3): 60-63.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 88 (December 2007) Invasive prenatal testing for aneuploidy. Obstetrics and gynecology 10(6): 1459-1467.
- Wang JW, Lyu YN, Qiao B, Li Y, Zhang Y, et al. (2021 Dec) Cell-free fetal DNA testing and its correlation with prenatal indications. BMC Pregnancy and Childbirth 21(1): 585.
- Sekizawa A, Yokokawa K, Sugito Y, Iwasaki M, Yukimoto Y, et al. (2003) Evaluation of bidirectional transfer of plasma DNA through placenta. Human genetics 113(4): 307-310.
- Nelson JL, Furst DE, Maloney S, Gooley T, Evans PC, et al. (1998) Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. The Lancet 351(9102): 559-562.
- Lo YD, Leung TN, Tein MS, Sargent IL, Zhang J, et al. (1999) Quantitative abnormalities of fetal DNA in maternal serum in preeclampsia. Clinical chemistry 45(2): 184-188.
- Leung TN, Zhang J, Lau TK, Chan LY, Lo YD, et al. (2001 Jan 1) Increased maternal plasma fetal DNA concentrations in women who eventually develop preeclampsia. Clinical chemistry 47(1): 137-139.
- Lo YD, Lau TK, Zhang J, Leung TN, Chang AM, et al. (1999) Increased fetal DNA concentrations in the plasma of pregnant women carrying fetuses with trisomy 21. Clinical chemistry 45(10): 1747-1751.
- Caramelli E, Rizzo N, Concu M, Simonazzi G, Carinci P, et al. (2003) Cell‐free fetal DNA concentration in plasma of patients with abnormal uterine artery Doppler waveform and intrauterine growth restriction-a pilot study. Prenatal Diagnosis: Published in Affiliation with the International Society for Prenatal Diagnosis 23(5): 367-371.
- Sekizawa A, Jimbo M, Saito H, Iwasaki M, Matsuoka R, et al. (2003) Cell-free fetal DNA in the plasma of pregnant women with severe fetal growth restriction. American journal of obstetrics and gynecology 188(2): 480- 484.
- Leung TN, Zhang J, T K Lau, N M Hjelm, Y M Lo, et al. (1998) Maternal plasma fetal DNA as a marker for preterm labour. The Lancet 352(9144): 1904-1905.
- Hahn S, Jackson LG (2008) Prenatal diagnosis. Preface. Methods in Molecular Biology (Clifton, NJ) 444: v-i.
- Gomez Lopez N, Romero R, Schwenkel, Garcia Flores V, Panaitescu B, et al. (2020) Cell-free fetal DNA increases prior to labor at term and in a subset of preterm births. Reproductive Sciences 27(1): 218-232.
- Clark SL, DeVore GR (1989) Prenatal diagnosis for couples who would not consider abortion. Obstetrics & Gynecology 73(6): 1035-1037.
- LO YM, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. (1997) Presence of fetal DNA in maternal plasma and serum. Lancet 350(9076): 485-487.
- Benhaourech S, Drighil A, Hammiri AE (2016) congenital heart disease and Down syndrome: various aspects of a confirmed association. Cardiovascular journal of Africa 27(5): 287-290.
- Wynn J, Yu L, Chung WK (201) Genetic causes of congenital diaphragmatic hernia. In Seminars in Fetal and Neonatal Medicine19(6): 324-330.
- Lo YD (2022) Discovery of Cell-Free Fetal DNA in Maternal Blood and Development of Noninvasive Prenatal Testing: 2022 Lasker-DeBakey Clinical Medical Research Award. JAMA 328(13): 1293-1294.
- Rafaeli Yehudai T, Imterat M, Douvdevani A, Tirosh D, Benshalom Tirosh N, et al. (2018) Maternal total cell-free DNA in preeclampsia and fetal growth restriction: Evidence of differences in maternal response to abnormal implantation. PLoS One 13(7): e0200360.
- Marschalek J, Wohlrab P, Ott J, Wojta J, Speidl W, et al. (2018) Maternal serum mitochondrial DNA (mt DNA) levels are elevated in preeclampsia-a matched case-control study. Pregnancy Hypertension 14: 195-199.
- Busnelli A, Lattuada D, Ferrari S, Marco Reschini, Barbara C, et al. (2019) Mitochondrial DNA copy number in peripheral blood in the first trimester pre-eclampsia and different preeclampsia clinical phenotypes development: a pilot study. Reprod Sci 26(8): 1054-1061.
- Palei AC (2021) Cell-free DNA as a potential biomarker for preeclampsia. Expert Review of Molecular Diagnostics 21(12): 1253-1256.
- Galbiati S, Causarano V, Pinzani P, Francesca S, Orlando C, et al. (2010) Evaluation of a panel of circulating DNA, RNA and protein potential markers for pathologies of pregnancy. Clinical chemistry and laboratory medicine 48(6):791-794.
- Borna S, Tarafdari A, Khenar H, Zabolian A, Ajir A, et al. (2021) Association of Cell-Free Fetal DNA at 11-17 Weeks of Pregnancy and the Outcome of Pregnancy.
- Kwak DW, Kim SY, Kim HJ, Lim JH, Kim YH, et al. (2020) Maternal total cell-free DNA in preeclampsia with and without intrauterine growth restriction. Scientific Reports 10(1): 11848.






 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.