Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
HUC-MSCs Inhibit the Expression of Cx43 Through JNK Pathway to Improve Hypoxic-Ischemic Brain Damage
*Corresponding author:Jiaowei Gu, Department of Pediatrics, Taihe Hospital, Hubei University of Medicine, China. Wenting Liu, Healthcare Big Data Center, School of Public Health, Hubei University of Medicine, China.
Received:January 18, 2023 Published:February 24, 2023
DOI: 10.34297/AJBSR.2023.18.002437
Abstract
Background: Hypoxic-ischemic brain damage (HIBD) always causes neonatal death and long-term neural system diseases. Previous studies showed that human umbilical cord blood mesenchymal stem cells (hUC-MSCs) could play a protective role in HIBD, but the specific mechanism is unclear. Connexin 43 (Cx43) widely spreads in the central nervous system, especially in astrocytes. Cx43 is also an important component of gap junctions and half channels, which involves maintaining the dynamic balance of the brain environment. The change of Cx43 expression was shown to be closely related to the occurrence and development of neural system diseases. We speculate the protective effect of hUC-MSCs transplantation of HIBD may be achieved by adjusting the expression of Cx43. Objective: To study the role of Cx43 in the treatment of HIBD with hUC-MSCs, and to explore the mechanism of hUC-MSCs in the treatment of HIBD. Methods: In this study, we use both HIBD rat models and human astrocytes cell-cultured oxygen glucose deprivation (OGD) model to test the Cx43 expression change and recovery by hUC-MSCs. Besides, we also use JNK pathway activator, anisomycin, to reverse the hUC-MSCs recovery on Cx43 expression of HIBD model built on 7-day Sprague Dawley (SD) rats. Modified Neurological Severity Score (mNSS) test was used to test the HIBD model and hUC-MSCs transplanted HIBD model. The western blotting, quantitative polymerase chain reaction (qPCR) was performed to test the expression of Cx43 and the JNK pathway. Results: We found that the expression of Cx43 in hippocampus tissue of HIBD rats significantly increased and reduced by hUC-MSCs treatment. With the activation of JNK pathway on hUC-MSCs transplanted HIBD, the Cx43 expression was increased again. The same change of Cx43 expression were observed on OGD-induced astrocytes: Cx43 significantly increased on OGD-induced astrocytes, reduced by MSCs-CM, This demonstrated that hUC-MSCs improve neurological function by inhibiting Cx43 expression through JNK pathway in HIBD rats.
Keywords: Hypoxic-Ischemic brain damage, Astrocytes, Human umbilical cord mesenchymal stem cells, Connexin 43, Inflammatory response, JNK
Abbreviations: hUC-MSCs: Human Umbilical Cord Blood Mesenchymal Stem Cells; HIBD: Hypoxic-Ischemic Brain Damage; OGD: Oxygen-Glucose Deprivation; CM: Conditioned Medium; SD rats: Sprague Dawlay Rats; Cx43: Connexin 43; p-JNK: Phospho-JNK; TNF-α: Tumornecrosis Factor-α; IL-1β: Interleukin-1β; IL-4: Interleukin-4; GJCs: Gap Junction Channels; Hcs: Hemichannels
Introduction
Hypoxic-ischemic brain injury (HIBD) is a common neurological disease in children, especially in neonatal period, which leads to high disability rate and high mortality rate. Aside from mild hypothermia within 6 hours after birth, there is no treatment proven to be effective in improving the prognosis of HIBD [1]. However, due to the narrow time window and strict inclusion criteria, their clinical applications are very limited. So, it is urgent to explore new, safe and effective treatment methods. Recent studies showed that the hUC-MSCs treatment can promote the recovery of neurological function in HIBD rats, but the specific mechanism is still unclear.
In central nervous system, intercellular communication is mainly carried out through gap junction channels (GJCs) [2], which allows direct intercellular communication, energy metabolites and molecular diffusion to maintain the balance of the internal environment [3]. GJCs include two half-channels (Hcs) between adjacent cells. Hcs mainly involves ion and small molecule exchange between intracellular and extracellular environment [4]. Each Hcs consists of six connexins, and there are eleven connexin subtypes expressed in the brain. Cx43 subtype is one of the most abundant connexins, which widely spreads in NVU components, especially in astrocytes [5]. In the physiological state, GJCs in astrocytes remains open, while Hcs shows a low open rate [6]. Under pathological conditions, the over-expression of Cx43 is accompanied by the abnormal open of C43 Hcs [7]. On one hand, many toxic substances such as glutamate, Ca2+, ATP, etc. spread to undamaged cells to aggravate the damage [8,9]. On the other hand, the release of ATP will activate microglia to release many inflammatory factors, which further up-regulate the expression of Cx43 and form a vicious circle [10-12]. Previous studies showed that Cx43 in astrocytes were rapidly and continuously up regulated after spinal cord injury [13], which further mediated the production of CXCL12 and bone cancer pain in rats. It also showed that inhibition of Cx43 expression alleviated bone cancer pain [14]. It is reported that Gap19 has anti-inflammatory and neuro-protective effects by inhibiting the over-expression of Cx43 and opening of Cx43 Hcs in astrocytes after intracerebral hemorrhage [15]. Zhao, et. al. [16] proved that vinpocetine mediated Cx43 in astrocytes to protect against cerebral ischemia-reperfusion injury through PI3K/AKT signal pathway. Therefore, we speculate that over-expression Cx43 in astrocytes plays an important role in HIBD and hUC-MSCs may inhabit it.
JNK is a kind of stress-activated protein kinase, which can strongly respond to external environmental stimuli and inflammatory cytokines and participates in varieties of physiological and pathological processes. It is reported that aspirin inhibits inflammation and scar formation by regulating JNK/STAT-3 signaling pathway and reduces the risk of tendon injury re-healing [17]. Huang, et. al., [18] confirmed that MSCs could secrete the paracrine factors which promoted the survival of astrocytes and down-regulated GFAP after cerebral ischemia through p38/MAPK/ JNK pathway. Recent studies also showed that the activation of JNK may involve in the gap junction remodeling and Cx43 expression induced by uremic toxin [19].
Therefore, we speculate that over-expression Cx43 in astrocytes plays an important role in HIBD and hUC-MSCs may inhabit it through JNK pathway. In this study, we used two models to test this hypothesis: a well-established HIBD rat model rescued by hUC-MSCs and OGD-induced human astrocytes cultured with MSC-conditioned medium (MSCs-CM) in vitro. It showed that Cx43 expression increase in HIBD rats and OGD-induced human astrocytes, reduced by hUC-MSCs treatment, and increase again with activation of JNK pathway. This demonstrated that hUC-MSCs may inhibit the expression of Cx43 through JNK pathway to rescue the hypoxic-ischemic brain damage.
Methods
Animal Groups
The experimental 7-day-old Sprague Dawley (SD) rats were purchased from the Animal Experimental Center of Hubei University of Medicine. All the experimental rats were raised in a specific pathogen-free (SPF) laboratory with a constant temperature of 22- 24°C, 40-60% relative humidity and artificial daylight from 07:00 to 19:00 every day. The food and water are freely accessed. The newborn young rats were fed by their mothers. All the experimental programs were approved by the Animal Ethics Committee of Hubei University of Medicine (license number: SYXK (E) 2019-0031).
Healthy 7-day-old SD rats were anesthetized with 10% chloral hydrate (3ml/kg) and their body temperature was kept at 37.0±0.5°C with a heating blanket. With the head and limbs fixed, their neck skin was cut into a 0.5-1cm incision to ligate the right carotid artery permanently. The incision was then sutured and disinfected. The whole operation was finished within 10minutes. Two hours later, the mice were exposed to 8% oxygen and 92% nitrogen at 37°C for 2.5h and then returned to their dams in a conscious state. The sham group was only subjected to a separation of the right carotid artery without ligation and hypoxia.
The experiments include two steps. The first one included three groups: sham, HIBD and HIBD+hUC-MSCs. On the 7th day after successful modeling, 12ul phosphate buffer was injected into the sham group and HIBD group through intranasal pathway, and the same volume of phosphate buffer containing hUC-MSCs was injected into the HIBD+hUC-MSCs group. The total number of cells was 0.5×106. Behavioral assessment was performed 14 days after HIBD. Western blot was used to detect the protein expressions of Cx43, JNK and p-JNK.QPCR was used to measure the expression of Cx43 mRNA.
The experiments in the second step include four groups: HIBD, HIBD+anisomycin, HIBD+hUC-MSCs and HIBD+anisomycin+hUCMSCs. After 7days of HIBD, the rats in HIBD+ anisomycin group were intra-peritoneal injected with anisomycin, an activator of JNK pathway. The rats in HIBD+anisomycin+hUC-MSCs were intranasal injected with hUC-MSCs and intra-peritoneal injected with anisomycin. Anisomycin was injected continuously for 5days according to the dose of 5mg/kg.
The Modified Neurological Severity Score (Mnss) Test
The modified Neurological Severity Score (mNSS) test includes the following four aspects: movement, sensation, balance and reflex, whose total score is 18 [20]. The higher the score, the more serious the neurological impairment.
hUC-MSCs Culture
HUC-MSCs were derived from the stem cell research center of our hospital. First, the cryopreserved hUC-MSCs were resuscitated in the 10cm petri dish. The standard medium with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin were used. After 72hours, the non-adherent cells were removed, and the fresh medium was added. When it reached 80-90% in the petri dish area, cells were digested with 0.25% trypsin and passaged. The hUC-MSCs in third to fifth passages were selected for the following experiments and all cultures were carried out in a humidified atmosphere with 5% CO2 and 37°C condition. The MSCs-CMs were taken from the second passage of hUC-MSCs cultured in standard medium for 24h.
Construction of OGD Model
The human brain astrocytes were from the Institute of Divinity in our Hospital. OGD was performed as previously described [18]. The third to fifth passages astrocytes with good growth condition were cultured into 6-well plates. 24hours later, the normal cell culture medium was replaced with FBS and glucose-free DMEM medium, and the cells were cultured in hypoxia environment of 5%O2 and 95%N2 for 6hours to establish the model of OGDdamaged astrocytes.
Experimental Grouping and Treatment in vitro
The rats were divided into three groups: normal control group (Cont group), oxygen-glucose deprivation injury group (OGD group) and hUC-MSCs CM treatment group after oxygen-glucose deprivation injury (0GD+CM group). The control group was cultured in standard medium supplemented with glucose at 37° in a regular 5%CO2 cell incubator (normal condition). After OGD, the cells were incubated with standard medium (the OGD group) or MSCs-CM (the OGD+MSCs-CM group) under normal conditions for 24h.
Western Blot Analysis
The total protein was extracted from hippocampus tissue or astrocytes, and the protein concentration in the supernatant was measured by BCA protein detection kit. The protein samples were separated by an 10% gel for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a PVDF membrane. All membranes were blocked with Western blot sealing solution at room temperature for 1 h and incubated at 4℃ overnight with the following primary antibodies: mouse anti- Tubulin (1:2000), rabbitanti-Cx43 (1: 1000), rabbit anti-p-JNK (1:1000), and rabbit anti-JNK (1:1000). Then, all membranes were washed three times with TBST and incubated with HRPconjugated anti-mouse or anti-rabbit secondary antibodies at room temperature for 1h. Finally, the protein bands were detected by enhanced chemiluminescence (ECL). The results were quantified by ImageJ software. All experiments were repeated three times independently.
Quantitative Real-Time Polymerase Chain Reaction (RTqPCR)
According to the instructions of manufacturer, the total RNA of hippocampus tissue or astrocytes was extracted with TRIzol reagent and transcribed into cDNA with reverse transcription kit. Cx43 mRNA expression levels were quantitated by a Kangwei century Ultra SYBR One Step RT-qPCR Kit system. β-actin was considered as endogenous control. The relative mRNA expression levels were calculated by 2-ΔΔCt method. Forward (F) and reverse (R) primers of the above genesas follows: β-actin-F:CCTTCCTGGGCATGGAGTC, β - a c t i n - R : T G A T C T T C A T T G T G C T G G G T G ; Cx43-F:AGGAGTTCAATCACTTGGCGT, Cx43- R:CTCCAGCAGTTGAGTAGGCTT.
Statistics Analysis
Graph Pad Prism 8.0 was used to analyze the data. All the values were expressed as the mean±standard error of the mean (SEM). The T-test was used to compare the average values of the two groups. One-way analysis of variance (ANOVA) was used for multiple groups of comparisons. P<0.05 was considered statistically significant
Results
HUC-MSCs Transplantation Significantly Improve Neurological Function in HIBD Rats
The experiment’s timeline was shown in Figure 1A. The neurological function of the three groups, Sham, HIBD and HIBD+MSCs, were assessed by mNSS test at 14days after HIBD. Their neurological function score was shown in Figure 1B. It shows that the neurological function score of the HIBD rats was significantly higher than that of the sham group. This shows the effectiveness of our HIBD model, where the neurological deficit was serious. After hUC-MSCs transplantation, the mNSS score was significantly reduced on HIBD rats. This suggested that hUC-MSCs could alleviate neurological deficit in HIBD rats (Figures 1,2&3).
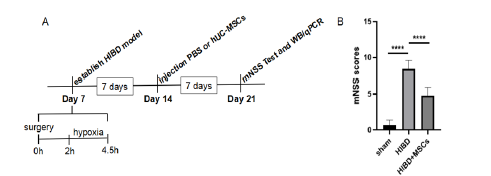
Figure 1:HUC-MSCs transplantation can improve the neurological function in HIBD rats.
(A) A schematic of experiments timeline.
(B) MNSS scores of the sham group, the HIBD group and the HIBD+hUC-MSCs group. The results are presented as the
mean±SEM.****P<0.0001.
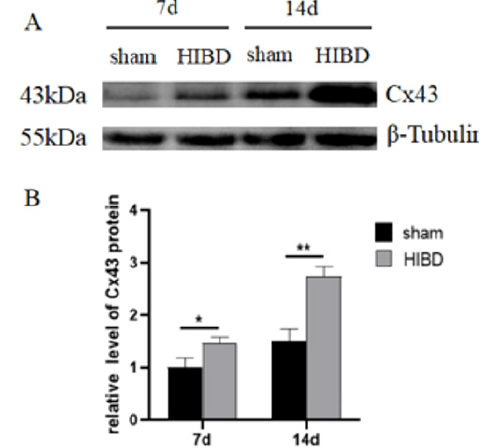
Figure 2:Hypoxia-ischemia treatment promoted the expression of Cx43 in hippocampus of SD rats.
(A) The protein expression level of Cx43 was analyzed by Western blot.
(B) Cx43 expression was quantitatively analyzed by Image J.*P<0.05, **P<
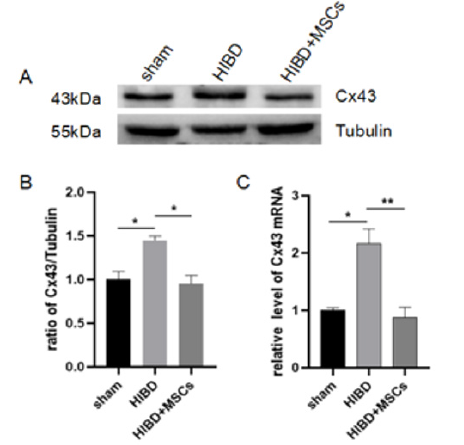
Figure 3:Cx43 expression in hippocampus of three groups.
(A) The protein expression of Cx43 analyzed by Western blot.
(B) Cx43 expression was quantitatively analyzed by Image J.
(C) The expression Cx43 mRNA detected by qPCR. The results are presented as mean±SEM, n=3. *P<0.05, **P<0.01.
Hypoxia-ischemia Significantly Increased Cx43 expression in hippocampus of SD rats
The Cx43 protein expression of Sham and HIBD and HIBD+MSCs on different time points, were shown in Figure 2. Compared with the Sham group, the expression of Cx43 in HIBD group significantly increased, especially on day 14. This suggested that Cx43 may play an important role in the pathological process of HIBD. Therefore, the 14th day of HIBD was selected as the observation time point (Figures 4,5).
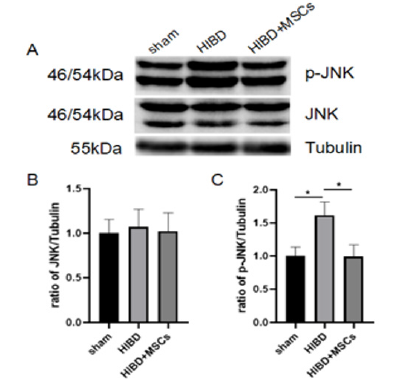
Figure 4:HUC-MSCs transplantation inhibited the activation of JNK pathway.
(A) The expression levels of JNK and p-JNK were detected by Western blot.
(B) JNK expression was quantitatively analyzed by Image J.
(C) P-jnk expression was quantitatively analyzed by Image J. The results are presented as the mean±SEM, n=3. *P<0.05.
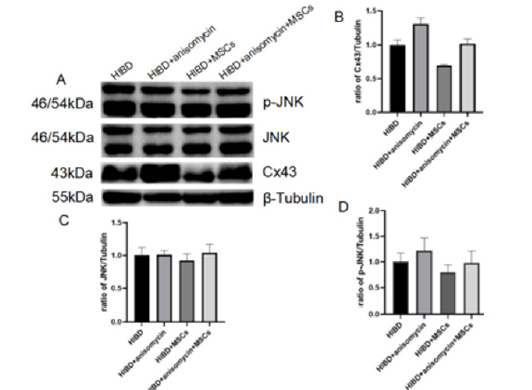
Figure 5:JNK activator reversed the down regulation of Cx43 expression by hUC-MSCs transplantation in HIBD rats. (A-D) The protein expression of Cx43, JNK and p-JNK detected by Western blot on HIBD and HIBD+hUC-MSC with and without JNK activator. The results are presented as the mean±SEM, n=3.
HUC-MSCs Transplantation Inhibited the Increase of Cx43 in Hippocampus of HIBD Rats
The Cx43 protein expression of the three groups, Sham and HIBD and HIBD+MSCs, The Drug Discovery and Development in Indian on New Chemical Entities (NCEE’s) were shown in Figure 3. From Figure 3A-B, Cx43 significantly increased on HIBD, and was decreased after hUC-MSCs transplantation. Consistent with this, the similar Cx43 mRNA expression change was tested by qPCR, hUCMSCs significantly reduce the increased expression of Cx43 mRNA in HIBD Figure 3C. Both results indicate that hUC-MSCs inhibit the increase of Cx43 expression in hippocampus of HIBD rats.
HUC-MSCs Transplantation Inhibited the Activation of JNK Pathway
As JNK may be involved in the gap junction remodeling and Cx43 expression induced by uremic toxin [19], the expression change of JNK pathway were tested on three groups. The protein expression of JNK pathway (JNK and p-JNK) on the three groups, Sham and HIBD and HIBD+MSCs at 14days after HIBD were shown in Figure 4. There is no significant difference of JNK expression among the three groups. Compared with Sham, p-JNK significantly increased in hippocampus of HIBD rats, and hUC-MSCs transplantation inhibited it. This suggested that hUC-MSCs may modulate the expression of Cx43 after HIBD by inhibiting the activation of JNK pathway.
JNK Activator Reversed the Downregulation of Cx43 Expression by hUC-MSCs Transplantation on HIBD Rats
To further improve whether the hUC-MSCs inhabits Cx43 expression increase of HIBD through JNK pathway, the JNK activator, anisomycin, was added on the two groups, HIBD and HIBD+hUCMSCs model. The expression of Cx43 and JNK pathway (JNK/p- JNK) on the four groups, HIBD and HIBD+hUC-MSCs model with and without anisomycin were shown in Figure 5. With anisomycin, the JNK pathway activated, p-JNK and Cx43 significantly increased on HIBD. From previous results, hUC-MSCs transplantation inhibit their increase, but with JNK activation, p-JNK and Cx43 significantly increased again Figure 5. This indicates hUC-MSCs may inhabit Cx43 through JNK pathway.
The Expression of Cx43 in Astrocytes Increased After OGD Injury
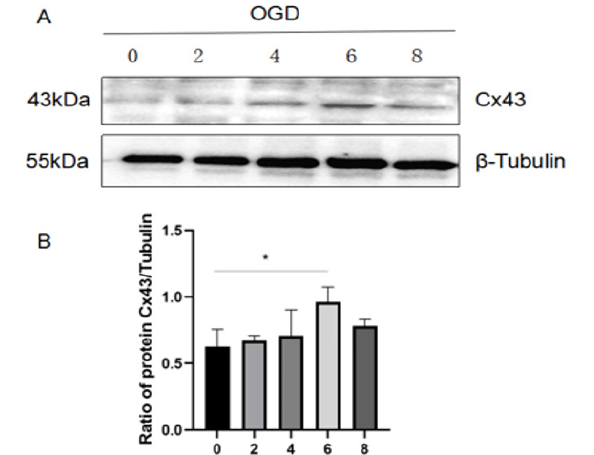
Figure 6:The expression of Cx43 in astrocytes increased after OGD injury.
(A) The expression of Cx43 in astrocytes was detected by western blot at different oxygen-glucose deprivation time (0,2,4,6 and 8 h).
(B) (B)Cx43 expression was quantitatively analyzed by Image J. The results are presented as the mean±SEM
Astrocytes injury is a common outcome of ischemic conditions associated with energy depletion and metabolic disruption [21]. OGD-induced astrocytes were therefore used to mimic ischemic insult. The Cx43 expression in astrocytes at different oxygenglucose deprivation time was assessed by western blot. As shown in Figure 6A and B, the expression of Cx43 in OGD-induced astrocytes constantly increased until the 6 h time point and then showed a decreasing trend. Based on this, 6hours of oxygen and glucose e privation was selected for the following experiments (Figures 6,7).
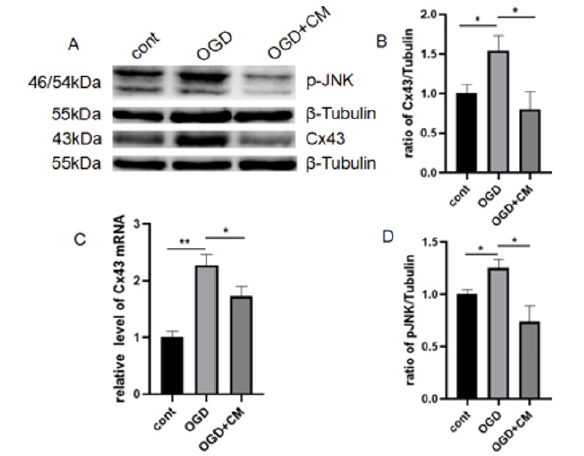
Figure 7:HUC-MSCs CM treatment inhibited the expression of Cx43 and p-JNK in OGD-induced astrocytes.
(A) The levels of Cx43 and p-JNK were detected by Western blot.
(B) Cx43 expression was quantitatively analyzed by Image J.
(C) The expression of Cx43 mRNA was detected by qPCR.
(D) P-jnk expression was quantitatively analyzed by Image J. The results are presented as the mean±SEM, n=3. *P<0.05, **P<0.01.
HUC-MSCs CM Treatment Inhibited the Expression of Cx43 and p-JNK in OGD-Induced Astrocytes
The expression of Cx43 and p-JNK on OGD-induced astrocytes with and without hUC-MSCs CM treatment were shown in Figure 7. Similar as in HIBD model, the same change was observed: both protein and mRNA expression of Cx43 and p-JNK in OGD-induced astrocytes significantly higher than the control group, and then inhibited by hUC-MSCs CM. These results demonstrated that hUCMSCs CM inhibited the Cx43 expression and the JNK pathway in OGD-induced astrocytes.
Discussion
HIBD accounts for most neonatal brain injury cases and is one of the main causes of infant acute death and severe neurological disorders (such as cerebral palsy, epilepsy, and cognitive impairment). Although there are many treatments for HIBD, the rate of poor prognosis is still high. In addition, the cost of treatment is expensive, which brings a heavy burden to the family and society. Therefore, it is greatly urgent to develop more effective and economical methods for HIBD. In recent years, stem cells have become a promising treatment for nerve injury. Because of the advantages of easy access, non-invasion, no ethical problems, and low immunogenicity, hUC-MSCs were considered as the first choice of stem cells for clinical application research. Many animal experiments and clinical studies have proved that hUC-MSCs are safe and effective in the treatment of brain injury [22-24], but the underlying mechanism remains unclear. In this study, Both HIBD animals’ models and OGD-induced astrocytes were used to explore the role of Cx43 and JNK pathways on hUC-MSCs treatment in hypoxic-ischemic brain injury. Our results demonstrated that hUCMSCs transplantation reduced the expression of Cx43, inhibited JNK pathway, and further rescued the neurological damage in HIBD rats.
Cx43 exists widely in the central nervous system and plays an important role in balancing its internal environment. It is reported that Cx43 is involved in the regulation of many immune responses, such as antigen presentation, T cell activation, macrophage migration, cytokine induction and inflammatory body activation [25-27]. Inhibition or down-regulation of Cx43 can reduce local inflammatory responses and accelerate the process of disease recovery [28-30]. Previous studies have shown that changes in the expression and function of Cx43, including GJCs coupling and Hcs activity, are closely related to the occurrence and development of many nervous system diseases [31-34]. Our study suggested that the Cx43 expression exhibit marked increase at 7-14days after HIBD in vivo, especially on day 14 and within 6 h of OGD injury in vitro, as well as a significant increase. We also found Both MSCs transplantation and in vitro co-culture could inhibit the increase of Cx43 expression and promoted the recovery of neurological function after brain injury. It was reported that GJCs and Hcs formed by Cx43 play an important role in neuropathic pain, and the Cx43 inhibitors significantly relieve neuropathic pain [35]. Almad, et al., [36] found that astrocyte-specific knockout of Cx43 slowed disease progression in space and time and improved the survival of motor neuron in the ALS mice model. Chen, et al., [37] proved that inhibition of Cx43 expression can alleviate cerebral ischemia / reperfusion injury through TLR4 signal pathway in middle cerebral artery occlusion (MCAO). Yu, et al., [15] illustrated that targeting Cx43 exerts the anti-inflammatory effect after intracerebral hemorrhage by regulating YAP signal pathway. Therefore, we speculated that hUC-MSCs might protect the HIBD rats partially through the regulation of Cx43 expression.
JNK signaling pathway is an important part of MAPK pathway, which is highly active in the central nervous system and plays an important role in many physiological and pathological processes such as inflammatory response, cell growth, differentiation, apoptosis, and stress. One study has shown that JNK can be used as an upstream mediator of Cx43, and inhibiting the activation of JNK signal pathway can reduce the expression of Cx43, whose decrease further reduces sevoflurane-induced hippocampus cell apoptosis and improves long-term biological behavior [38]. It is reported that Gap26, a specific Cx43 blocker, could antagonize morphine analgesia tolerance and significantly inhibit the activation of JNK and c-jun induced by chronic morphine analgesia tolerance [34]. In our study, we found that the process of HIBD was accompanied by the activation of the JNK pathway, and hUC-MSCs transplantation inhibited the JNK pathway activation. Moreover, our results also suggested that anisomycin, an activator of JNK, suppressed the decreases of Cx43 and p-JNK protein expression induced by hUC-MSCs transplantation. Liang et al. [39] confirmed that the downregulation of Cx43 expression mediated by JNK signal pathway in IL-22-induced psoriatic dermatitis mice. The above findings suggest that hUC-MSCs transplantation improved the neural function in HIBD rats by inhibiting Cx43 expression, which may be related to the JNK pathway.
Conclusions
In summary, our results indicate that hUC-MSCs transplantation may regulate Cx43 expression through JNK pathway to improve neurological deficits. This provides a novel mechanism for the treatment of hUC-MSCs in HIBD. Our study only explores the role of Cx43 in hUC-MSCs transplantation for HIBD and is not designed to investigate the effects of GJCs and Hcs. Secondly, whether the protective effect of hUC-MSCs transplantation for HIBD is achieved by secreting some factors still needs to be further explored.
Funding
This study was supported by the Science and Technology Research Project of Hubei Province, China (No. 2013BCB002, NO. 2021CFB158), Education Research Project of Hubei Province(No. D20222106) and National Natural Science Foudation of China (No. 32060150)
References
- Midori Yenari, Kazuo Kitagawa, Patrick Lyden, Miguel Perez Pinzon (2008) Metabolic downregulation: a key to successful neuroprotection? Stroke 39(10): 2910-2917.
- Md Mirazul Islam, Zahurin Mohamed (2015) Computational and Pharmacological Target of Neurovascular Unit for Drug Design and Delivery. Biomed Res Int 2015: 731292.
- Christian C Naus, Christian Giaume (2016) Bridging the gap to therapeutic strategies based on connexin/pannexin biology. J Transl Med 14(1): 330.
- Christian Giaume, Luc Leybaert, Christian C Naus, Juan C Saez (2013) Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol 4: 88.
- Christian Giaume, Annette Koulakoff, Lisa Roux, David Holcman, Nathalie Rouach (2010) Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11(2): 87-99.
- Yeri Kim, Joanne O Davidson, Katherine C Gunn, Anthony R Phillips, Colin R Green, et al. (2016) Role of Hemichannels in CNS Inflammation and the Inflammasome Pathway. Adv Protein Chem Struct Biol 104: 1-37.
- Mauricio A Retamal, Nicolas Froger, Nicolas Palacios Prado, Pascal Ezan, Pablo J Saez, et al. (2007) Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27(50):13781-13792.
- Juan C Saez, Mauricio A Retamal, Daniel Basilio, Feliksas F Bukauskas, Michael VL Bennett (2005) Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta 1711(2): 215-224.
- Zu Cheng Ye, Megan S Wyeth, Selva Baltan Tekkok, Bruce R Ransom (2003) Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23(9): 3588-3596.
- Simon JO Carroll, Catherine A Gorrie, Sailakshmi Velamoor, Colin R Green, Louise FB Nicholson (2013) Connexin43 mimetic peptide is neuroprotective and improves function following spinal cord injury. Neurosci Res 75(3): 256-267.
- Carmen Vazquez, Rosa Maria Tolon, Maria Ruth Pazos, Marta Moreno, Erin C Koester, et al. (2015) Endocannabinoids regulate the activity of astrocytic hemichannels and the microglial response against an injury: In vivo studies. Neurobiol Dis 79: 41-50.
- Xiang Yin, Liangshu Feng, Di Ma, Ping Yin, Xinyu Wang, et al. (2018) Roles of astrocytic connexin-43, hemichannels, and gap junctions in oxygen-glucose deprivation/reperfusion injury induced neuroinflammation and the possible regulatory mechanisms of salvianolic acid B and carbenoxolone. J Neuroinflammation 15(1): 97.
- I-Hui Lee, Eva Lindqvist, Ole Kiehn, Johan Widenfalk, Lars Olson (2005) Glial and neuronal connexin expression patterns in the rat spinal cord during development and following injury. J Comp Neurol 489(1): 1-10.
- LiHua Hang, Shu Na Li, Hong Luo, Wei Wei Shu, Zu Min Mao, et al. (2016) Connexin 43 Mediates CXCL12 Production from Spinal Dorsal Horn to Maintain Bone Cancer Pain in Rats. Neurochem Res 41(5): 1200-1208.
- Hailong Yu, Xiang Cao, Wei Li, Pinyi Liu, Yuanyuan Zhao, et al. (2020) Targeting connexin 43 provides anti-inflammatory effects after intracerebral hemorrhage injury by regulating YAP signaling. J Neuroinflammation 17(1): 322.
- Mingming Zhao, Shuai Hou, Liangshu Feng, Pingping Shen, Di Nan, et al. (2020) Vinpocetine Protects Against Cerebral Ischemia-Reperfusion Injury by Targeting Astrocytic Connexin43 via the PI3K/AKT Signaling Pathway. Front Neurosci 14: 223.
- Anhui Wang, Changshui Xu (2019) The role of connexin43 in neuropathic pain induced by spinal cord injury. Acta Biochim Biophys Sin (Shanghai) 51(6): 555-561.
- Weiyi Huang, Bingke Lv, Huijun Zeng , Dandan Shi, Yi Liu, et al. (2015) Paracrine Factors Secreted by MSCs Promote Astrocyte Survival Associated With GFAP Downregulation After Ischemic Stroke via p38 MAPK and JNK. J Cell Physiol 230(10): 2461-2475.
- Chih Ying Changchien, Meng Ho Sung, Hsin Han Chang, Wen Chiuan Tsai, Yu Sen Peng, et al. (2020) Uremic toxin indoxyl sulfate suppresses myocardial Cx43 assembly and expression via JNK activation. Chem Biol Interact 319: 108979.
- Zhuoyu Wen, Xiaomeng Xu, Lili Xu, Lian Yang, Xiaohui Xu, et al. (2017) Optimization of behavioural tests for the prediction of outcomes in mouse models of focal middle cerebral artery occlusion. Brain Res 1665: 88-94.
- Jiabin Guo, Sue P Duckles, John H Weiss, Xuejun Li, Diana N Krause, et al. (2012) 17β-Estradiol prevents cell death and mitochondrial dysfunction by an estrogen receptor-dependent mechanism in astrocytes after oxygen-glucose deprivation/reperfusion. Free Radic Biol Med 52(11-12): 2151-2160.
- Jiaowei Gu, Li Huang, Che Zhang , Yong Wang, Ruibo Zhang, et al. (2020) Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: a randomized, controlled trial. Stem Cell Res Ther 11(1): 43.
- Li Huang, Che Zhang, Jiaowei Gu, Wei Wu, Zhujun Shen, et al. (2018) A Randomized, Placebo-Controlled Trial of Human Umbilical Cord Blood Mesenchymal Stem Cell Infusion for Children with Cerebral Palsy. Cell Transplant 27(2): 325-334.
- Huajiang Dong, Gang Li, Chongzhi Shang, Huijuan Yin, Yuechen Luo, et al. (2018) Umbilical cord mesenchymal stem cell (UC-MSC) transplantations for cerebral palsy. Am J Transl Res 10(3): 901-906.
- Aaron M Glass, Elizabeth G Snyder, Steven M Taffet (2015) Snyder and S.M. Taffet, Connexins and pannexins in the immune system and lymphatic organs. Cell Mol Life Sci, 2015. 72(15): 2899-2910.
- Yanru Huang, Zhimin Mao, Zhen Zhang, Fumiko Obata, Xiawen Yang, et al. (2019) Connexin43 Contributes to Inflammasome Activation and Lipopolysaccharide-Initiated Acute Renal Injury via Modulation of Intracellular Oxidative Status. Antioxid Redox Signal 31(16): 1194-1212.
- Daniel Rodjakovic, Lilian Salm, Guido Beldi (2021) Function of Connexin-43 in Macrophages. Int J Mol Sci 22(3): 1412.
- Kongping Li, Huarong Zhou, Lixuan Zhan, Zhe Shi, Weiwen Sun, et al. (2018) Hypoxic Preconditioning Maintains GLT-1 Against Transient Global Cerebral Ischemia Through Upregulating Cx43 and Inhibiting c-Src. Front Mol Neurosci 11: 344.
- Michel Dosch, Joel Zindel, Fadi Jebbawi, Nicolas Melin, Daniel Sanchez Taltavull, et al. (2019) Connexin-43-dependent ATP release mediates macrophage activation during sepsis. Elife 8: 42670.
- Akshata A Almad, Arpitha Doreswamy, Sarah K Gross, Jean Philippe Richard, Yuqing Huo, et al. (2016) Connexin 43 in astrocytes contributes to motor neuron toxicity in amyotrophic lateral sclerosis. Glia 64(7): 1154-1169.
- LingYan Xing, Tuo Yang, ShuSen Cui, Gang Chen, et al. (2019) Connexin Hemichannels in Astrocytes: Role in CNS Disorders. Front Mol Neurosci 12: 23.
- Juan A Orellana, Pablo J Saez, Kenji F Shoji, Kurt A Schalper, Nicolas Palacios Prado, et al. (2009) Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal 11(2): 369-399.
- Shen Ning, et al. (2013) Spinal cord Cx43 mediates chronic morphine analgesic tolerance in rats through JNK pathway. Chinese Pharmacology Bulletin 29(3): 372-376.
- Yeri Kim, Jarred M Griffin, Mohd N Mat Nor, Jie Zhang, Peter S Freestone, et al. (2017) Tonabersat Prevents Inflammatory Damage in the Central Nervous System by Blocking Connexin43 Hemichannels. Neurotherapeutics 14(4): 1148-1165.
- Yunjiao Wang, Gang He, Hong Tang, Youxing Shi, Xia Kang, et al. (2019) Aspirin inhibits inflammation and scar formation in the injury tendon healing through regulating JNK/STAT-3 signalling pathway. Cell Prolif 52(4): 12650.
- Akshata A Almad, Arens Taga, Jessica Joseph, Sarah K Gross, Connor Welsh, et al. (2022) Cx43 hemichannels contribute to astrocyte-mediated toxicity in sporadic and familial ALS. Proc Natl Acad Sci U S A 119(13): 2107391119.
- Yingzhu Chen, Liangzhu Wang, Lingling Zhang, Beilei Chen, Liu Yang, et al. (2018) Inhibition of Connexin 43 Hemichannels Alleviates Cerebral Ischemia/Reperfusion Injury via the TLR4 Signaling Pathway. Front Cell Neurosci 12: 372.
- Congjie Bi, Qiuping Cai, Yangyang Shan, Fan Yang, Shiwei Sun, et al. (2018) Sevoflurane induces neurotoxicity in the developing rat hippocampus by upregulating connexin 43 via the JNK/c-Jun/AP-1 pathway. Biomed Pharmacother 108: 1469-1476.
- Jingyao Liang, Pingjiao Chen, Changxing Li, Dongmei Li, Jianqin Wang, et al. (2019) IL-22 Down-Regulates Cx43 Expression and Decreases Gap Junctional Intercellular Communication by Activating the JNK Pathway in Psoriasis. J Invest Dermatol 139(2): 400-411.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.